Abstract
Background:
Pityriasis rosea (PR) is an acute inflammatory dermatosis. The association of human herpes virus 6 and 7 suggests the utility of use of antiviral agents in this disease.
Aims and Objectives:
To evaluate the effectiveness and safety of acyclovir in the treatment of PR.
Methods:
An observer-blind, randomized (1:1), parallel group, add-on trial was conducted on 24 adult patients with PR. Subjects of both Group A and B received the standard of care in the form of cetirizine 10 mg OD and calamine. Group A in addition received acyclovir 400 mg tablets thrice daily for 7 days. Both groups were followed up for four consecutive weeks for assessment of effectiveness and adverse events.
Results:
Group A complained of significantly fewer new lesions than Group B (P = 0.046). A complete response was obtained in all patients of Group A and 83% patients of Group B at the end of the follow up period. There was significant reduction in both lesional score and pruritus at second week follow-up in Group A and third week follow-up in Group B (P < 0.05). Minor adverse effects were observed in both treatment arms.
Conclusion:
Acyclovir offered rapid resolution of clinical severity of PR from second week onwards without significantly increased adverse events as compared to supportive therapy alone.
Keywords: Acyclovir, cetirizine, pityriasis rosea
INTRODUCTION
Pityriasis rosea (PR) is an acute inflammatory skin disorder. Though self-limiting, the bothersome symptoms often have a significant impact on the quality-of-life of patients. The incidence of PR varies from 0.39[1] to 4.80/100[2] dermatological patients. The association of human herpes virus 6 (HHV-6) and HHV-7 with PR suggest that antiviral agents can be tried to speed up recovery of PR.[3] Till date, supportive care with topical emollients and antihistamines is practiced by dermatologists for this disorder. Oral erythromycin was once reported to be of benefit to patients with PR,[4] but recent clinical experiences suggest that the use of macrolides may not be useful in the treatment of PR.[5,6] Studies evaluating acyclovir in PR are being conducted worldwide, however, data on Indian patients is scarce.[3,7] We undertook this trial to evaluate the safety and effectiveness of acyclovir among Indian patients with PR.
METHODS
The study was designed as a single center, observer-blind, randomized (1:1), parallel-group, add-on Phase IV trial. The patients were recruited from May 2013 to September 2013. Eligible subjects were consecutive, consenting adult patients (>18 years) of either sex clinically diagnosed as PR at the dermatology outpatient department of a teaching hospital in eastern India. Exclusion criteria were pregnant and lactating women, advanced disease of vital organs, history of sensitivity to acyclovir or cetirizine, and suspicion of mimicking diseases (fungal infection, psoriasis, or eczema). The study was approved by the Institutional Ethics Committee and written informed consent was obtained from all subjects. The trial was registered with the Clinical Trial Registry - India and bears the registration number - CTRI/2013/12/004240.
Subjects were allocated, using a computer-generated randomization schedule (simple randomization by 1:1 allocation), to one of two treatment groups, A and B. Standard of care in the form of tablet cetirizine (10 mg OD at bed time) and calamine lotion was given to both groups. Group A in addition received tablet acyclovir (400 mg thrice a day for 7 days). Allocation concealment was done by sequentially numbered opaque envelopes. Subjects appeared for follow-up visits weekly for four consecutive weeks following randomization. Acyclovir and cetirizine were obtained from the hospital pharmacy, acyclovir (Batch No. ACV-41, date of expiry October 2016, manufacturer C.I. Laboratories, West Bengal, India), cetirizine (Batch No. CT 20313, date of expiry June 2016, manufacturer Bengal Antibiotics, West Bengal, India), calamine (Linical, Batch No. A00812, date of expiry December 2013, manufacturer Palsons Drugs Private Limited, West Bengal, India) were used. Randomization and dispensing of medications were done by a person unrelated to the trial. The physician evaluating the effectiveness and safety parameters was thus unaware of the treatment group of the patient making the trial observer-blind.
The sample size was 11 evaluable PR patients in each treatment group. This was calculated to detect a difference of 3 units in Visual Analogue Scale (VAS) (vide infra) between groups, with 80% power and 0.05 probability of type 1 error, assuming a standard deviation of 2.5 for this parameter. Considering 10% possible dropout rate, this translated to a recruitment target of approximately 24 subjects overall.
