Abstract
Objective:
This study was designed to evaluate the efficiency of microwave sterilization of orthodontic instruments and molar bands immersed in plain distilled water with and without oral rinse, and to ascertain the minimum time of exposure required to sterilize.
Materials and Methods:
The orthodontic instruments (hinged and nonhinged), molar bands and mouth mirrorsused in the patient 's mouth were selected for the study. The instruments were divided into two groups – Group I with oral rinse-set A (0.01% chlorhexidine gluconate) and set B (0.025% betadine) and Group II (included sets C and D without oral rinse). The instruments of set A, B and C were microwaved at 2,450 MHz, 800 W for 5 min, whereas, set D was microwaved for 10 min at the same above mentioned specifications. The efficacy of sterilization was assessed by stab inoculation of the instruments onto trypticase soya agar plates. The plates were checked for bacterial growth following incubation at 37 °C for 24 h. For sterility control,Geobacillus stearothermophilus (MTCC 1518) was included.
Results:
No growth was observed in the plates that were inoculated with the microwaved orthodontic instruments of sets A, B and D, whereas scanty bacterial growth was observed in the plates inoculatedwith the microwaved set C instruments.
Conclusion:
Effective sterilization was achieved when the orthodontic instruments and molar bands were immersed in distilled water without oral rinse and microwaved for 10 min as also for those that were immersed in distilled water with oral rinse and microwaved for 5 min.
KEY WORDS: Biological indicator, microwave sterilization, molar bands, orthodontic pliers
Awareness of efficient sterilization techniques occupies center-stage in the prevention of the spread of infectious diseases. Many oral and systemic disease causing organisms are easily transmitted from the oral cavity having long latent period of incubation. The concern about the transmission of infectious–contagious diseases, such as AIDS, hepatitis B, herpes, pneumonia, and tuberculosis, between the dental patients, orthodontists, dental personnel, dental office, and dental laboratory has brought the importance of the control of cross infection back into the limelight.[1,2] Orthodontists are at an ever greater risk to exposure of serious pathogens and must take adequate precautions to guard themselves against their transfer. The preferred method to sterilize orthodontic pliers has always been debatable, with the common methods being moist heat by autoclave and dry-heat with hot air oven.[3] Orthodontic offices use dry-heatsterilizers as it protects the delicate cutting surfaces of the orthodontic instruments as also because of the contention that the moisture in steam autoclave can cause corrosion and rapid deterioration of the instruments.[4,5,6,7] The process of cleaning and sterilizing orthodontic instruments presents special problems as these instruments have large hinge areas that are difficult to clean and sterilize. They also have cutting edges, sharp angles, and pointed ends that are easily damaged. Matlack[8] stated that autoclaving causes severe rusting and corrosion on pliers ' joints. Jones[9] opined that on comparison with cold disinfection and autoclaving there was no significant difference in the corrosion or efficiency of orthodontic pliers. In another study, Jones et al.[10] was of the opinion that after routine use and steam autoclaving, stainless steel pliers performed better than chrome-plated pliers.
Cottone et al.[11] recommended the use of microwave irradiation as a practical physical sterilization method and Tate et al.[12] opined that it was as effective as autoclaving. The lethal action of microwaves on microorganisms is well-established in the literature, but the mechanism of destruction by microwaves is yet not completely understood. Fitzpatrick et al.[13] Jeng et al.[14] Yeo et al.[15] reported that the effect of microwave irradiation on microorganisms is directly of thermal character, whereas Carrol and Lopez,[16] Culkin and Fung,[17] and Olsen,[18] opined that the effects were of a nonthermal one. Biological indicators provide the only real method for verifying the effectiveness of sterilization procedures since the sterilization of the bacterial spores provides a guarantee that effective sterilizing conditions have been achieved. Geobacillus stearothermophilus (formerly Bacillus stearothermophilus) is a commonly used biological indicator for testing the efficiency of steam autoclave and microwave oven.[19] The use of microwave sterilization in dentistry has been widely reported, but its usein orthodontics finds scant mention.
