Abstract
Background:
Neonatal jaundice refers to yellow coloration of the skin and the sclera (whites of the eyes) of newborn babies that result from the accumulation of bilirubin in the skin and mucous membranes. Because bilirubin is potentially toxic to the central nervous system. Genetic disorders of bilirubin conjugation, particularly the common Gilbert's syndrome, can also contribute to neonatal hyperbilirubinemia.
Objective:
The aim of this study was to evaluate the lipid per-oxidation and antioxidant enzyme activities in patients with neonatal jaundice before and after phototherapy.
Materials and Methods:
The study includes 50 neonatal jaundice patients with average age 2-15 days. All patients of neonatal jaundice receiving phototherapy except feeding, cleaning. Subjects selected were from the patients attending Pediatrics Department. Plasma malondialdehyde (MDA), erythrocyte glutathione peroxidase (GPX), superoxide dismutase and catalase (CAT) to monitor the bilirubin level.
Results:
The results show increased levels of bilirubin compared with controls (P < 0.001) shows the level of plasma MDA in control, before and after phototherapy. Represents the level of GPX was significantly increased in after the phototherapy group when compared with before phototherapy and control SPSS soft ware: (P < 0.001). Shows the reduced glutathione (GSH) level in plasma was significantly decreased in the after phototherapy group when compared with before phototherapy and control (P < 0.001). And finally with ascorbic acid and CAT.
Conclusion:
It is evident from the study that increased oxidative stress in neonatal jaundice babies leads to decrease in the levels of antioxidants like GSH and ascorbic acid and disturb their metabolism, that weaken their ability to fight the growing stress. Intense oxidative stress and decreased antioxidants may contribute to neural cell death and alter the erythrocytomembrane structure processing in neonatal jaundice.
KEY WORDS: Antioxidants, bilirubin, Gilbert's syndrome, neonatal jaundice
Neonatal jaundice refers to yellow coloration of the skin and the sclera (whites of the eyes) of newborn babies that result from the accumulation of bilirubin in the skin and mucous membranes. This is associated with a raised level of bilirubin in the circulation, a condition known as hyperbilirubinemia.[1] Some 60% of normal newborns become clinically jaundiced sometime during the 1st week of life. Unconjugated (indirect) hyperbilirubinemia occurs as a result of excessive bilirubin formation and because the neonatal liver cannot clear bilirubin rapidly enough from the blood.[2,3]
Although most newborns with jaundice are other-wise healthy, they need to be monitored.
Because bilirubin is potentially toxic to the central nervous system. Sufficiently elevated levels of bilirubin can lead to bilirubin encephalopathy and subsequently kernicterus, with devastating, permanent neuro-developmental handicaps.[4] Bilirubin is normally cleared from the body by hepatic conjugation with glucuronic acid and elimination in bile in the form of bilirubin glucuronides [Figure 1]. Neonatal jaundice stems from a transient deficiency of conjugation (exacerbated in preterm infants) combined with increased turnover of red cells. Pathologic conditions that can increase bilirubin production include isoimmunization, heritable hemolytic disorders, and extravasated blood (e.g. from bruises and cephalhematomas).[5]
Figure 1.
