Abstract
The surface area of the titanium dental implant materials can be increased by surface treatments without altering their shape and form, thereby increasing the biologic properties of the biomaterial. A good biomaterial helps in early cell adhesion and cell signaling. In this study, the commercially pure titanium surfaces were prepared to enable machined surfaces to form a control material and to be compared with sandblasted and acid-etched surfaces, laser treated surfaces and titanium dioxide (20 nm) Nano-particle coated surfaces. The surface elements were characterized. The biocompatibility was evaluated by cell culture in vitro using L929 fibroblasts. The results suggested that the titanium dioxide Nano-particle coated surfaces had good osteoconductivity and can be used as a potential method for coating the biomaterial.
KEY WORDS: Biocompatibility, cell attachment, cell culture, nano-particles dental implants, surface treatment, titanium
In prosthodontics, endosseous dental implants are currently the most happening treatment modality. Various techniques and biomaterials have been evolved and are used successfully in the field of implantology. An ideal biomaterial is a nonviable material used in medical devices, intended to interact with the biological systems.[1] The surface characteristics of the implant material determine the osseointegration potential by its biocompatibility.[2] However, in many situations, success of the implant treatment may not be predictable due to poor bone quality or inadequate quantity of bone.[3] Precise aseptic technique and an osteoconductive implant material not only decrease the healing time, and promote greater vascularity in the cortical bone, but also enhance osseointegration, and hence, increase the success rate of the treatment.[3] Beginning in the late 1960s, the focused efforts of P-I Branemark led to the detailed microscopic characterization of interfacial bone formation around machined metallic endosseous implants. Commercially pure titanium (CpTi) and some Titanium alloys have relatively low modulus of elasticity, excellent strength-to-weight ratio, good fracture toughness, superior biocompatibility, and durable corrosion resistance making it suitable for medical implants.[4,5] It can be machined to any required shape with surface modification due to the high reactivity to oxygen, carbon, nitrogen and hydrogen.[6] Still failures in osseointegration of CpTi do exist. The cause of these failures, while not precisely determined, has been largely attributed to inadequate osseointegration. Studies show that four material related factors surface composition, surface energy, surface roughness and surface topography can influence the bone – implant interfaces.[5,7] The surface characteristics of titanium dental implants can be modified by additive methods, such as plasma spraying, ion sputtering as well as by subtractive methods, such as acid etching, acid etching associated with sand blasting and laser ablation. Various surface textures such as macro, micro and Nano have been created on the surface of titanium and used to successfully influence cell and tissue responses.[8,9] Recently, Nano technology was introduced in dentistry to stimulate a better biomimetics in restorative and prosthetic dentistry. Different physical relationships exist between cells at Nano scale level and micron scale level. Surface Nano topography of the implanted surface improves cell interactions by cell signaling, which regulates cell attachment, spreading, migration and differentiation.[10,11] In this study, the CpTi (grade II) surfaces were prepared to enable machined, acid-etched and sandblasted, laser etched, and titanium dioxide Nano-particle coated surfaces. The chemical composition and morphology of the surfaces were evaluated. The cellular attachment and cell growth on the disk surfaces were evaluated. This study demonstrated that the titanium dioxide Nano-particle coated titanium surface is a new method to treat implant surfaces.
Materials and Methods
Sample preparation
Disks of CpTi (CpTi grade II) were obtained from cylindrical bars with a diameter of 6 mm and a thickness of 2.3 mm by electrical discharge machining (EDM) (ECOCUT India and ELAPT Software–India and Japan). The 40 samples obtained were finished with no surface treatments. About 10 machined samples were assigned as control and grouped as TM. The remaining 30 samples were divided into three groups based on the surface treatment to be subjected. The groups were assigned according to the surface treatment, and they were exposed to, group TSA – sand blasted and acid etched, group TL – laser treated, group TN – titanium Nano-particle coated. The machined (TM) discs were sandblasted with a sandblasting machine, with Alumina as an abrasive. Alumina of 110 μm under 4 kg/cm2 pressure was forced on the sample surfaces and then the samples were treated with acids 30%HCL, 30% H2SO4W/W for 20 min in mixture boiling point to form group (TSA). The machined disks were Laser irradiated with Nd-YAG, Q-switched laser; power, 4.0 kW; frequency, 3 kHz (GSI Industrial LASERS-UK). The samples were grouped as (TL). The TM discs were treated with titanium dioxide Nano-particles (spherical shaped, size – 20 nm) (Anna Univ, Chennai, India), the titanium Nano-particles were made into slurry and applied on the disc surfaces. Discs were subjected to pulse laser deposition for coating of Nano-particles on the discs using Nd-YAG, Q-switched type with a density of 1.5–4.5 J/cm2 (GSI – UK). The discs were grouped as (TN). Thereafter, disks were thoroughly degreased, washed and sonicated in a series of solvents (ethanol, acetone and de ionized water). All disks were ultrasonically rinsed with distilled water, air-dried at room temperature, and then stored in a sealed auto clavable pouch. All samples were sterilized before use.