Objective
This study was designed to evaluate the efficiency of microwave sterilization of orthodontic instruments and molar bands immersed in plain distilled water with and without oral rinse and to ascertain the minimum time of exposure required to completely sterilize the same.
Materials and Methods
The present study was conducted in the Department of Orthodontics and Dentofacial Orthopedics, Sree Balaji Dental College and Hospital,Chennai, in collaboration with the Department of Microbiology, Sree Balaji Dental College and Hospital, Chennai.
A combination of hinged and nonhinged orthodontic instruments and orthodontic molar bands were selected for the study. The hinged orthodontic instruments were distal end cutter, band cutting scissor, pin and ligature cutter, bracket positioner, weingart plier, and posterior band removing plier and the nonhinged orthodontic instruments were band pusher and band seater. Mouth mirror and orthodontic molar bands too were included in the study.
Four sets (Set A, Set B, Set C, Set D) comprising of hinged and nonhinged orthodontic instruments and molar bands were used in the patient's mouth and then stabbed onto trypticase soya agar plate (Himedia India Pvt. Ltd., India) and incubated at 37°C, while, the biological indicator, G. stearothermophilus MTCC 1518 (IMTECH, Chandigarh, India) impregnated on a filter paper strip was also inoculated onto trypticase soya agar plate and incubated at 52°C. After 24– 48 h the plates were checked for the presence of bacterial colonies. Group I consisting of set A and Set B instruments were immersed in distilled water with 0.01% of chlorhexidine gluconate, and distilled water with 0.025% of betadine, respectively, and were irradiated for 5 min. Group II was without oral rinse and the set C and set D instruments under this group were immersed in plain distilled water and irradiated for 5 min and 10 min respectively. All the four sets of instruments in their respective microwave containers were irradiated separately in a microwave oven IFB model: 20PG3S exposed to microwaves of 2450 MHz frequency and 800 W power. For sterility control, G. stearothermophilus MTCC 1518 (IMTECH, Chandigarh, India) was impregnated on a sterile whatmann no. 1 filter paper strip and placed in a sterile 1.5 ml microfuge (Tarsons Products Pvt. Ltd., India). along with the instruments for sterilization. The whole protocol was performed in triplicate.
Results
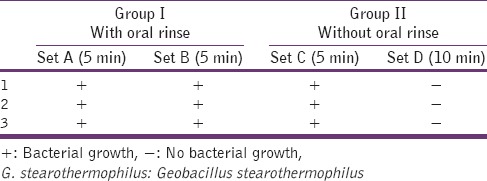
No bacterial growth was observed in the cultures stabbed with the Group I (set A and set B instruments), that were imm [Figures 1 and 2]. The Group II (set C instruments) that was immersed in plain distilled water and microwaved for 5 min showed scanty bacterial growth. The Group II (set D instruments) that was immersed in plain distilled water and microwaved for 10 min did not show any bacterial growth when inoculated onto trypticase soya agar plates [Table 1]. The biological indicator G. stearothermophilus, when microwaved for 10 min and subsequently inoculated on trypticase soya agar plates, showed no bacterial growth whereas the one microwaved for 5 min showed scanty bacterial growth[Table 2].
Figure 1.

Before and after microwave sterilization
Figure 2.
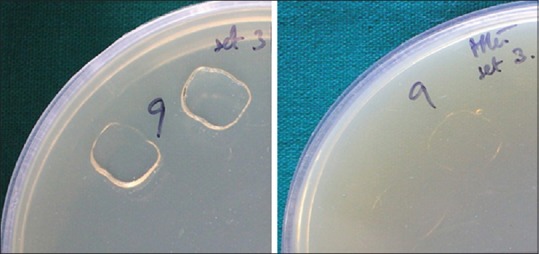
Before and after microwave sterilization
Table 1.
