Abstract
Direct rigid fixation of the implant into jawbones has become an accepted and proven treatment for edentulism. This paper reviews the concept of osseointegration.
KEY WORDS: Implants, osseointegration, prosthesis, titanium
The goal of modern dentistry is to return patients to oral health in a predictable fashion.[1] The science of osseointegration has widened the scope of treatment options for edentulous patients. The consequences of partial or complete edentulism are well-known to both patients and dentists. The dental profession in general and prosthodontists in particular, have demonstrated impressive evidence of different techniques and methods used to compensate for tooth loss.
The genesis of osseointegration as a concept was introduced by Per-Ingvar Branemark (1969), professor at the Institute of Applied Biotechnology, University of Goteborg. He called it as “A direct structural and functional connection between ordered living bone and the surface of the load – covering implant.”
Concept of Osseointegration
When Titanium O2 forms over the surface of Titanium implant due to oxidation, this surface O2 formation causes osseointegration.[2] This new field of clinical usage of biotechnology has been a boon to the dental cripples who have lost natural teeth and adnexae. Further studies on this phenomenon gave rise to different implant systems.
Definition
Osseointegration
“A direct connection between living bone and a load-carrying endosseous implant at the light microscopic level.”[3]
-Branemark
“Contact established without interposition of nonbone tissue between normal remodeled bone and an implant entailing a sustained transfer and distribution of load from the implant to and within the bone tissue.”
-American Academy of Implant Dentistry (1986)
“The apparent direct attachment or connection of osseous tissue to an inert, alloplastic material without intervening connective tissue.”
-G.P.T. 83
History
Since 1952, the concept of tissue-integrated prostheses was studied at the laboratory of vital microscopy at the University of Lund, and subsequently at the laboratory for experimental biology at the University of Goteberg.
The initial concept of osseointegration stemmed from vital microscopic studies of the bone marrow of the rabbit fibula, which was uncovered for visual inspection in a modified intravital microscope at high resolution in accordance with a very gentle surgical preparation technique. With special instrumentation, the marrow could be studied in transillumination in vivo, and in situ, after the covering bone was ground down to a thickness of only 10–20 μm. Circulation was maintained in this thin layer of bone and with very few signs of microvascular damage, which is the earliest and most sensitive indication of tissue injury. These intravascular studies of bone marrow circulation also revealed the intimate circulatory connection among marrow, bone, and joint tissue compartments. Subsequent studies of the regeneration of bone and marrow emphasized the close functional connection between marrow and bone in the repair of bone defects.
Branemark performed a series of in vivo studies on bone marrow, and joint tissue with particular emphasis on tissue reaction to various kinds of injury: Mechanical, thermal, chemical, and rheologic. These studies in the early 1960s strongly suggested the possibility of osseointegration. He observed that the titanium chambers were inseparably incorporated within the bone tissue, which actually grew into very thin spaces in titanium [Figures 1 and 2].
Figure 1.
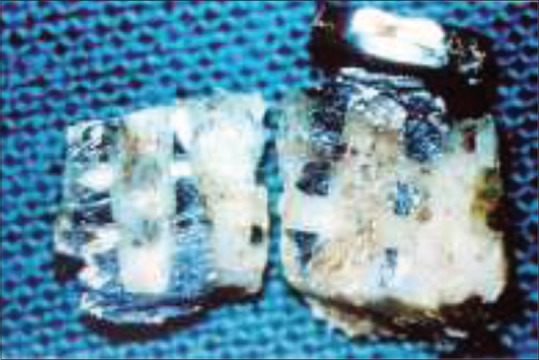
Macroscopic level of osseointegration
Figure 2.
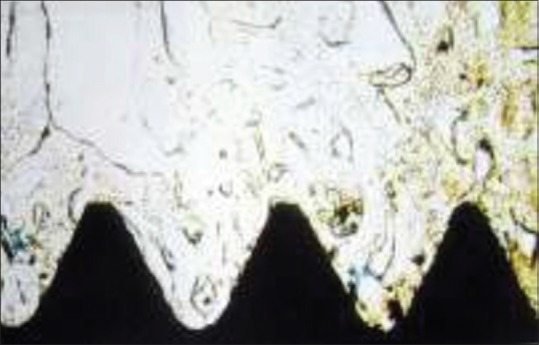
Histologic level of osseointegration
The basis for osseointegration, leading to permanent, functional and interactive, coexistence between titanium and bone, is established when a topographically harmonious and precise recipient site – geometrically adjusted to the anatomy of the fixture – is gently prepared in bone.[3]
Interdisciplinary clinical cooperation with plastic surgeons and otolaryngologists enabled them to study the repair of mandibular defects and replacement of ossicles by means of autologous bone grafts. Desired anatomic shapes of bone grafts were performed in rabbits and dogs and subsequently applied clinically with long-term follow-up. In an extensive series, the repair of major mandibular and tibial defects in dogs was studied.