Surface analysis
Scanning electron microscope (SEM) was used to examine the surface morphology. They were analyzed under magnification from ×500 to ×5000 and the surface analyses of the samples were evaluated by energy dispersive X-ray spectrometer (EDS) (Hitachi-Japan). The degree of surface contamination of the titanium discs was evaluated.
Cell culture study
The sterile samples were placed in multi-well plate using flat tipped forceps. A control material was also placed in the multi-well plate. A glass cover slip was used as a control material. The extraction vehicle was the culture medium, Minimum Essential Medium (SIGMA) (Sigma-Aldrich Co. LLC. Allied machine and engineering corp) supplemented with fetal bovine serum and 1% antibiotics (streptomycin and penicillin). The cells used were L929 murine fibroblasts, (NCCS, Pune). The cells were trypsinized and counted using a hemocytometer before seeding in the multi well plates. Individual wells of multi well plates were seeded with L929 cells at a density of 5 × 103 cells/well with minimum media so that the cell suspension will be on top of the samples. The cells were incubated for 48 h at 37°C under humidified atmosphere containing 5% CO2. The samples at 48 h were washed with phosphate buffer solution, fixed with 2.5% of gluteraldehyde for minimum 2 h at room temperature and dehydrated using graded (90%, 70%, 50%, 30%) of methanol solution. The samples were immersed in isomyl acetate solution for 1 min. The samples were dried at the critical point, with CO2 as medium, sputter coated with gold and observed under SEM (Hitachi S3000N, Japan) Anna Univ, India. The morphological changes of the cells attached to the samples were evaluated.
Results
The surface composition of each sample was analyzed using EDS.
Machined samples (TM)
The results for surface composition of the machined surfaces showed traces of carbon and copper, which could be due to surface contamination during spark erosion of the machined implants. Negligible quantities of chloride and silica were found, which could have been observed when the surface oxide reacted with the elements in the air.
Sand blasted and acid etched samples (TSA)
Surface composition of acid-etched samples TSA showed traces of alumina and silica in small amounts, which could have been incorporated from the sandblasting procedure. Acid etched samples showed amounts of oxygen - 14.8% reduced the concentrations of carbon and nitrogen which were found to be absent. Chemically activated titanium surfaces exhibit a high surface energy due to the oxide layer that grows spontaneously in contact with the environment and tissue fluids. The formation of stable oxides on the titanium surface may also explain the biocompatibility of this material. The oxide surface is hydrophilic, binding to water and forming −OH and O2 groups in the outermost layer. Concomitantly, these groups can nucleate and form calcium phosphate precipitates when in contact with physiological fluids. The number of atoms and molecules present on the titanium surface is important for the formation of hydroxyapatite and may enhance osseointegration.
Laser treated surface (TL)
Surface composition of laser treated samples showed a high Ti content-90.43% and oxide layer of 8.73% indicates that laser treatment can produce a high degree of purity on the treated surface there was a very small negligible amount of calcium - 0.86% with no other additional elements on the surface when compared with machined samples.
Nano-particle coated surface (TN)
The EDS analysis of Nano-particle coated titanium surface showed increased oxide layer of 23.94% compared to all other groups. This indicates that Nano-particle coating could increase the surface oxide composition on the titanium surface which could contribute to the increased biocompatibility.
Surface morphology of samples
Machined samples
At low magnification, the surface of CpTi discs (TM) exhibited shallow grooves resulting from machining and polishing [Figure 1a]. At high magnification, the machined metal surfaces did not reveal significant topographical changes. The mild irregularity may be due to [Figure 1b] mechanical processing resulted in micro structures with a diameter of 2–8 μm in an irregular pattern in the form of flat extensions [Figure 1b].
Figure 1.
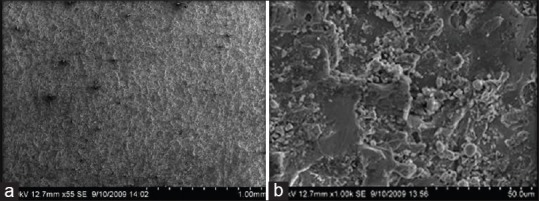
(a and b) Scanning electron microscope images of TM
Sand blasted and acid etched samples
At low magnification, the chemical treatment along with sand blasting produced uniform shallow irregularities [Figure 2a]. At higher magnification, [Figure 2b] there is a formation of a unique texture characterized by Nano-pitting with honey comb like appearance. Protrusion showed an average size of 30–90 μm depth and base width of 10–40 μm. The primary structure of the surface consisted of covering protrusion without troughs that may be ascribed to the preparation and particle size of aluminum dioxide [Figure 2b].
