Abstract
Context:
Adequate amount of keratinized gingiva is necessary to keep gingiva healthy and free of inflammation. Autografts have been used for years with great success to increase the width of attached gingiva. Autografts, however, have the disadvantage of increasing postoperative morbidity and improper color match with the adjacent tissues. Alloderm® allograft has been introduced as an alternative to autografts to overcome these disadvantages.
Aim:
In this study, the efficacy of alloderm® in increasing the width of attached gingiva and the stability of gained attached gingiva was evaluated clinically.
Materials and Methods:
Five patients with sites showing inadequate width of attached gingiva (≤1 mm) were enrolled for the study. The width of keratinized gingiva and other clinical parameters were recorded at baseline and 9th month postoperatively.
Result:
In all cases, there is the average increase of about 2.5 mm of attached gingiva and was maintained for 9-month. Percentage shrinkage of the graft is about 75% at the end of 3rd month in all cases. Excellent colors match with adjacent tissue has been obtained.
Conclusion:
The study signifies that Alloderm® results in an adequate increase in the amount of attached gingiva and therefore can be used successfully in place of autografts.
Keywords: Alloderm®, allograft, attached gingiva, case series, keratinized gingiva, periodontal plastic surgery
INTRODUCTION
The presence of a thick keratinized gingival covering serves as an effective barrier that is resistant to damage by various types of insults. As such, it forms the basis for a healthy gingival function.[1] There are certain situations in which an adequate width of attached gingiva is important. A narrow zone of keratinized tissue favors gingival recession and inflammation in patients with subgingival restorations, impedes proper impression taking in prosthodontics, and results in an unstable attachment level after orthodontic treatment.[2,3,4] A band of keratinized tissue around the neck of implants is desirable from a clinical standpoint because it facilitates surgery, prosthodontics, cosmetics, and maintenance.[5] An adequate amount of attached gingiva makes plaque control more effective, decreases susceptibility to infection, and possibly prevents further recession. Various gingival augmentation procedures have been proposed using autografts and allografts.[6]
First used in burn surgery in 1992, freeze-dried acellular dermal matrix (Alloderm®) allografts were subsequently introduced in periodontal surgery in 1994 as an alternative to autogenous free gingival grafts (FGGs) to achieve increased attachment of keratinized gingiva around natural teeth or implants,[7] root coverage,[8] and ridge preservation procedures.[9]
Allografts are freeze-dried, cell-free dermal matrices comprised of a structurally integrated basement membrane complex and an extracellular matrix in which collagen bundles and elastic fibers are the main components.[10] Cell components in the allografts are the targets of rejection response. In addition, the ultrastructural integrity of the extracellular matrix, if damaged, would induce an inflammatory response in the body. A major advantage of Alloderm® over skin preparations is the absence of the undesirable dead cells with their associated Class I and II HLD antigens and potential transmission of cell-associated viruses. Furthermore, Alloderm® has essentially undamaged collagen and elastin matrices and does not initiate an inflammatory response by the host recipient tissues, making it completely biocompatible.[11,12]
The use of autografts has been considered the most predictable procedure for keratinized tissue augmentation and vestibular deepening. When compared to autografts, however, Alloderm® eliminates the need for a second surgical site, thus decreasing postoperative morbidity. It is easy and less time-consuming to use, and esthetically it blends well with the adjacent tissue; and multiple sites can be treated in a single visit.[7,10,13]
Therefore, the case study was undertaken to clinically evaluate the use of an Acellular Dermal Matrix Allograft (Alloderm®) to increase the width of attached gingiva and to evaluate stability of the gained attached gingival tissue.
CASE REPORT
A total of 5 patients of which 3 were males and 2 were females in the age range of 20–60 years were selected from out-patients of the Department of Periodontology, Bapuji Dental College and Hospital, Davangere. The study was carried out on subjects having sites with attached gingiva equal to or <1 mm on the facial aspect of the teeth [Figure 1]. In addition to having a limited amount of attached gingiva, patients had to fulfill the following criteria: (1) Good oral hygiene; (2) Facial probing depths ≤2 mm; (3) No removable partial denture in the area to be treated; (4) Not allergic to amoxicillin or clindamycin; (5) No dermal or autoimmune disease; (6) No systemic disease; and (7) Were a nonsmoker. The procedure to be used and the potential risks and complications were discussed with the patient. An informed consent was obtained from each participating patient. The study was approved by the Ethics Committee of Bapuji Dental College, Davangere.