Studies were performed on the healing and anchorage stability of titanium tooth root implants or fixtures of various sizes and designs. They found that when such an implant was introduced into the marrow cavity, and following an adequate immobilized healing period, a shell of compact cortical bone was formed around the implant without any apparent soft tissue intervention between normal bone and the surface of the implant.
They carried out vital microscopic studies on human microcirculation and intravascular behavior of blood cells at high resolution by means of an implanted optical titanium chamber in a twin-pedicled skin tube on the inside of the left upper arm of healthy volunteers. The long-term experiments in these chambers revealed no inflammatory processes. It was assumed that bone anchorage according to the principle of osseointegration might also work in humans, and first edentulous patients were treated in 1965.
In order to create osseointegration
A minimal amount of remaining bone should be removed
The basic topography of the region should not be changed
The retention of the original or transitional denture should be maintained during the healing period.
After 20 years of experimental and clinical development, a screw-shaped implant made of pure titanium was selected. It had an outer diameter of 3.7 mm and a length of 10 mm. This allowed its use in almost every edentulous jaw, regardless of the volume and topography of the remaining bone tissue.
A minimum of four fixtures appears to be adequate for support of full-arch prosthesis in the edentulous jaw. However, if morphologically feasible, six fixtures are installed.
Extra oral application of titanium fixtures has been used since 1976. A specially designed fixture has been used to anchor hearing aids for bone-conducting devices. Similar fixtures have also been used as anchorage for auricular prostheses.
Osseointegration has also been applied to long bones in the reconstruction of damaged or diseased joints, lost fingers, hands, arms and lower legs.
Mechanism of Osseointegration
The healing process in the implant system is similar to primary bone healing. Initially, blood is present between the fixture and bone, then blood clot forms. The blood clot is transformed by phagocytic cells, such as polymorphonuclear leukocytes, lymphoid cells, and macrophages [Figure 3]. The phagocytic activity level peaks during the time between the 1st and 3rd day after surgery.
Figure 3.
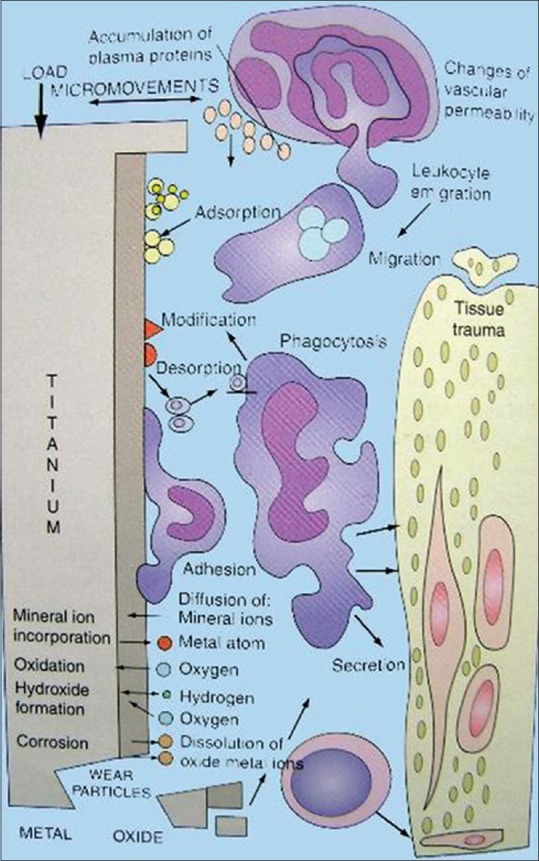
Mechanism of osseointegration
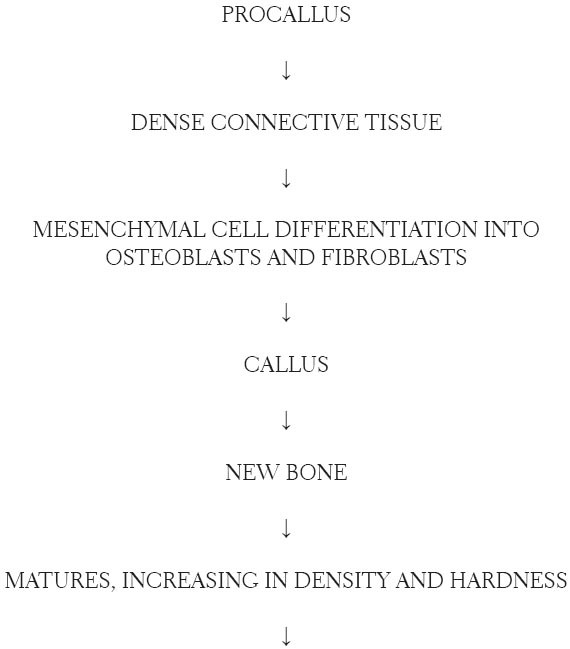
About this time, the prosthesis is attached to the fixtures and with stimulation, bone remodeling occurs. Haversian bone calcifies becoming dense and homogenous. Occlusal stresses stimulate the surrounding bone. As remodeling occurs, the osseointegrated fixtures can withstand masticatory functions.
