Abstract
Context:
Urticaria, also referred to as hives or wheals is a common and distinctive reaction pattern characterized by spontaneous eruption of wheals. About 30–50% of patients categorized as idiopathic urticaria have autoimmune urticaria, needing immunosuppressive agents. Immunosuppressive agents are either too costly or have serious side effects. Azathioprine seems to address both these issues, being less costly.
Aims:
The aim was to evaluate the role of azathioprine in autologous serum skin test (ASST) positive chronic urticaria (CU).
Settings and Design:
A single-blind randomized control trial of ASST positive patients of CU.
Materials and Methods:
Patients with positive ASST were allotted into two treatment groups, named group A and group B. Patients in group A were administered azathioprine (50 mg/day) for a period of 8 weeks and followed up till 36 weeks, while patients in group B were given placebo pills. All patients were directed to take levocetirizine (5 mg) on as and when basis, in addition. Urticaria was assessed by total severity score.
Statistical Analysis Used:
MedCalc statistical software (v 12.5 for Windows) to calculate P values in independent samples by t-test, Mann–Whitney test, Friedmann test, and ANOVA.
Results:
Administration of azathioprine in group A resulted in a significant diminution of the intensity of the disease as well as in the requirement of rescue antihistamine (positive primary and secondary outcome).
Conclusion:
Azathioprine not only had immunomodulatory properties during the treatment period, but also had lasting therapeutic effect as well. There were some gastrointestinal side effects in the initial stages but no incidence of hematological or biochemical disturbances.
Keywords: Autologous serum skin test positive, azathioprine, chronic urticaria
INTRODUCTION
Urticaria, also referred to as hives is a common and distinctive reaction pattern characterized by the appearance of short-lived swellings on the skin and mucous membranes called wheals,[1] described as evanescent, circumscribed, erythematous or white, nonpitting, edematous, usually pruritic plaques that change in size and shape [Figures 1a and b].
Figure 1.
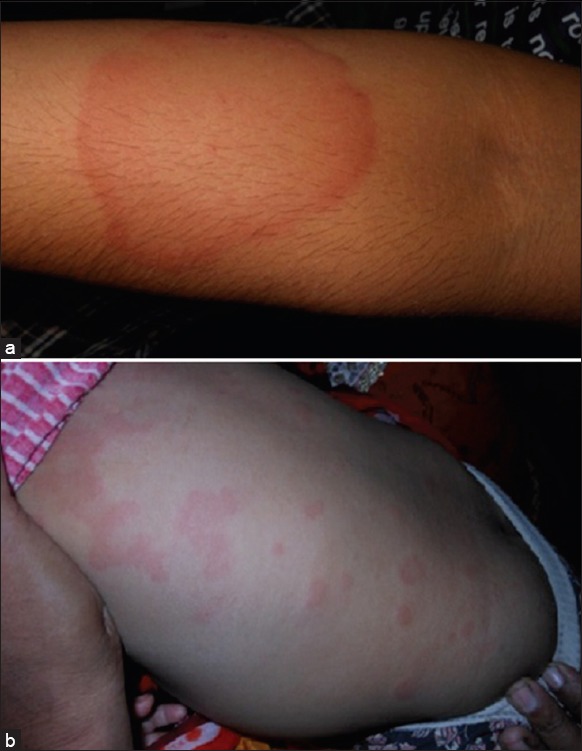
(a) Typical wheal on the forearm, (b) many wheals on the trunk of a child
Urticaria is said to be acute if it lasts less than six weeks, and chronic (chronic urticaria, [CU]) if it lasts more than six weeks with daily or nearly daily symptoms. Etiologically, urticaria may be classified as idiopathic (cause unknown), immunological (cause - autoimmune, IgE/contact, or immune complex-related), nonimmunological (cause - physical, drug-related, or dietary), and medical condition-related (urticaria pigmentosa, cryopyrin-associated, and periodic syndromes).[2] About 30–50% of patients categorized as idiopathic urticaria have autoantibodies to IgE or FcεRIα subunit of high-affinity mast cell receptors; these patients are said to have autoimmune urticaria.[3] Clinically, these patients have a propensity to have somewhat more severe, long-standing course and are refractory to conventional treatment, needing immunosuppressive agents.
Autologous serum skin test (ASST) has been found to be a useful screening test for autoimmune urticaria with sensitivity of 65–81% and specificity of 71–78%.[3]
For the widespread problem of CU refractory to antihistamines, the use of alternative, non-steroidal therapies is suitable and preferable, especially in the long term. Therefore, alternative therapies and immunosuppressive agents have been used in the management of CU, especially autoimmune urticaria. These therapies include dapsone, sulfasalazine, colchicine, and immunosuppressive agents such as mycophenolate mofetil, cyclosporine, and methotrexate.[3] There have been no randomized controlled trials of azathioprine in autoimmune urticaria; we therefore studied its use in ASST positive cases of CU.
MATERIALS AND METHODS
Setting
An outpatient dermatology department of a tertiary care hospital in eastern India.
Type of study
Single blind, randomized control study. The trial was registered with the clinical trials registry of India.
Inclusion criteria
ASST positive patients of CU aged 12 years and above, after obtaining informed consent.
Exclusion criteria
Pregnancy, immunosuppressive drug intake within the last two months, consumption of alcohol on a regular basis, severely ill, debilitated patients, and patients unwilling to take part in the study.
Method of generating random sequence
Sequentially numbered opaque envelopes with an allocation ratio of 1:1.
Method of concealment
Case record numbers.
Blinding
Single blinding (participant blinded): The participants did not know which treatment group they belonged to.