Figure 2.
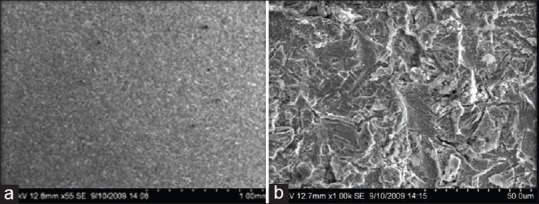
(a and b) Scanning electron microscope images of group TSA
Laser treated surface
Electron microscope pictures of increasing magnification showed the regular processing tracks of the laser on the samples surfaces [Figure 3a]. The surface exhibited troughs of 50–80 μm diameter. A typical, regular pattern of the secondary landscape of the laser processed titanium surface was exhibited at higher magnification. The pattern consisted of regular protrusions and troughs with a scaled projection, producing a secondary roughness of 10 μm with a typical troughed structure formed due to laser impulse transfer.
Figure 3.
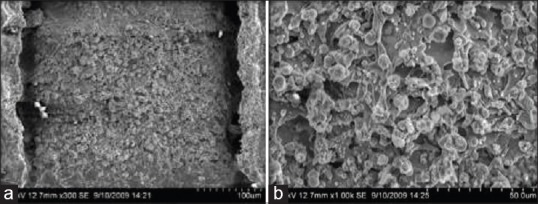
(a and b) Scanning electron microscope images of group TL
Nano-particle coated
At low magnification, the CpTi surface was not grossly affected by the Nano texturing treatment. At higher magnification (10 μm) Nano scale, the surface was characterized by anisotropic pitting due to adhesion of the titanium oxide particles on to the surfaces [Figure 4a]. At the micron scale, the procedure produced a Nano texturing of the surface that was densely covered with pits of averagely 1.5 μm depth and 3–5 μm diameter [Figure 4b].
Figure 4.

(a and b) Results of cell culture study. Group TN
Cell culture
The cell attachment and growth were observed under an SEM after 48 h. The morphology of L929 cells on the sample surface differed following;
Cell attachment and growth on the test samples
Machined surfaces
The cell cultures after 48 h showed that the cell layer at low magnification showed no specific organization [Figure 5a]. However, organization was seen along the groves produced by the machined tooling. The cells seen under higher magnification were fusiform with filamentous extensions and oriented in a parallel manner [Figure 5b]. On the machined surfaces, the cells appeared with uniform morphology with a single filamentous extension, seen anchored on the machined surface.
Figure 5.
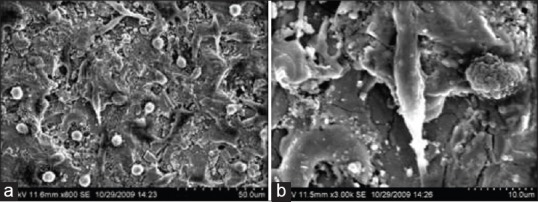
(a and b) Results of cell culture study. Group TM
Sand blasted, and acid etched surfaces
At low magnification, morphology of the cells cultured on the acid etched surface was different from that of the machined tool surface. The surface roughness created by the acid etching and sand blasting procedures influenced the initial attachment and spread of the cells [Figure 6a]. The cells did not show uniform arrangement but were found all throughout the etched surface. The cells showed fusiform and stellate appearance. At higher magnification cells appeared with filamentous extensions into the etched surfaces [Figure 6b]. The results show that blasting and acid etching the surface favor initial attachment of the cell. At high magnification, the cells appeared to flatten and spread well indicating good attachment to the etched surface.
Figure 6.