Figure 1.
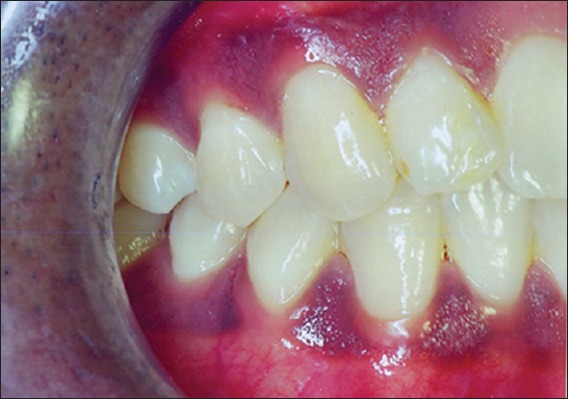
Preoperative photograph showing less width of attached gingiva in relation to first premolar
The junction of the attached and movable tissue was determined by rolling the alveolar mucosa coronally with the side of the probe (a roll-test). The width of the attached gingiva was measured at baseline and 3, 6, and 9-month postoperatively using the subtracting method. The apicocoronal dimension of the graft used was measured at the time of placement onto the recipient bed. The amount of graft shrinkage was calculated by subtracting the apicocoronal dimension of the graft from the amount of gained attached gingiva. Plaque index,[14] gingival index,[15] probing depth, and recession depth were also measured at baseline and 3, 6, and 9-month postoperatively.
Presurgical and postsurgical measurements were made by one examiner. Measurements were made to the nearest 0.5 mm using a UNC 15 periodontal probe (Hu Freidy, U.S.A.) and an occlusal stent (with guiding grooves).
Surgical procedure
All patients underwent Phase-1 periodontal therapy that included oral hygiene instructions, supragingival and subgingival scaling, and root planning. The case was selected for surgery only when patient compliance about oral hygiene was found to be satisfactory. Patients were instructed to rinse with 0.12% chlorhexidine solution twice daily. After adequate anesthesia, a superficial horizontal incision was made just coronal to the mucogingival junction. The surgical blade (No. 15) was held perpendicular to the gingival surface, and the lips were retracted firmly as the incision was made. Two vertical incisions were made at either end of the horizontal incision [Figure 2]. The periosteal recipient bed was then prepared by sharp dissection in an apical direction. Muscle and loose connective tissue fibers were thoroughly scraped with a scalpel to prevent subsequent graft mobility [Figure 3]. Following preparation, the required dimension of Alloderm® allograft was procured and rehydrated in a Petri dish with 50 ml of sterile saline solution for 5 min. After the protective backing paper had been floated, the Alloderm allograft was transferred to another dish with 50 ml of sterile saline solution for 5 min. The allograft was placed with the connective tissue surface toward the recipient beds and the basement membrane surface facing externally [Figures 4 and 5]. The allograft was stabilized on the recipient bed by resorbable sutures [Figure 6].
Figure 2.
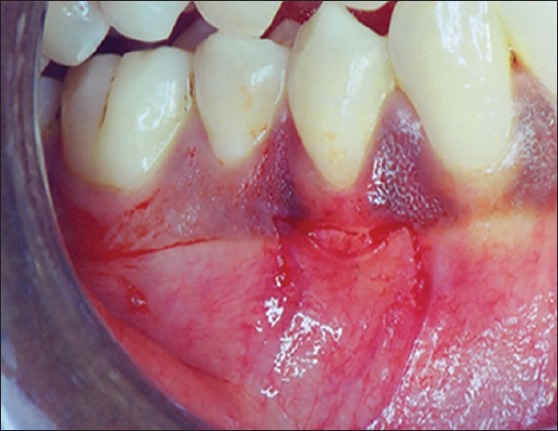
Horizontal and vertical incision given at recipient site
Figure 3.