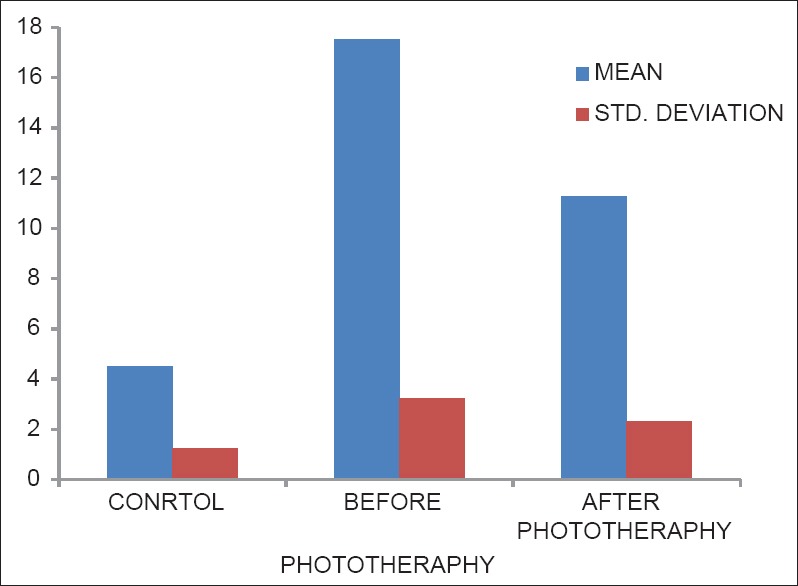
Levels of bilirubin in control, before phototherapy and after phototherapy groups
Genetic disorders of bilirubin conjugation, particularly the common Gilbert's syndrome, can also contribute to neonatal hyperbilirubinemia.[6] The largest group of other-wise healthy infants at increased risk for hyperbilirubinemia are late-preterm infants and those who are exclusively breast-fed[7,8] (particularly if breast-feeding is not going well). Breast-feeding and the poor caloric intake associated with breast-feeding difficulties are both thought to cause an increase in the enterohepatic circulation of bilirubin.[9] The goal of therapy is to lower the concentration of circulating bilirubin or keep it from increasing. Phototherapy achieves this by using light energy to change the shape and structure of bilirubin, converting it to molecules that can be excreted even when normal conjugation is deficient Figures 1 and 2.[10] Absorption of light by dermal and subcutaneous bilirubin induces a fraction of the pigment to undergo several photochemical reactions that occur at very different rates. These reactions generate yellow stereoisomers of bilirubin and colorless derivatives of lower molecular weight [Figure 2]. The products are less lipophilic than bilirubin, and unlike bilirubin, they can be excreted in bile or urine without the need for conjugation. The relative contributions of the various reactions to the overall elimination of bilirubin are un-known, although in vitro and in vivo studies suggest that the photoisomerization is more important than photodegradation.[10] Bilirubin elimination depends on the rates of formation as well as the rates of clearance of the photoproducts. Photoisomerization occurs rapidly during phototherapy, and isomers appear in the blood long before the level of plasma bilirubin begins to absorbs light most strongly in the blue region of the spectrum near 460 nm [Figure 3], a region in which penetration of tissue by light increases markedly with increasing wavelength.
Figure 2.
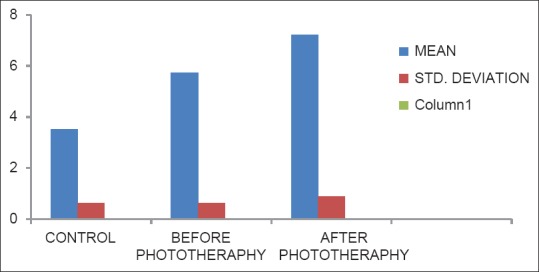
Status of lipid peroxidation mda in control, before phototherapy and after phototherapy groups
Figure 3.
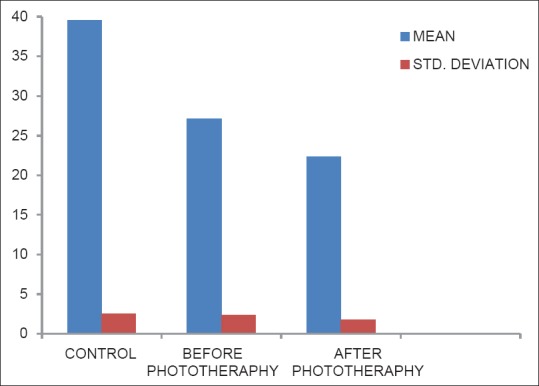
Levels of reduced glutathione in controls, before phototherapy and after phototheraphy groups
The rate of formation of bilirubin photoproducts is highly dependent on the intensity and wavelengths of the light used. Only wavelengths that penetrate the tissue and are absorbed by bilirubin have a phototherapeutic effect. Taking these factors into account, lamps with output predominantly in the 460–490-nm blue region of the spectrum is probably the most effective for treating hyperbilirubinemia. A common misconception is that ultraviolet (UV) light (<400 nm) is used for phototherapy. Phototherapy lights in current use do not emit significant erythemal UV radiation. In addition, the plastic cover of the lamp and in the case of preterm infants, the incubator, filter out UV light. Oxidative stress causes imbalance between reactive oxygen species (ROS) generation and antioxidant system that scavenge or reduce ROS concentration. Redox imbalance caused by increased ROS production and/or reduced antioxidant reserve, leading to pathological consequences including damage to proteins, lipids and DNA (Westhuyzen et al., 1997).