The effectiveness parameters were VAS, appearance of new lesions, lesional score and pruritus. Decrease in itch was assessed by the patients using VAS of 1-10.[7] Lesional score was calculated by addition of erythema score, scaling score and number of lesions score, wherein presence or absence of erythema meant an erythema score of 1 and 0 respectively; presence or absence of scaling meant a scaling score of 1 and 0 respectively; <30 lesions was given a score of 1, 30–100 lesions were given a score of 2 and >100 lesions scored 3. Pruritus was graded as 0 (absent itching); 1 - mild (itching present but not disturbing life); 2 - moderate (disturbing, but not hampering daytime activities and/or sleep); 3 - severe/intense (disturbing and hampering daytime activities and/or sleep). Response to therapy[8] was categorized as: (a) Complete response - no new lesions, disappearance of all previous lesions, with or without residual postlesional pigmentation; (b) partial response - a few new lesions, regression or disappearance of some previous lesions; (c) no response - no regression of lesions, appearance of new lesions.
Safety assessment included vital signs, treatment-emergent clinical adverse events and routine hematological and biochemical tests. The laboratory tests were done at baseline and after one week of therapy with trial medication, and included hemoglobin, total and differential leukocyte count, platelet count, creatinine, total and conjugated bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and fasting blood glucose.
The numerical data were analyzed using unpaired t-test or Mann–Whitney U-test (as applicable) and the qualitative data were analyzed using the Chi-square test and Fisher's exact test. P value within a group was evaluated by Friedman's ANOVA followed by post-hoc Dunn's test. Medcalc version 10.2 (Mariakerke, Belgium: MedCalc Software, 2011) and GraphPad Prism version 5 (San Diego, California: GraphPad Software Inc., 20057) was used for statistical analysis, and P < 0.05 was considered statistically significant. Modified intention to treat analysis was done with subjects reporting for at least one postbaseline follow-up.
RESULTS
The flow chart of study participants is depicted in Figure 1. Of the 27 subjects screened, 24 subjects were randomized equally into two groups.
Figure 1.
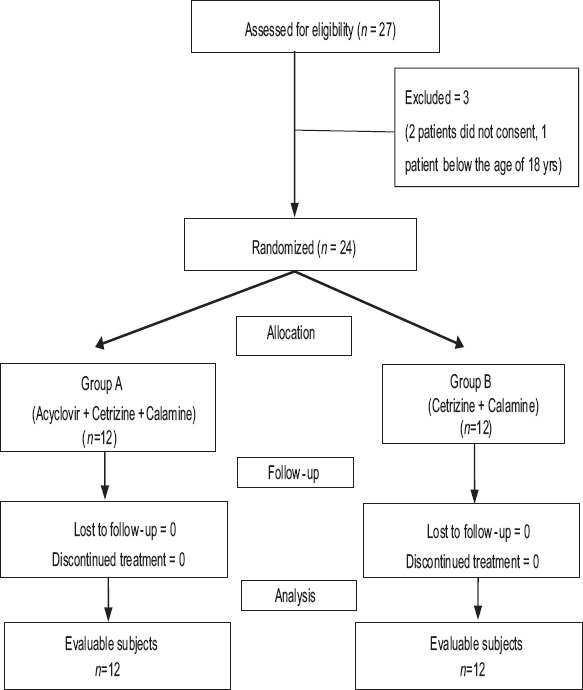
Consolidated Standards of Reporting Trials flowchart of study participants
In Table 1, showing the clinico-demographic variables, study groups were comparable at baseline with respect to age, sex, residence, income, occupation and duration of illness.
Table 1.
Clinico-demographic variables
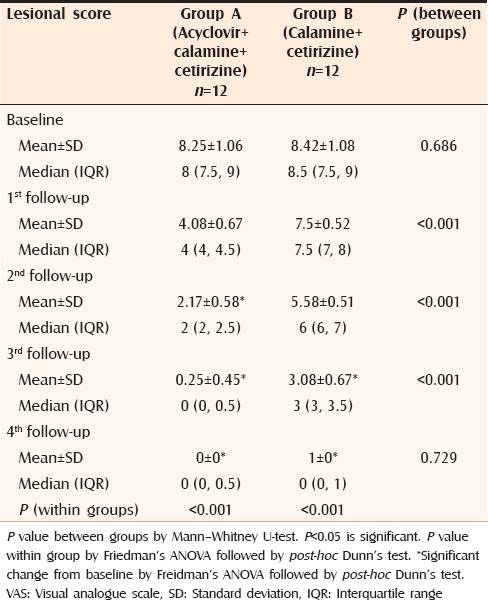
After one week, only 2 out of 12 patients in Group A complained of appearance of new lesions which was significantly less than Group B where 7 out of 12 patients reported new lesions (P = 0.046). A promising result was obtained on analyzing the changes on objective, as well as subjective scoring. When lesional scores were evaluated, significant reduction (P < 0.05) was observed in patients of Group A from second follow-up itself [Table 2] whereas, in patients of Group B, the lesional score did not touch the baseline even at the end of the study. The results were similar in case of pruritus score as well [Table 3]. Complete response was obtained in all patients of Group A and 83% patients of Group B [Table 4]. Comparison of VAS in the two groups showed similar results as for lesional and pruritus scores [Table 5].