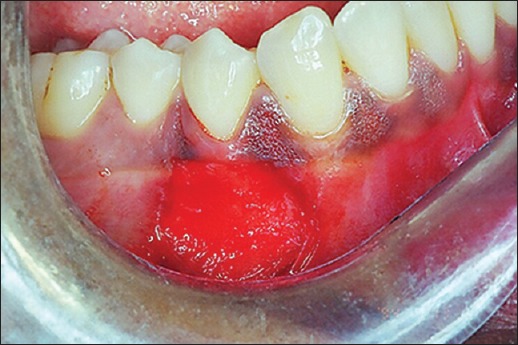
Recipient site prepared
Figure 4.
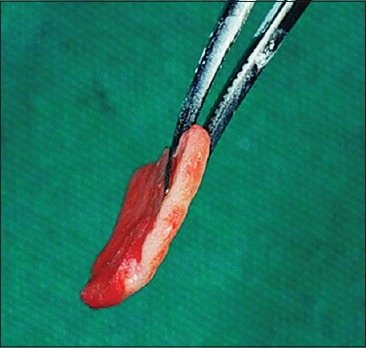
Rehydrated alloderm® showing the connective tissue side of the graft
Figure 5.
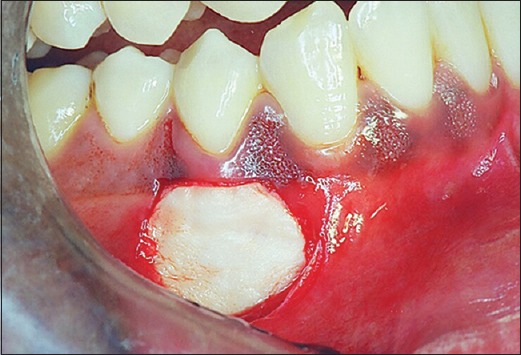
Alloderm® placed at the site
Figure 6.
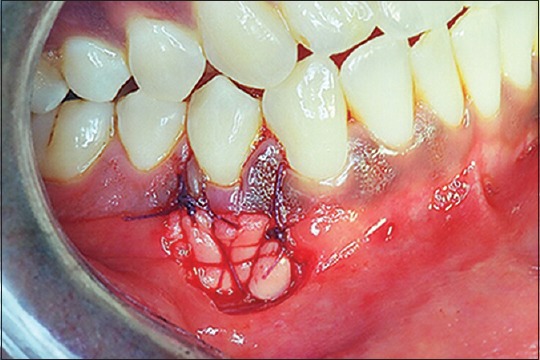
Alloderm® sutured and stabilized
Postsurgical care
The patients were given postoperative instructions and medications. Amoxicillin (500 mg 3 times a day for 5 days) and ibuprofen (3 times a day for 3 days) were prescribed. Continuous rinsing with 0.12% chlorhexidine solution twice daily for 3 weeks was also prescribed. The patient was advised to refrain from retracting the lips and cheeks and to avoid brushing or flossing in the grafted area for 6 weeks. Suture removal was done at the end of 10 days. The patient was seen at 6 weeks [Figure 7], 12 weeks [Figure 8], 24 weeks [Figure 9], and 36 weeks [Figure 10] to monitor wound healing and plaque control. Localized supragingival scaling was done if required and oral hygiene instructions were reinforced routinely at each visit.
Figure 7.
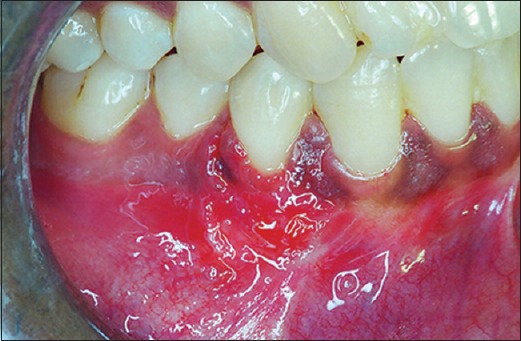
Postoperative photograph at 15th days after suture removal
Figure 8.