Osseointegrated fixtures under occlusal loads are surrounded by cortical and spongy bone. When osseintegration is established, and prosthesis is designed for good stress distribution, cortical bone forms along the fixture surface a few millimeters in thickness. The cortical bone-to-fixture surface interface has canaliculi participating in the electrolyte transportation near the oxide layer. A network of collagen bundles surround the osteocytes and insert into a glycoprotein layer. A 100 Angstrom glycoprotein layer is formed. The Haversian bone becomes well organized and forms osteon.
Biological Seal
During the developmental years, implant dentists began to recognize that for implants to be successful and survive for extended periods of time in the hostile environment of the oral cavity, there had to be an effective biologic seal between the implant material and the tissues of the jaws. Weinmann theorized the concept of a seal around implants. More recently, Lavelle emphasized the necessity for the attached gingiva to adapt to the implant, providing a barrier to the bacteria and oral toxins into the space between Implant posts and biologic tissues.
A systematic scientific study to investigate this seal phenomenon was conducted later, using a combination of light microscopy and electron microscopy. It was shown that the gingival epithelium regenerated a series of epithelial cells following surgery that were consistently similar to those seen in the natural tooth crevicular epithelium and junctional epithelial zones. Other reports showed the presence of hemidesmosomes associated with the regenerated epithelial cells and the presence of an oricin-positive deposition on the implant surface that suggested the presence of a dental cuticle-like or basal lamina-like structure that would assist in creating a positive attachment between gingival epithelium and the implant surface, and that this mechanism developed very rapidly following implantation. Thus, the presence of a gingival attachment apparatus with epithelial components similar to that seen around natural teeth has been firmly established [Figure 4].
Figure 4.
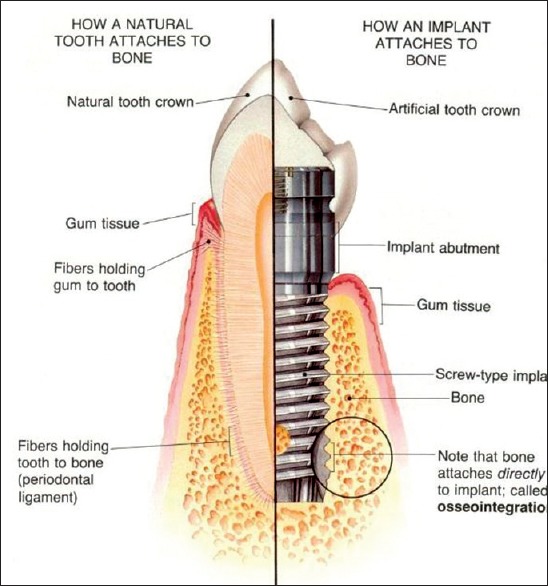
Natural Tooth versus an implant
All dental implants, whether endosteal, transosteal, or subperiosteal, must have a superstructure or coronal portion supported by a post that must pass through the submucosa (lamina propria) and the covering stratified squamous epithelium into the oral cavity. A “weak link” in the permucosal passage is created between the prosthetic attachment and the predicted bony support of the implant. This permucosal zone is the area where initial tissue breakdown begins that can result in eventual tissue necrosis and destruction around the implant.
The biologic seal thus becomes an important and pivotal factor in dental implant longevity. It serves as a physiologic barrier effective enough to prevent the ingress of toxins, bacterial plaque, oral debris, and other deleterious substances in the oral cavity. All these agents are known initiators of tissue and cell injury and must be prevented from gaining access to the internal environment that provides support for the implant device.
If the seal is violated, adjacent soft tissues will become inflamed. This will be followed by osteoclastic activity of the underlying hard tissue and chronic resorption of the supporting bone. With continued loss of the supporting bone, the discrepancy will fill with granulation tissue and the implant will become increasingly mobile, resulting in entry of the bacterial toxins and degenerative agents further into the internal environment around the implant. Ultimately, sufficient destruction will occur to give rise to an acute suppurative inflammation or acute inflammation with pain, particularly upon mastication or extensive mobility that renders support of the dental prosthesis impractical. If degenerative processes are allowed to progress to this extent, the only effective treatment is removal of the implant and debridement of the lesion. Furthermore, if sufficient bone is lost due to this destructive procedure, subsequent support of additional implants or other restorative devices may be severely compromised.
Conclusion
The successful replacement of lost natural tooth by means of tissue-integrated implants represents a major advance in clinical treatment. The knowledge of understanding of implant prosthesis has evolved from experimental laboratory phases into evidence based clinical practice over past four decades.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Misch CE. 3rd ed. USA: Evolve Elsevier; 2008. Contemporary Implant Dentistry; p. 21. [Google Scholar]
- 2.Glossary of prosthodontic terms. J Prosthet Dent. 2005;94:10–92. doi: 10.1016/j.prosdent.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Branemark PI. USA: Quintessence Books; 2005. The Osseointegration Book – From Calvarium to Calcaneus; p. 24. [Google Scholar]