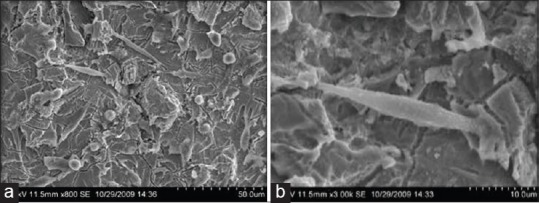
(a and b) Group TSA
Laser treated cells
At low magnification, the cells cultured on the laser treated surfaces showed a wide variety of cells grown on the modified surface showing good adhesion and good spreading of shapes [Figure 7a]. The cells had a variety of shapes including round and spindle. Some spindle shaped cells showed multi polar extensions. The majority of fibroblasts showed more spherical, polygonal and triangular cell shape with fine and long filopodia. At high magnification, filopodious extensions of the cells cultured shows strong adhesion and wide spreading to the laser treated surface [Figure 7b]. Cell growth seems to be denser compared to machined and acid etched surfaces. However, the cells did not seem to be having a particular orientation to particular surfaces. The appearance proves the fact that laser treating the surface enhances the titanium oxide layer on the surface (90.43%) there by increasing the potential for good adhesion and cell spreading.
Figure 7.
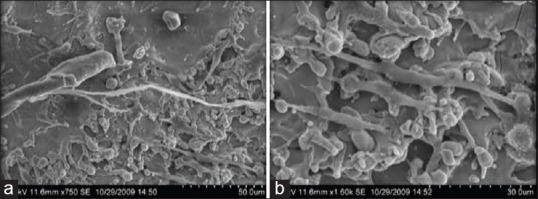
(a and b) Group TL
Nano-particle coated surfaces
The cell culture results after 48 h of culturing showed cells with variable shape and dimensions from polygonal, stellate and fusiform cells. Some of them showed long cytoplasmic extensions. Typical migrating cell morphology with leading and trailing edges was observed in areas of confluence of the Nano texturing. The predominant cells were polygonal showing finger like projections with an uniform pericellular cytoplasmic spreading [Figure 8a]. These filopodia appear to span the larger surface topographical feature. The CpTi surface was not smooth and some of its irregularities attached to the ridges of the Nanocavitation, created by the Nano texturing treatment. At high magnification, the cells showed filopodia attached to the edge of the periphery of the Nano cavitation [Figure 8b]. The results show that Nano surface treatment could modulate initial cell attachment response. At high magnification, the cell seems to attach selectively to the Nano scale topographic cavitation which may be an important factor for initial adhesion and proliferation of the cell. The results prove that Nano scale topography for implant surface may have potential advantage in promoting initial osteogenesis and stability of the implant.
Figure 8.
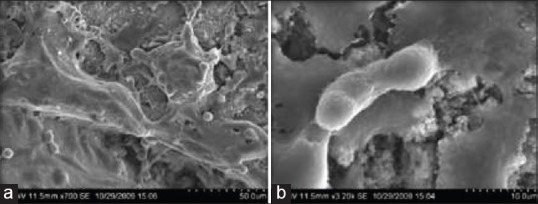
(a and b) Group TN
Discussion
The ideal success of a dental titanium implant is determined by the degree of purity of titanium, native oxide layer and a rough implant surface. When these factors are present, high mechanical stability can be achieved by a complete osseointegration of a large implant surface. Since the screw diameter and length of the implant cannot be increased beyond a limit (to have an increased surface area), alterations in the surface topography of the implants have become the next viable option. Surface roughness is one of the key factors determining implant prognosis and success.[12,13,14] Surface roughness may be of three types macro, micro and Nano. It is known that fibroblasts respond to the micro topography of the surface of the dental implants. They align in relation to the micro texture of the substrate. This is called contact guidance. The process by which cells become established on a surface includes (a) initial attachment (b) cellular spreading (c) secretion of extracellular matrix (d) cellular migration and (e) growth and differentiation. Surface profiles in the Nano meter range play an important role in the adsorption of proteins, adhesion of cells and thus the rate of osseointegration.[15] Nano scale topography is a powerful way of altering protein interactions with the surface. This leads to increased cell adhesion in the Nano coated implants when compared to other the other groups.[16] The cell adhesion over the machined samples (TM) showed good cell adhesion with parallel orientation towards the grooved surfaces with typical morphology of L929 cells. The cell adhesion over the sand blasted and acid-etched surfaces (TSA) showed few filopodial extensions at the end of the cells. The cell adhesion over the Laser treated (TL) surfaces showed cells with multipolar filopodial extensions and dense spreading of cells. The cells over the titanium dioxide Nano-particle coated sample (TN) showed an intact monolayer and extensions into the Nano cavitation surfaces. The surfaces of all groups were nontoxic and favored cell migration and attachment. In this study, the fibroblast spreading was seen more in the Nano titanium dioxide coated (TN) implant surface. The increased vitronectin adsorption on Nanostructured surfaces favored better cell adhesion when compared to conventional surfaces.[16] In conclusion, the modified surface characteristics of the group (TN) led to better cell response and attachment. The difference between the surface characteristics of the (TN) group from other groups could have served as a scaffold for early cell attachment and growth. This indicates the hypothesis that the Nano textured implants improve osseointegration, and can be used in dental implants.