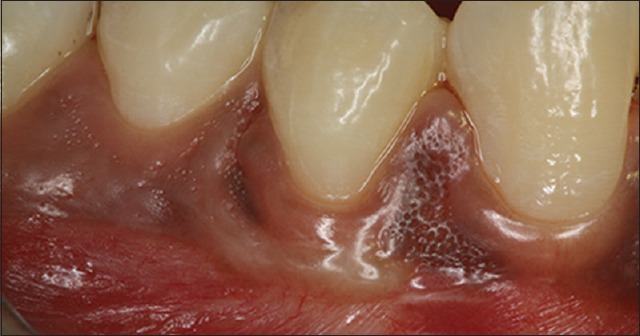
Postoperative photograph at 3-month
Figure 9.
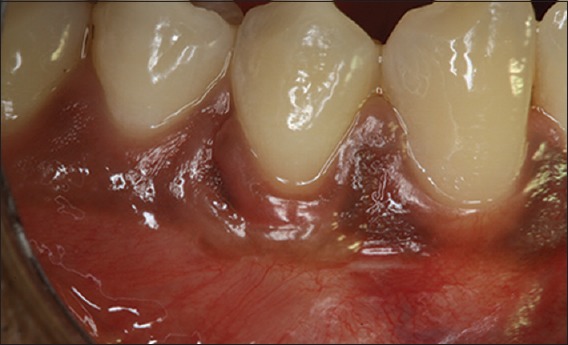
Postoperative photograph at 6-month
Figure 10.
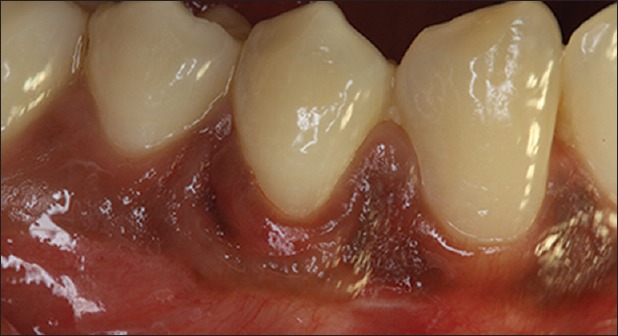
Postoperative photograph at 9-month
RESULTS
All of the sites treated with Alloderm® demonstrated uneventful healing 15 days after surgery. Graft rejection and mobility of the newly created tissues were not found in any of the patients. However, at the 4th week postoperatively, a substantial amount of graft shrinkage could be seen. Excellent color blend was also noticed at this stage.
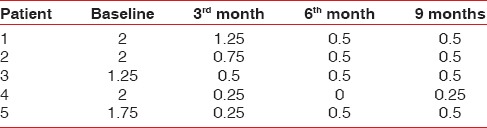
Tables 1–3 show changes in plaque index, gingival index, and probing depth, respectively. All of these three parameters show a consistent decrease in value in all patients, which indicates improved oral hygiene by the patients after the grafting procedure. There was no change in recession depth throughout the follow-up period. Table 4 shows an increase in the width of the attached gingiva in all patients during the 9-month follow-up period. Complete keratinization of the newly formed attached tissue was observed at the end of 12 weeks [Figure 8] and was maintained for 9-month [Figure 10]. In all cases, there is an average increase of about 2.5 mm of attached gingiva. Table 5 shows the percentage shrinkage of the graft, which on average was 75% at the end of the 3rd month in all cases and was stabilized for 9-month.
Table 1.
Data showing changes in plaque index

Table 3.
Data showing changes in probing depth
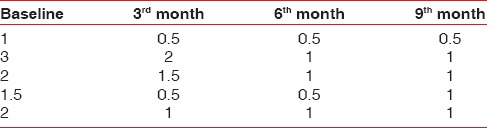
Table 4.