Efficacy of microwave irradiation on clinically contaminated orthodontic instruments
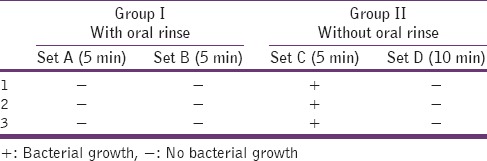
Table 2.
Microwave irradiation efficacy on the biological control (G. Stearothermophilus MTCC 1518)
Discussion
The prevention of transmission of diseases by contaminated instruments from one patient to another and to the dental staff has gained enhanced importance in recent years. Hepatitis B virus and human immuno deficiency virus pose a potential hazard among the practicing orthodontists.[20,21] Hence, the Occupational Safety and Health Administration Standards on Occupational Exposure to Bloodborne Pathogens and Hazard Communications have mandated by law that dental practitioners follow infection control procedures to protect employees.[22] The preferred method to sterilize orthodontic pliers has been the content of the debate in the orthodontic community.[23] The ideal regimen for sterilization would therefore include the following requirements namely, prevention of transmission of microbial pathogens from one patient to another and to sterilize a wide assortment of instruments in a minimal amount of time without damage.[24] The various disinfection techniques that are used are quarternary ammonium compounds, glutaraldehyde, isopropyl alcohol, ethyl alcohol, ultraviolet light, gamma radiation, mercuric salts, phenolic compounds, 1%sodium hypochlorite, iodophors, and microwaves. However, the sterilization methods as recommended by the American Dental Association are steam under pressure (autoclave), dry-heat, chemical vapor, and ethylene oxide gas.
In the practice of orthodontics, great care has to be taken during sterilization procedures as they can cause damage to the cutting edges, sharp angles and tips of the orthodontic pliers. Although steam sterilization is the least expensive and the quickest method of sterilization, it has several disadvantages. Though it is a time-tested procedure, it causes corrosion and rusting of orthodontic pliers and damages the cutting edges.
Hot air oven is used at 160°C –180°C for a period of one to one and half hours.[8] Dry-heat sterilization is a low cost sterilization procedure. It is a protective method as it prevents corrosion and rusting of sharp cutting edges and tips and hence is the method of choice when preservation of metal cutting edge is essential.[25] Dry-heat causes oxidative destruction of bacterial protoplasm. The thermal death point of bacterial cells and spores is semi-logarithmic with respect to concentration and exposure time. However, it has two drawbacks. First, it requires longer exposure time as the hot air tends to stratify, and hence, penetrates slowly and unevenly and secondly, the time and temperature varies for different instruments.
The low cost, speed and simplicity of sterilization by microwaves have encouraged its widespread use in dentistry. It has been reported that it has been used for the sterilization of dental burs,[26] dental hand pieces,[27] polyvinyl siloxane and polyether impression materials,[28] dental casts,[29,30] dental mirrors,[31] composite finishing and polishing instruments,[12,32] removable and complete dentures,[33] and chair side reline resins;[34] however, the exposure time and the frequency of microwave irradiation differs for each.