Free radicals are atoms or molecules that contain one or more unpaired electrons the presence of unpaired electron make the species highly reactive. They play an important role in human diseases. Free radicals include free oxygen related reaction compounds collectively known as “ROS”. The reactive oxygen includes superoxide (O2), hydrogen peroxide (H2O2), and hydroxyl (OH) the superoxide radical is formed when electrons leak from the electron transport chain. The dismutation of superoxide results in the formation of hydrogen peroxide. The hydroxyl ion is highly reactive and can modify purines and pyrimidines that cause strand breaks resulting in DNA damage. Some oxidase enzymes can directly generate the hydrogen peroxide radical. ROS have been implicated in more than 100 diseases.
Free radicals result in per-oxidation of polyunsaturated fatty acids in the cell membrane and subsequent generation of further unstable radicals leading to a chain of events. This attack makes cell membrane leaky and functions of absorption and secretion are lost finally leads to cell death (Zachee et al., 1997) Lipid per-oxidation is an ideal marker of oxidant stress. During lipid per-oxidation, unstable hydroperoxides resulting from peroxyl radicals-dependent chain reactions among unsaturated fatty acyl moieties, break down to smaller and more stable products, e.g. aldehydes such as acrolein malondialdehyde (MDA), thiobarbituric acid reacting substance (TBARS) are primarily products of arachidonic acid oxidations and may serve as stable markers of free radicals attack of the cell membrane phospholipids in vivo (Meister et al., 1993). Free radicals are utilized by macrophages, and neutrophils use them to destroy bacteria and foreign invaders. However, too much production or production in the wrong place can be harmful. That is why the body needs antioxidant compounds. Cells are equipped with both nonenzymatic and scavenger system to eliminate radicals from the body.
Enzymatic antioxidants
Superoxide dismutase (SOD)
Catalase (CAT)
Glutathione peroxidase (GPX)
Reduced glutathione (GSH).
Superoxide dismutase is a metalloenzyme. It catalyzes the dismutation of the superoxide radical anion and hence diminish toxic effects due to this radical or other free radical derived secondary reactions.
The H2O2 thus formed is detoxified either by CAT or reduced GSH dependent reactions. CAT is a hemoprotein. It is 10 times faster than the peroxidase. It has four protein subunits each containing Fe at its active sites. A true catalytic role in decomposition of H2O2.
2H2O2→2H2O + O2.
Glutathione peroxidase a tetrameric selenoprotein plays an important role in the detoxification of numerous toxic compounds including xenobiotics carcinogens and ROS (Zachee et al., 1996).
It removes H2O2 and other hydroperoxidase and thus protect against lipid per-oxidation. GPX catalyzes the reaction.
ROOH +2GSH→ROH + H2O + GSSH.
Where R may be H or alkyl moiety. GPX uses GSH as co-substrate. GSH exited in both reduced (GSH) and oxidized Glutathione (GSH) forms, the former predominating (>99%) under normal condition. GSH is synthesized in the cytosol and can be regenerated by the enzyme GSH reductase utilizing NADPH.
Materials and Methods
The study includes 50 neonatal jaundice patients with average age 2-15 days. All patients of neonatal jaundice receiving phototherapy except feeding, cleaning. Subjects chosen were from the patients attending Pediatrics Department SRM Medical College Hospital and Research Centre. The control group composed of 30 full-term healthy neonates of appropriate weight delivered normally in the labor room of SRM General Hospital, Chennai.
Blood samples were obtained from the concerned patients before and after phototherapy samples were collected from the same patients and analyzed for the following parameters. Plasma MDA was estimated by the method (Hunter,1984) erythrocyte GPX was estimated by the method (Rotruck,1973) SOD was determined by the method (Kakkar,1984), and CAT was measured by the method of Sinha 1972 and reduced GSH was determined by the method of Maron 1979. Data are presented as mean ± standard deviation. Student's unpaired t-test was used to find the significance between the groups.