Data showing width of AG
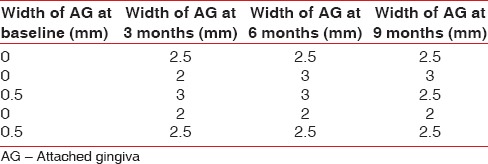
Table 5.
Data showing amount of shrinkage of alloderm
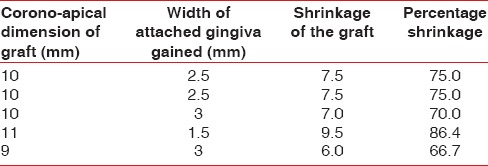
Table 2.
Data showing changes in gingival index
DISCUSSION
In this case study, an acellular dermal matrix (Alloderm®) was used to increase the width of attached gingiva at sites with <1 mm of attached gingiva. For years, free gingival autografts and connective tissue autografts have been used with great success. However, certain obvious limitations of the autografts forced clinical researchers to search for some alternative allografts that patients would find comfortable, compatible, and acceptable. Autografts require a second surgical site for the donor tissue. In the FGG technique, the donor site is healed by secondary intention and may result in postoperative pain and morbidity. The FGGs result in a tyre patch appearance of the recipient site and are unaesthetic. Furthermore, autografts cannot be used to increase width of attached gingiva on multiple teeth at the same visit because of the limited supply of donor tissue.[16] The use of Alloderm® has been shown to be effective in increasing the width of attached gingiva[11,12,17] and can be used as a substitute for FGGs and connective tissue grafts. The studies in which Alloderm® and connective tissue grafts have been used to increase the width of attached gingiva have shown similar results.[18] The main aim of using this allograft was to minimize postsurgical complications and patient discomfort. Studies have shown that an acellular dermal matrix allograft provides a uniform thickness and is easily trimmed, well-adaptable material, and requires a short time (<10 min) to rehydrate before it can be used.[10] On the other hand, harvesting of a connective tissue graft from the palate is time-consuming and the size of the graft that can be harvested limited. A second surgical site and the amount of time consumed adds to patient discomfort as compared to the lesser degree of patient discomfort with Alloderm®.
Studies comparing FGG and Alloderm® to increase the width of attached gingiva have shown that Alloderm® is not as effective in increasing width of attached gingiva but more predictable in its esthetics and blending with the surrounding tissue as compared to FGG. The amount of attached gingiva gained with Alloderm®, however, is clinically sufficient to prevent persistent inflammation.[10,18]
The mechanism by which Alloderm® results in an increased width of keratinized attached gingiva is still controversial. Though most of the studies support the fact that the Alloderm® graft itself has little influence on epithelial differentiation, the type of epithelium that covers the allograft seems to be determined by the surrounding tissues. Alloderm® acts as a scaffold to allow repopulation of fibroblasts, blood vessels, and epithelium from surrounding tissues, and it is eventually completely replaced by host tissues.[19] Studies have also shown that this material (Alloderm®) exhibited more shrinkage than an autogenous FGG.[8,10] The results of this study also showed considerable shrinkage (an average of 75%) in the allograft, which can be explained by the fact that Alloderm® has a nonvital matrix, and it lacks epithelial differentiation. The study further supports the use of an Alloderm® allograft for periodontal plastic surgical procedures.