Microorganism inhibition depends on the energy absorbed or the heat transferred. It has been reported that microwaves caused water molecules to vibrate producing friction that results in water heating (heat and humidity). The effect of microwave irradiation on microorganisms is not only thermal[12,13,14] but also nonthermal, i.e. interaction of the electromagnetic field.[15,16,17] Efficacy of microwave irradiation is depending on the vehicle in which the pliers are immersed, time of exposure, level of power of microwave oven and type of microorganisms. Microbial cells are heated by the microwaves depending on the chemical composition of microorganisms and the surrounding medium. Therefore, a certain frequency of microwave energy may be absorbed by the nucleic acids. The structural changes in the more peripheral layer around the biological macromolecules may alter their stability and function and be denatured in an irreversible manner.[16]
It has been reported that microwave irradiation for 20 min was ineffective in killing Bacillus subtilis transferred to stone casts; however, the microorganisms exhibited less growth.[29] Studies have also demonstrated that the exposition of bacterial suspensions to microwave irradiation caused reduction in viable cell counts and increased the leaching of DNA and protein.[35] Microwaves cause changes in structural integrity and permeability of cell wall and cell membrane leading to detrimental cell metabolism and cell death.[36]
Irrespective of the mechanism of microwaves on pathogenic microorganisms being thermal or nonthermal, the effect of inactivation occurs mainly in the presence of water, indicating that humidity plays a vital role in microwave energy absorption, this being an important factor for sterilization in microwave oven.[37,38] The water molecules present in the medium or inside the cells, being diploid, interact with the electromagnetic field of the microwaves, producing numerous collisions and the vibrations so caused produces the desired heating.[39] This increase in temperature in turn causes protein and DNA denaturation.[40]
Biological indicators provide the only real method of verifying the effectiveness of any sterilization procedure. They consist of ampoules or strips enclosed in glassine envelopes that contain a known quantity of spores of G. stearothermophilus. In our study, G. stearothermophilus was used as a biological indicator. The spores were completely destroyed after 10 minutes exposure to microwave irradiation. This indicated that proper sterilization of all hinged and nonhinged instruments and molar bands was achieved by microwave irradiation.
The results of the present study indicated that the utilization of microwave oven for bacterial decontamination is entirely feasible in clinical orthodontics. In this study, no sterilization failure was observed with the use of microwave oven for both the hinged and nonhinged instruments, orthodontic molar bands and mouth mirrors. Sets A, B and D exhibited complete sterilization whereas set C showed scanty bacterial growth when immersed in plain distilled water and microwaved for 5 min only. This reiterates the fact that microwave irradiation is effective when the instruments are dipped in distilled water along with oral rinse either 0.01% chlorhexidine gluconate or 0.025% betadine and irradiated for 5 min. Microwave irradiation of the orthodontic instruments was also equally effective when they were dipped in just plain distilled water but the exposure time had to be increased to 10 min. It was, however, found to be ineffective when the orthodontic instruments were dipped in plain distilled water and exposed to microwave irradiation for 5 min, reiterating the fact that immersion in plain distilled water warrants microwaving for 10 min to ensure complete and effective sterilization. Effective sterilization was confirmed with the destruction of G. stearothermophilus used as a test strip in the microwave oven. Analysis of the cultures confirmed no growth of test spores (G. stearothermophilus) after microwave sterilization. The advantages of microwave sterilization are that it is easy, cost-effective, less time consuming, does not require special storage, and minimizes stocking of many sets of instruments as quick, repeated sterilization between patients becomes a reality. Therefore, microwave sterilization could find a potential use in dental offices and dental laboratories as it effectively inactivates microorganisms.