Conclusion
The surface morphology of treated samples showed more uniform roughness in sand blasted and acid etched samples, and Laser treated samples. The cell adhesion over the machined samples showed good adhesion of cells with parallel orientation towards the grooved surfaces with typical morphology of L929 cells. The cell adhesion over the sand blasted, and acid-etched surfaces showed few filopodial extensions at the end of the cells. The cell adhesion over the Laser treated surfaces showed cells with multipolar filopodial extensions and dense spreading of cells. The cells over the titanium dioxide Nano-particle coated sample showed an intact monolayer and extensions into the Nano cavitation surfaces. Within the limitations of the study, the Nano-particle coated surfaces (TN) proved to be better than the other two groups (TSA) sand blasting and acid etching and (TL) laser treated surfaces in cell adhesion. Nano-particle coating creates a better scaffold of titanium for enhancing cell adhesion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Branemark PJ. Long term follow up study of osseo integrated implant in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387–416. doi: 10.1016/s0300-9785(81)80077-4. [DOI] [PubMed] [Google Scholar]
- 2.Zarb GA, Schmitt A. The longitudinal clinical effectiveness of osseointegrated dental implants: The Toronto study. Part III: Problems and complications encountered. J Prosthet Dent. 1990;64:185–94. doi: 10.1016/0022-3913(90)90177-e. [DOI] [PubMed] [Google Scholar]
- 3.Goodacre CJ, Kan JY, Rungcharassaeng K. Clinical complications of osseointegrated implants. J Prosthet Dent. 1999;81:537–52. doi: 10.1016/s0022-3913(99)70208-8. [DOI] [PubMed] [Google Scholar]
- 4.Masuda T, Salvi GE. Cell and matrix reactions at titanium implants in surgically prepared rat tibiae. Int J oral maxilla Implants. 1997;12:470–85. [PubMed] [Google Scholar]
- 5.Lee Guehennec. surface treatments of titanium dental implants for rapid Osseo integration. Dent mater. 2007;23:844–854. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Cooper LF. Biologic determinants of bone formation for osseointegration: Clues for future clinical improvements. J Prosthet Dent. 1998;80:439–49. doi: 10.1016/s0022-3913(98)70009-5. [DOI] [PubMed] [Google Scholar]
- 7.Wilke HJ. clinical implant materials edited by Heimke. 9 of ADV biomater [Google Scholar]
- 8.A Gagge, scaning electronic microscopical analysis of lazer- treated titanium implant surfaces – A comparative study. Biomaterials. 2000;21:1067–1073. doi: 10.1016/s0142-9612(00)00002-8. [DOI] [PubMed] [Google Scholar]
- 9.Cooper L LF. Role of surface topography in creating and maintaining bone at titanium endosseous implants. JPD. 2000;84(5):522–534. doi: 10.1067/mpr.2000.111966. [DOI] [PubMed] [Google Scholar]
- 10.Brunette DM, et al. Material science, surface science, engineering biological responses and medical applications: USA. 2001 [Google Scholar]
- 11.Baraton MI, Chen X, Gonsalves KE. Study of nanostructured alumina nitride powder surface - determination of the acidic/ basic sites by CO, Co2, and acetic acid adsorption nanostructure materials. 1997;8:435. [Google Scholar]
- 12.Albrektsson T. Wennerberg Oral implant surface: Part 1 Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:534–543. [PubMed] [Google Scholar]
- 13.Kasemo B. Biocompatibility of titanium implants; surface Science aspects. J Prostho Dent. 1983;’49:832–7. doi: 10.1016/0022-3913(83)90359-1. [DOI] [PubMed] [Google Scholar]
- 14.Johansson CB, Albrektsson T. A removal torque and histomorphometric study of commercially pure nobium and titanium implants in rabbit bone. Clin Oral Implant Res. 1991;2:24–9. doi: 10.1034/j.1600-0501.1991.020103.x. [DOI] [PubMed] [Google Scholar]
- 15.Lie, Liao H, Fartash B, Hermansson L, Johnsson T. Surface-dimpled commercially pure titanium implant and bone ingrowth. Biomaterials. 1997;18:691–6. doi: 10.1016/s0142-9612(96)00185-8. [DOI] [PubMed] [Google Scholar]
- 16.Mendonça G, Mendonça DB, Aragão FJ, Cooper LF. Advancing dental implant surface technology-from micron-to nanotopography. Biomaterials. 2008;29:3822–35. doi: 10.1016/j.biomaterials.2008.05.012. [DOI] [PubMed] [Google Scholar]