CONCLUSION
It can be concluded that a lesser but adequate amount of attached gingiva can be obtained using Alloderm® that is sufficient to maintain oral hygiene and resolve persistent gingival inflammation. Viewed subjectively, the sites showed better esthetics and blended with the surrounding tissue and caused less postoperative pain. These findings suggest that Alloderm® can be effectively used as an alternative to autogenous grafts for gingival augmentation procedures.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Camargo PM, Melnick PR, Kenney EB. The use of free gingival grafts for aesthetic purposes. Periodontol 2000. 2001;27:72–96. doi: 10.1034/j.1600-0757.2001.027001072.x. [DOI] [PubMed] [Google Scholar]
- 2.Haeri A, Serio FG. Mucogingival surgical procedures: A review of the literature. Quintessence Int. 1999;30:475–83. [PubMed] [Google Scholar]
- 3.Maynard JG, Jr, Wilson RD. Physiologic dimensions of the periodontium significant to the restorative dentist. J Periodontol. 1979;50:170–4. doi: 10.1902/jop.1979.50.4.170. [DOI] [PubMed] [Google Scholar]
- 4.Randall J. Consensus report. Mucogingival therapy. Ann Periodontol. 1996;1:702–6. doi: 10.1902/annals.1996.1.1.702. [DOI] [PubMed] [Google Scholar]
- 5.Wennström JL, Bengazi F, Lekholm U. The influence of the masticatory mucosa on the peri-implant soft tissue condition. Clin Oral Implants Res. 1994;5:1–8. doi: 10.1034/j.1600-0501.1994.050101.x. [DOI] [PubMed] [Google Scholar]
- 6.Henry HT, Robert RA, Thomas JH. Periodontal plastic and esthetic surgery. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Clinical Periodontolgy. 10th ed. St. Louis: WB Saunders Company; 2006. pp. 1005–29. [Google Scholar]
- 7.Silverstein LH, Callan DP. An acellular dermal matrix allograft substitute for palatal donor tissue. Postgrad Dent. 1996;3:14–21. [Google Scholar]
- 8.Henderson RD, Greenwell H, Drisko C, Regennitter FJ, Lamb JW, Mehlbauer MJ, et al. Predictable multiple site root coverage using an acellular dermal matrix allograft. J Periodontol. 2001;72:571–82. doi: 10.1902/jop.2001.72.5.571. [DOI] [PubMed] [Google Scholar]
- 9.Fowler EB, Breault LG, Rebitski G. Ridge preservation utilizing an acellular dermal allograft and demineralized freeze-dried bone allograft: Part I. A report of 2 cases. J Periodontol. 2000;71:1353–9. doi: 10.1902/jop.2000.71.8.1353. [DOI] [PubMed] [Google Scholar]
- 10.Wei PC, Laurell L, Geivelis M, Lingen MW, Maddalozzo D. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 1. A clinical study. J Periodontol. 2000;71:1297–305. doi: 10.1902/jop.2000.71.8.1297. [DOI] [PubMed] [Google Scholar]
- 11.Callan DP, Silverstein LH. Use of acellular dermal matrix for increasing keratinized tissue around teeth and implants. Pract Periodontics Aesthet Dent. 1998;10:731–4. [PubMed] [Google Scholar]
- 12.Shulman J. Clinical evaluation of an acellular dermal allograft for increasing the zone of attached gingiva. Pract Periodontics Aesthet Dent. 1996;8:201–8. [PubMed] [Google Scholar]
- 13.Scarano A, Barros RR, Iezzi G, Piattelli A, Novaes AB., Jr Acellular dermal matrix graft for gingival augmentation: A preliminary clinical, histologic, and ultrastructural evaluation. J Periodontol. 2009;80:253–9. doi: 10.1902/jop.2009.080326. [DOI] [PubMed] [Google Scholar]
- 14.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 15.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 16.Cohen ES. Mucogingival surgery. In: Cohen ES, editor. Atlas of Cosmetic and Reconstructive Periodontal Surgery. 3rd ed. Shelton: People's Medical Publishing House; 2007. pp. 45–85. [Google Scholar]
- 17.Harris RJ. Gingival augmentation with an acellular dermal matrix: Human histologic evaluation of a case – Placement of the graft on bone. Int J Periodontics Restorative Dent. 2001;21:69–75. [PubMed] [Google Scholar]
- 18.Harris RJ. Clinical evaluation of 3 techniques to augment keratinized tissue without root coverage. J Periodontol. 2001;72:932–8. doi: 10.1902/jop.2001.72.7.932. [DOI] [PubMed] [Google Scholar]
- 19.Wei PC, Laurell L, Lingen MW, Geivelis M. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 2. A histological comparative study. J Periodontol. 2002;73:257–65. doi: 10.1902/jop.2002.73.3.257. [DOI] [PubMed] [Google Scholar]