Estimation of lipid peroxides
Malondialdehyde was measured using the established thiobarbituric acid (TBARS) method of Hunter (1963).
Reagents
Malondialdehyde standard: 1,1,3,3 tetra methoxy propane in 3% acetic acid. Concentration: 50 nM/ml
H2SO4 – 4 m
Phosphotungstic acid: 10% in water
Thiobarbituric acid (TBA): 670 mg of TBA was weighed and mixed in 100 ml of 50% acetic acid
Butanol.
Procedure
To 0.2 ml of plasma, 2.0 ml of 4M H2SO4 and 0.25 ml of 10% phosphotungstic acid were added and centrifuged for 10 min. The supernatant was discarded. Then, 2.0 ml of TBA and 2.0 ml of distilled water were added to the residue. The tubes were kept in boiling water for 1 h, cooled, and the color developed was read at 533 nm. 2.0 ml of butanol was added if the solution was turbid and the colored chromogen was extracted in the butane layer. Standard MDA at different concentrations ranging from 10 to 50 nm was also treated similarly, and the standard graph was constructed.
The lipid peroxide content was expressed as nanomoles of MDA/ml of serum.
Assay of catalase
Catalase activity was assayed colorimetrically by the method of Sinha (1972).
The method is based on the fact that the dichromate in acetic acid is reduced to chromic acetate when heated in the presence of hydrogen peroxide with the formation of perchloric acid as an unstable intermediate. The chromic acetate is measured colorimetrically at 620 nm. The CAT preparation is allowed to split hydrogen peroxide for different periods of time. The reaction is stopped at different time intervals by the addition of dichromate acetic acid mixture and the remaining H2O2 determined colorimetrically.
Reagents
Dichromate – acetic acid reagent: 5% potassium dichromate solution was mixed with glacial acetic acid in the ratio 1:3
Hydrogen peroxide, 0.2 M
Phosphate buffer, 0.01 M, pH 7.0.
Procedure
To 1.0 ml of phosphate buffer taken in four tubes, 0.1 ml of enzyme preparation was added. To this 0.4 ml of hydrogen peroxide was added. The reaction was stopped at 0, 15, 30 and 60 s by the addition of 0.2 ml dichromate acetic acid reagent. The tubes were boiled for 10 min, cooled and read at 620 nm. For standards, different amounts of hydrogen peroxide ranging from 20 to 100 moles were taken and proceeded as above along with a blank.
The activity of CAT was expressed as U/g Hb.
Assay of glutathione peroxidase
The activity of GSH was estimated by the method of Rotruck (1973).
A known amount of enzyme preparation was allowed to react with H2O2 in the presence of reduced GSH. After a specific time period, the remaining GSH content was measured.
Reagents
Phosphate buffer, 0.4 M, pH 7.0
Sodium oxide solution,10 mM
Trichloroacetic acid (TCA) – 10%
Ethylenediaminetetraacetic acid (EDTA) – 0.2 mM
H2O2 – 0.2 m M
GSH, 2 mM
Procedure
The reaction mixture in a total volume of 100 ml, contained 0.2 ml of phosphate buffer, 0.2 ml of EDTA, 0.1 ml of sodium azide and 0.2 ml of enzyme. To the reaction mixture 0.2 ml of GSH and 0.1 ml of H2O2 were added and incubated at 37°C for 10 min. The reaction was arrested by the addition of 0.5 ml of 10% TCA. After centrifugation, the supernatant was assigned for GSH content using 5,5’-dithio- bis(2-nitrobenzoic acid) (DTNB). A blank was treated similarly to which 0.2 ml of the enzyme was added after incubation. The activity of GSH was expressed as U/g Hb activity.
Estimation of total reduced glutathione
The total reduced GSH in the serum sample was determined by the method of Moron (1979).
Reagents
TCA 5%
DTNB (0.6 mM)-5.5 dithiobio [2-nitro benzoic acid] in 0.2 M phosphate buffer-pH 8
Phosphate buffer: 0.2M pH 8
GSH standard-100 mg of GSH in 100 ml of distilled water (concentration-1 mg/ml)
Working standard: 10 ml of stock solution was made upto 100 ml with distilled water.