Conclusion
Immersion of orthodontic instruments and molar bands in distilled water without oral rinse and microwaving for 10 min ensures complete and effective sterilization
Immersion in distilled water with oral rinse and microwaving for 5 min ensures complete and effective sterilization
Microwave irradiation is indeed an effective, quick and economic alternative for orthodontic instrument sterilization.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Codino RJ, Marshall WE. Control of infection in the dental operatory. Dent Surv. 1976;52:42–50. [PubMed] [Google Scholar]
- 2.Sande MA, Gadot F, Wenzel RP. Point source epidemic of Mycoplasma pneumoniae infection in a prosthodontics laboratory. Am Rev Respir Dis. 1975;112:213–7. doi: 10.1164/arrd.1975.112.2.213. [DOI] [PubMed] [Google Scholar]
- 3.Starnbach H, Biddle P. A pragmatic approach to asepsis in the orthodontic office. Angle Orthod. 1980;50:63–6. doi: 10.1043/0003-3219(1980)050<0063:APATAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Crowell WS. Why dental instruments rust and how to prevent it. Dent Cosmo. 1925;67:752–5. [Google Scholar]
- 5.Custer F, Andersen R. A study of surface changes in instruments during sterilization procedures. N Y J Dent. 1968;38:8–17. [PubMed] [Google Scholar]
- 6.Custer F, Coyle T. Instrument changes during sterilization. J Dent Res. 1970;49:487–95. doi: 10.1177/00220345700490030401. [DOI] [PubMed] [Google Scholar]
- 7.Custer F, Addingtion L. Physical changes of instruments during sterilization. J Periodontol. 1965;36:382–6. doi: 10.1902/jop.1965.36.5.382. [DOI] [PubMed] [Google Scholar]
- 8.Matlack RE. Instrument sterilization in orthodontic offices. Angle Orthod. 1979;49:205–11. doi: 10.1043/0003-3219(1979)049<0205:ISIOO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Jones ML. An initial assessment of the effect on orthodontic pliers of various sterilization/disinfection regimes. Br J Orthod. 1989;16:251–8. doi: 10.1179/bjo.16.4.251. [DOI] [PubMed] [Google Scholar]
- 10.Jones M, Pizarro K, Blunden R. The effect of routine steam autoclaving on orthodontic pliers. Eur J Orthod. 1993;15:281–90. doi: 10.1093/ejo/15.4.281. [DOI] [PubMed] [Google Scholar]
- 11.Cottone JA, Tererhalmy GT, Molinari JA. 1st ed. Malvern, PA: Lea and Febiger; 1991. Practical Infection Control in Dentistry. [Google Scholar]
- 12.Tate WH, Goldschmidt MC, Ward MT, Grant RL. Disinfection and sterilization of composite polishing instruments. Am J Dent. 1995;8:270–2. [PubMed] [Google Scholar]
- 13.Fitzpatrick JA, Kwao-Paul J, Massey J. Sterilization of bacteria by means of microwave heating. J Clin Eng. 1978;3:44–7. doi: 10.1097/00004669-197801000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Jeng DK, Kaczmarek KA, Woodworth AG, Balasky G. Mechanism of microwave sterilization in the dry state. Appl Environ Microbiol. 1987;53:2133–7. doi: 10.1128/aem.53.9.2133-2137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo CB, Watson IA, Stewart-Tull DE, Koh VH. Heat transfer analysis of Staphylococcus aureus on stainless steel with microwave radiation. J Appl Microbiol. 1999;87:396–401. doi: 10.1046/j.1365-2672.1999.00832.x. [DOI] [PubMed] [Google Scholar]
- 16.Carrol DE, Lopez A. Letthality of radio-frequency energy upon microorganisms in liquid, buffered, and alcoholic food systems. J Food Sci. 1969;34:320–4. [Google Scholar]
- 17.Culkin KA, Fung DY. Destruction of Escherichia coli and salmonella typhimurium in microwave-cooked soups. J Milk Food Technol. 1975;38:8–15. [Google Scholar]
- 18.Olsen CM. Microwaves inhibit bread mold. Food Eng. 1965;37:51–3. [Google Scholar]
- 19.Biological indicators for verifying sterilization. Council on dental materials, instruments, and equipment. Council on dental therapeutics. J Am Dent Assoc. 1988;117:653–4. [PubMed] [Google Scholar]