Table 2.
Changes in lesional score in both groups
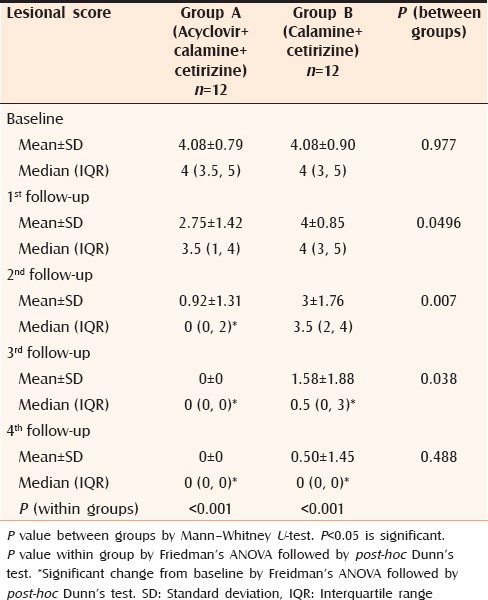
Table 3.
Changes in pruritus score in both groups
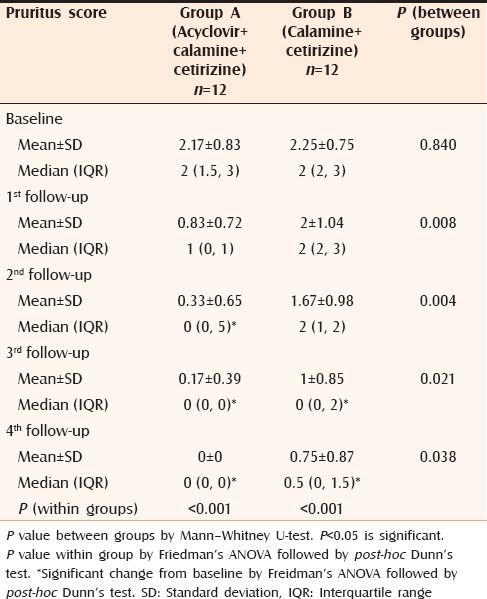
Table 4.
Response to therapy
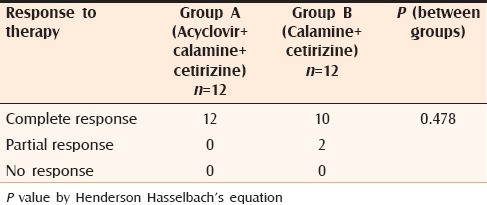
Table 5.
Changes in VAS in both groups
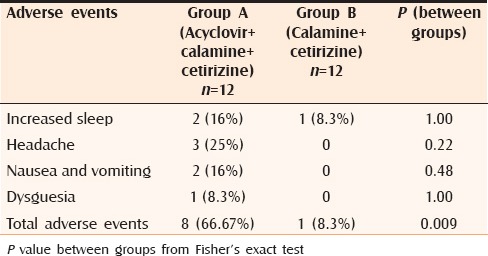
Minor side-effects in the form of sedation, headache, nausea, vomiting, dysguesia were observed in the treatment arms but had comparable incidence in both [Table 6]. None of the patients discontinued the trial due to these side-effects though. Laboratory parameters were within normal limits in both groups.
Table 6.