Procedure
To 0.25 ml of serum, 0.5 ml of 5% cold TCA was added. The precipitate was removed by centrifugation at 4,000 rpm for 10 min. To 0.3 ml of supernatant, 2 ml of DTNB and 1 ml of buffer was added and the color intensity was read at 412 nm, against the blank containing TCA (instead of sample) and series of standards [10-50 μg] was treated in a similar way to determine the GSH content.
Results
Table 1 shows increased levels of bilirubin compared with controls (P < 0.001). The levels of bilirubin were significantly lower (P < 0.001) in after the phototherapy group compared to before phototherapy [Figure 1].
Table 1.
Levels of bilirubin in control, before phototherapy and after phototherapy groups
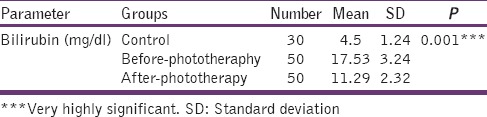
Table 2 shows the level of Plasma MDA in control, before and after phototherapy. Increased lipid peroxidation in after the phototherapy group when compared with before phototherapy and control group (P < 0.001). There was also a significant difference between before phototherapy and control group (P < 0.001) [Figure 2].
Table 2.
Status of lipid peroxidation MDA in control, before phototherapy and after phototherapy groups

Table 3 represents the level of GPX was significantly increased in after the phototherapy group when compared with before phototherapy and control (P.001). There was also statistical significance between control and before phototherapy group (P < 0.001) [Figure 3].
Table 3.
Levels of reduced glutathione in controls, before phototherapy and after phototheraphy groups
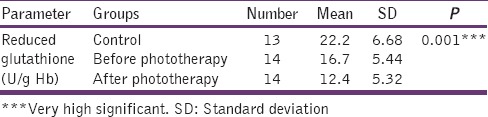
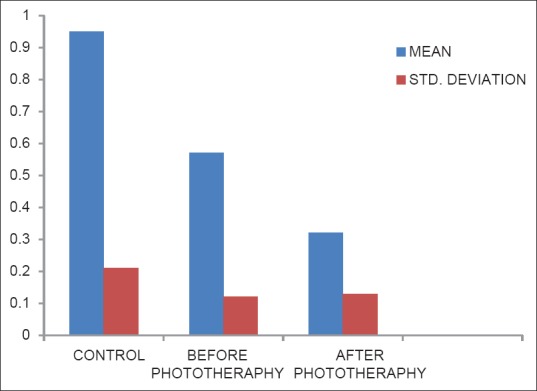
Table 4 shows the reduced GSH level in plasma was significantly decreased in the after phototherapy group when compared with before phototherapy and control (P < 0.001). The value observed was higher in the control group when compared with before phototherapy (P < 0.001) [Figure 4].
Table 4.
Levels of serum glutathione peroxidase in control, before phototheraphy and after phototheraphy groups
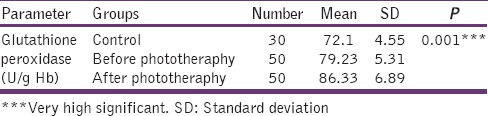
Figure 4.
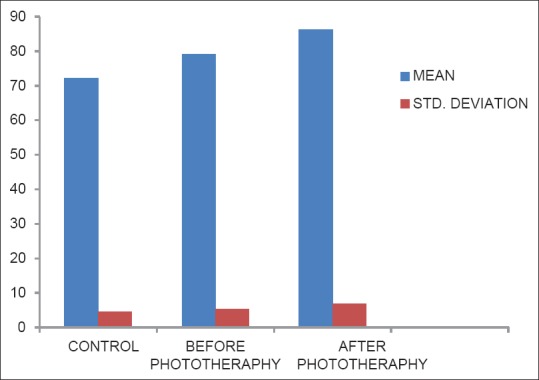
Levels of serum glutathione peroxidase in control, before phototheraphy and after phototheraphy groups
Table 5 shows the erythrocyte activity of CAT was significantly decreased in after the phototherapy group when compared with before phototherapy and control group (P < 0.001). There was a significant difference between before phototherapy and control (P < 0.001) [Figure 5].
Table 5.