- 20.Cottone JA. Hepatitis B virus and the dental profession. J Am Dent Assoc. 1985;110:617–621. doi: 10.1016/s0002-8177(15)30011-8. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control (CDC). Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1986;35:237–42. [PubMed] [Google Scholar]
- 22.Council on dental materials, instruments and equipment, council on dental practice, and council on dental therapeutics. Infection control recommendations for the dental office and the dental laboratory. J Am Dent Assoc. 1996;127:672–80. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]
- 23.Hohlt WF, Miller CH, Neeb JM, Sheldrake MA. Sterilization of orthodontic instruments and bands in cassettes. Am J Orthod Dentofacial Orthop. 1990;98:411–6. doi: 10.1016/s0889-5406(05)81649-x. [DOI] [PubMed] [Google Scholar]
- 24.Eshleman JR. Methods used for sterilization or disinfection of instruments. J Dent Educ. 1968;32:330–3. [PubMed] [Google Scholar]
- 25.Burnett GW, Schuster GS. Baltimore: Williams and Wilkins Company; 1978. Oral Microbiology and Infectious Disease. Student Edition; pp. 57–73. [Google Scholar]
- 26.Fais LM, Pinelli LA, Adabo GL, Silva RH, Marcelo CC, Guaglianoni DG. Influence of microwave sterilization on the cutting capacity of carbide burs. J Appl Oral Sci. 2009;17:584–9. doi: 10.1590/S1678-77572009000600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohrer MD, Bulard RA. Microwave sterilization. J Am Dent Assoc. 1985;110:194–8. doi: 10.14219/jada.archive.1985.0250. [DOI] [PubMed] [Google Scholar]
- 28.Abdelaziz KM, Hassan AM, Hodges JS. Reproducibility of sterilized rubber impressions. Braz Dent J. 2004;15:209–13. doi: 10.1590/s0103-64402004000300009. [DOI] [PubMed] [Google Scholar]
- 29.Berg E, Nielsen O, Skaug N. High-level microwave disinfection of dental gypsum casts. Int J Prosthodont. 2005;18:520–5. [PubMed] [Google Scholar]
- 30.Davis DR, Curtis DA, White JM. Microwave irradiation of contaminated dental casts. Quintessence Int. 1989;20:583–5. [PubMed] [Google Scholar]
- 31.Tarantino L, Tomassini E, Petti S, Simonetti D’Arca A. Use of a microwave device for dental instrument sterilization: Possibilities and limitations. Minerva Stomatol. 1997;46:561–6. [PubMed] [Google Scholar]
- 32.Tate WH, Goldschmidt MC, Powers JM. Performance of composite finishing and polishing instruments after sterilization. Am J Dent. 1996;9:61–4. [PubMed] [Google Scholar]
- 33.Webb BC, Thomas CJ, Harty DW, Willcox MD. Effectiveness of two methods of denture sterilization. J Oral Rehabil. 1998;25:416–23. doi: 10.1046/j.1365-2842.1998.00266.x. [DOI] [PubMed] [Google Scholar]
- 34.Neppelenbroek KH, Pavarina AC, Palomari Spolidorio DM, Sgavioli Massucato EM, Spolidorio LC, Vergani CE. Effectiveness of microwave disinfection of complete dentures on the treatment of Candida-related denture stomatitis. J Oral Rehabil. 2008;35:836–46. doi: 10.1111/j.1365-2842.2008.01874.x. [DOI] [PubMed] [Google Scholar]
- 35.Woo IS, Rhee IK, Park HD. Differential damage in bacterial cells by microwave radiation on the basis of cell wall structure. Appl Environ Microbiol. 2000;66:2243–7. doi: 10.1128/aem.66.5.2243-2247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campanha NH, Pavarina AC, Brunetti IL, Vergani CE, Machado AL, Spolidorio DM. Candida albicans inactivation and cell membrane integrity damage by microwave irradiation. Mycoses. 2007;50:140–7. doi: 10.1111/j.1439-0507.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- 37.Dixon DL, Breeding LC, Faler TA. Microwave disinfection of denture base materials colonized with Candida albicans. J Prosthet Dent. 1999;81:207–14. doi: 10.1016/s0022-3913(99)70250-7. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe K, Kakita Y, Kashige N, Miake F, Tsukiji T. Effect of ionic strength on the inactivation of micro-organisms by microwave irradiation. Lett Appl Microbiol. 2000;31:52–6. doi: 10.1111/j.1472-765x.2000.00763.x. [DOI] [PubMed] [Google Scholar]
- 39.Najdovski L, Dragas AZ, Kotnik V. The killing activity of microwaves on some non-sporogenic and sporogenic medically important bacterial strains. J Hosp Infect. 1991;19:239–47. doi: 10.1016/0195-6701(91)90241-y. [DOI] [PubMed] [Google Scholar]
- 40.Ponne CT, Bartels PV. Interaction of electromagnetic energy with biological material-relation to food processing. Radiat Phys Chem. 1995;45:591–607. [Google Scholar]