Adverse events
DISCUSSION
Pityriasis rosea has long been suspected to have an infectious, mainly viral etiology because of its clinical course mimicking viral exanthems and thus, acyclovir supposedly holds promise in its management. Antibiotics such as erythromycin and other macrolides have been used to treat PR.[4] Macrolides have antiinflammatory and immunomodulatory effects that might affect the course of PR or other cutaneous eruptions. Studies have compared the effectiveness of these two drugs, acyclovir and erythromycin in PR. Such a study conducted by Ehsani et al. on 30 patients with PR showed that patients in the acyclovir group responded significantly better than the patients treated with erythromycin.[8] In another study conducted by Amatya et al., response to oral acyclovir compared with that to oral erythromycin was better and statistically significant in the first, second, fourth and sixth weeks.[9] Drago et al. conducted a similar study with either oral acyclovir (800 mg 5 times daily) or placebo.[3] On the day 14 of treatment, 79% of treated patients fully regressed compared with 4% of the placebo group. The lesions cleared in 18.5 days in treated patients and 37.9 days in the placebo group. All the above studies were done with high doses of acyclovir (4 g/day). In our study, substantial symptom relief was obtained with 1.2 g/day of acyclovir, thus exposing the patient to a lower yet effective dose of the drug. Earlier clearance of symptoms has a favorable effect on the quality of life of patients of PR treated with acyclovir. Our study found that acyclovir offers faster resolution of lesions than supportive symptomatic therapy with cetirizine and emollient thus allaying the anxiety in the minds of patients. Though at the end of four weeks we noted similar decrease in the number of new lesions in both treatment groups, itching was significantly less among those treated with acyclovir.
This study has its share of limitations. Double blinding could not be used, and the duration of therapy could not be extended beyond four weeks due to logistical reasons.
The unit price of the study drugs in the Indian market showed that the cost should not be a major impediment to treatment because the add-on cost for acyclovir for one week was 350 INR (approximately $5.83) only.
CONCLUSION
Acyclovir offered significantly rapid relief in clinical symptoms of erythema, scaling and pruritus compared with use of standard care of antihistamines and emollients. The appearance of new lesions was reduced. Acyclovir can be safely used in the treatment of PR to achieve faster control of the disease and improvement of the quality of life of patients. A more comprehensive long term trial can give us a deeper insight to the sustained effect of the drug. Comparative trials with different dosage schedules may establish the optimum dose and duration of acyclovir therapy in PR.
ACKNOWLEDGEMENTS
The authors acknowledge the contribution of the faculty, residents and staff of the Department of Dermatology, Medical College, Kolkata for their support during the conduct of the trial.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.de Souza Sittart JA, Tayah M, Soares Z. Incidence pityriasis rosea of Gibert in the Dermatology Service of the Hospital do Servidor Público in the state of São Paulo. Med Cutan Ibero Lat Am. 1984;12:336–8. [PubMed] [Google Scholar]
- 2.Olumide Y. Pityriasis rosea in Lagos. Int J Dermatol. 1987;26:234–6. doi: 10.1111/j.1365-4362.1987.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 3.Drago F, Vecchio F, Rebora A. Use of high-dose acyclovir in pityriasis rosea. J Am Acad Dermatol. 2006;54:82–5. doi: 10.1016/j.jaad.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Sharma PK, Yadav TP, Gautam RK, Taneja N, Satyanarayana L. Erythromycin in pityriasis rosea: A double-blind, placebo-controlled clinical trial. J Am Acad Dermatol. 2000;42:241–4. doi: 10.1016/S0190-9622(00)90132-4. [DOI] [PubMed] [Google Scholar]
- 5.Bukhari IA. Oral erythromycin is ineffective in the treatment of pityriasis rosea. J Drugs Dermatol. 2008;7:625. [PubMed] [Google Scholar]
- 6.Amer A, Fischer H. Azithromycin does not cure pityriasis rosea. Pediatr. 2006;117:1702–5. doi: 10.1542/peds.2005-2450. [DOI] [PubMed] [Google Scholar]
- 7.Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: Evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol. 2012;92:497–501. doi: 10.2340/00015555-1265. [DOI] [PubMed] [Google Scholar]
- 8.Ehsani A, Esmaily N, Noormohammadpour P, Toosi S, Hosseinpour A, Hosseini M, et al. The comparison between the efficacy of high dose acyclovir and erythromycin on the period and signs of pitiriasis rosea. Indian J Dermatol. 2010;55:246–8. doi: 10.4103/0019-5154.70672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amatya A, Rajouria EA, Karn DK. Comparative study of effectiveness of oral acyclovir with oral erythromycin in the treatment of pityriasis rosea. Kathmandu Univ Med J (KUMJ) 2012;10:57–61. doi: 10.3126/kumj.v10i1.6916. [DOI] [PubMed] [Google Scholar]