Levels of catalase in control, before phototheraphy and after phototheraphy groups
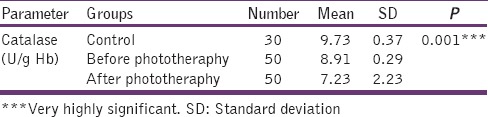
Figure 5.
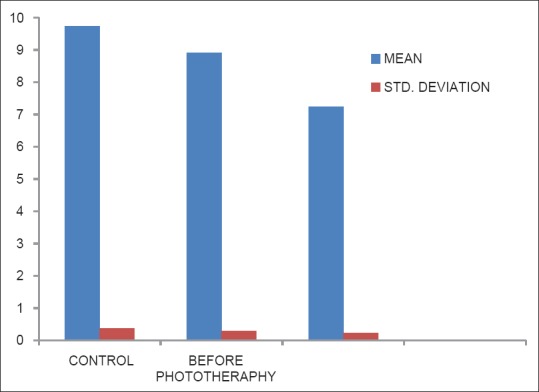
Levels of catalase in control, before phototheraphy and after phototheraphy groups
Table 6 shows the Ascorbic acid level in plasma was decreased in after the phototherapy group when compared to before phototherapy group (P < 0.001). There was a significant difference between before phototherapy and control group (P < 0.001) [Figure 6].
Table 6.
Levels of vitamin C in controls, before phototherapy and after phototherapy-groups
Figure 6.
Levels of vitamin c in controls, before phototherapy and after phototherapy-groups
Discussion
There are varying reports on changes in plasma lipid per-oxidation and erythrocyte antioxidant enzyme due to phototherapy. Some of the studies showed an increase while some other showed decrease. The results indicate that there is increase in free radical generation, and antioxidant defense is impaired in per-oxidation have been reported in neonatal jaundice patients. In the present study the lipid per-oxidation product, that is, MDA levels have been increased significantly in erythrocytes of the neonatal jaundice patients than that in the control group. This may show the presence of increased oxidative stress. Rise in MDA could be due to increased generation of ROS due to the excessive oxidative damage generated in these patients. These oxygen species in turn can oxidize many other important biomolecules including membrane lipids. The raised MDA level reflects the oxidative injury due to neonatal jaundice, which is attributed to free radical formation that abstracts hydrogenatoms from lipoproteins causing lipid per-oxidation, of which MDA is the main product. The membrane phospholipids, specifically polyunsaturated fatty acids are converted to MDA by per-oxidation, which can be analyzed by reactivity to TBA. Increased levels of TBA reaction products have been found in the erythrocytes of neonatal jaundice patients.[8]
We observed a significant decrease in the levels of erythrocyte reduced GSH, ascorbic acid and plasma Vitamin E (nonenzymatic antioxidant defense system) in neonatal jaundice patients when compared to controls. GSH, Vitamin E, and ascorbic acid are important chain breaking antioxidants responsible for scavenging the free radicals and suppression of per-oxidation in aqueous and lipid region of the cell.[11,12] The decrease in the levels of these nonenzymatic antioxidant parameters may be due to the increased turnover, for preventing oxidative damage in these patients suggesting an increased defense against oxidant damage in neonatal jaundice patients. Similar reports of decreased GSH, ascorbic acid and Vitamin E levels in neonatal jaundice patients were reported by various studies.
In our study, the erythrocyte antioxidant enzyme, that is, SOD and GPX activities have been increased significantly in patients with neonatal jaundice patients compared to controls. The increased activity of SOD may be indicative of increased superoxide generation by whichever mechanism like increased catecholamine metabolism. SOD is an important antioxidant enzyme having an antitoxic effect against superoxide anion. The overexpression of SOD might be an adaptive response, and it results in increased dismutation of superoxide to hydrogen peroxide in neonatal jaundice. But, on the other hand, low erythrocyte SOD activities have also been reported in various studies. GPX, an oxidative stress-inducible enzyme plays a significant role in the peroxyl scavenging mechanism and in maintaining functional integration of the cell membranes.[13] The rise in the activity of GPX could be due to its induction to counter the effect of increased oxidative stress. GPX provides an effective protective mechanism against cytosolic injury because it eliminates H2O2 and lipid peroxides by reduction utilizing GSH. Decrease in antioxidant enzyme status was reported in various studies.
In the present study, we have observed a significant decrease in the activity of CAT in patients with Neonatal jaundice compared to controls. CAT is the enzyme that protects the cells from the accumulation of hydrogen peroxide by dissimulating it to form water and oxygen or by using it as an oxidant in which it works as a peroxidase. A decrease in CAT activity in neonatal jaundice patients as compared with normal healthy subjects was also observed. The results of our present study have shown higher oxygen free radical production and decreased CAT activity, supports the higher oxidative stress hypothesis in neonatal jaundice patients. The increased activities of antioxidant enzymes may be a compensatory regulation in response to increased oxidative stress.
Conclusion
Although the MDA level was increased after phototherapy, phototherapy has a negative impact on numerous parts of the oxidant/antioxidant defense system in jaundiced full-term newborns, exposing them to potential oxidative stress. The results demonstrate that jaundice can produce an increased oxidative stress, which can be counteracted by increase in antioxidant response as evidenced by increase in Antioxidant enzymes. It is evident from the study that increased oxidative stress in neonatal jaundice babies leads to decrease in the levels of antioxidants like GSH and ascorbic acid and disturb their metabolism, that weaken their ability to fight the growing stress. Intense oxidative stress and decreased antioxidants may contribute to neural cell death and alter the erythrocyte membrane structure processing in neonatal jaundice.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Welsh A. London: Published by the Royal College of Obstetricians and Gynaecologists; 2010. Neonatal jaundice. [Google Scholar]
- 2.Sies H. Oxidative stress: From basic research to clinical application. Am J Med. 1991;91:31S–8S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- 3.Johnson J. Glenview, IL, United States of America: Mead Johnson and Company; 1993. Jaundice and your baby; pp. 1–4. [Google Scholar]
- 4.Melton K, Akinbi HT. Neonatal jaundice. Strategies to reduce bilirubin-induced complications. Postgrad Med. 1999;106:167–8. doi: 10.3810/pgm.1999.11.775. 71. [DOI] [PubMed] [Google Scholar]
- 5.Britton JR, Britton HL, Beebe SA. Early discharge of the term newborn: A continued dilemma. Pediatrics. 1994;94:291–5. [PubMed] [Google Scholar]
- 6.Modi N, Keay AJ. Phototherapy for neonatal hyperbilirubinaemia: The importance of dose. Arch Dis Child. 1983;58:406–9. doi: 10.1136/adc.58.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan JL, Newton RB. Serum antioxidant activity in neonates. Arch Dis Child. 1988;63:748–50. doi: 10.1136/adc.63.7_spec_no.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain SK. The neonatal erythrocyte and its oxidative susceptibility. Semin Hematol. 1989;26:286–300. [PubMed] [Google Scholar]
- 9.Ostrea EM, Jr, Cepeda EE, Fleury CA, Balun JE. Red cell membrane lipid peroxidation and hemolysis secondary to phototherapy. Acta Paediatr Scand. 1985;74:378–81. doi: 10.1111/j.1651-2227.1985.tb10987.x. [DOI] [PubMed] [Google Scholar]
- 10.Turgut M, Basaran O, Cekmen M, Karatas F, Kurt A, Aygün AD. Oxidant and antioxidant levels in preterm newborns with idiopathic hyperbilirubinaemia. J Paediatr Child Health. 2004;40:633–7. doi: 10.1111/j.1440-1754.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 11.Jain SK. The neonatal erythrocyte and its oxidative susceptibility. Semin Hematol. 1989;26:286–300. [PubMed] [Google Scholar]
- 12.McDonagh AF. The role of singlet oxygen in bilirubin photo-oxidation. Biochem Biophys Res Commun. 1971;44:1306–11. doi: 10.1016/s0006-291x(71)80228-0. [DOI] [PubMed] [Google Scholar]
- 13.Hulea SA, Smith TL, Wasowicz E, Kummerow FA. Bilirubin sensitized photooxidation of human plasma low density lipoprotein. Biochim Biophys Acta. 1996;1304:197–209. doi: 10.1016/s0005-2760(96)00120-8. [DOI] [PubMed] [Google Scholar]