Primary outcome
Decrease in disease severity at six months from the time of enrolment into the study.
Secondary outcome
Levocetrizine usage during the study.
Sample size
Approximately, 60 (Indian population) ASST positive patients of CU.
Duration of trial
One year (February 2012 to January 2013).
Criteria for a positive response in autologous serum skin test
A positive test was defined as a serum-induced wheal response with a diameter of 1.5 mm or more than that of saline-induced response at 30 min.
PROCEDURE
Patients with positive ASST were allotted into two treatment groups, A and B. Patients in group A were administered azathioprine (50 mg/day) supplied in unlabeled paper envelopes for a period of 8 weeks and followed up for another 28 weeks, while patients in group B were given placebo pills, also supplied in similar packages, to be taken daily for 8 weeks. In addition, all the participating patients were directed to take levocetrizine (5 mg) on as and when basis for the entire study period (36 weeks) up to a maximum of 4 tablets/day (20 mg). The sequence of events is depicted in the flow chart [Figure 2].
Figure 2.
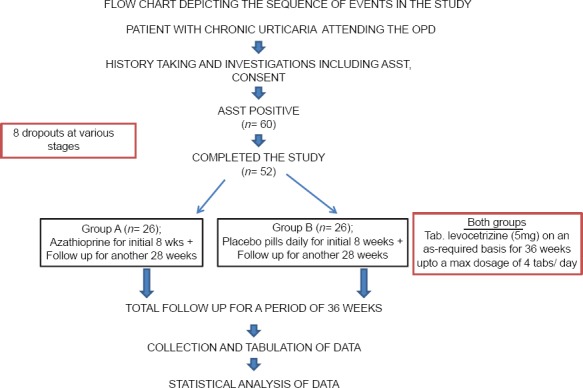
Flowchart depicting the sequence of events in the study
Thus, every patient was followed up every 4 weeks for a period of 36 weeks for the assessment of disease as well for monitoring of side effects, if any. In addition, patients in group A underwent baseline and monthly blood counts and liver function tests during the 8 weeks that they were on azathioprine. Patients in group B were unaware of this fact because they hardly ever came face to face as the number of patients studied per day was one or two. Furthermore, since no separate corner or room was reserved for the study, it was impossible for one patient to know what happened to another patient in the trial.
Disease assessment
Urticaria was assessed by total severity score (TSS).[4] Six separate parameters of disease activity and severity were recorded on scale of 0–18 at baseline (0 week), and then every 4 weeks for a total period of 36 weeks. Based on these, a TSS was generated, and overall disease severity classified as clear (TSS = 0), mild (TSS 1–6), moderate (TSS 7–12), or severe (TSS 13–18). This is represented in Table 1.
Table 1.
TSS scoring system
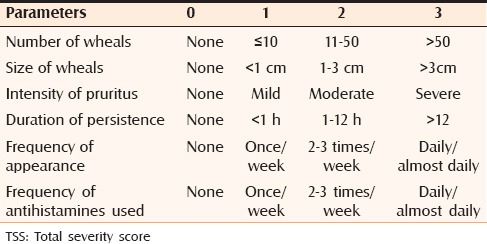
A follow-up score of 0 was considered complete remission, a score of 1–6 marked improvement, 6–12 moderate improvement, and 13–18 poor/no improvement.
Analysis of data
At the end of the study, the data was compiled, tabulated, and analyzed with suitable statistical tests using MedCalc statistical software (v 12.5 for Windows) by MedCalc Software bvba, Belgium. P value less than 0.05 was taken to be the cut off value of significance.
RESULTS AND ANALYSIS
Out of the 60 ASST positive patients considered for the study, eight were lost to follow-up at various stages, leaving 26 patients in each group who had completed the study. The groups were comparable with respect to age (two-tailed probability = 0.71).
Analysis of disease severity in the treatment groups at the beginning of the study
A P value of 0.60 indicated statistically comparable disease severity in the treatment groups at the beginning of the study.
Analysis of disease severity in the treatment groups at the subsequent follow-up
The P < 0.05 indicated a significant decrease in disease severity in group A at all the follow ups [Table 2].
Table 2.
Analysis of TSS values at 8, 12, 24, and 36 weeks of study (Friedman ANOVA test for TSS values during therapy)

Analysis of incidence of side effects
Two patients in group A (7%, P > 0.05) suffered from vomiting and loose stools during the initial 14 days of the study. Addition of antiemetic drugs resolved the issue. There were also no hematological or biochemical symptoms suggestive of hepatotoxicity.
Thus, administration of azathioprine in the first group resulted in a significant diminution of the intensity of the disease.
DISCUSSION
Azathioprine has been used as a corticosteroid-sparing agent with a favorable therapeutic index when compared to traditional immunosuppresants such as 6-mercaptopurine, methotrexate, 5-flourouracil and actinomycin-C. The drug has been in use for more than three decades for indications such as immunobullous disease, atopic dermatitis, and other severe skin conditions.[5,6] Azathioprine is quickly and almost completely absorbed from the digestive tract. Serum levels attain a peak approximately 2 h after administration, and the half-life is roughly 5 h when active metabolites are taken into consideration.[7] The mechanism of action is not fully understood. However, it has effects on DNA replication and the immune system. Azathioprine has been seen to impair gamma globulin synthesis and impair T-lymphocyte function and T-cell components such as interleukin-2.[8] These are the probable mechanisms of action of azathioprine in chronic autoimmune urticaria. Nausea, vomiting, diarrhea are the most common adverse effects. However, bone marrow suppression is a concern, and monthly complete blood cell counts are indicated.[9]
In the current study, we administered azathioprine at 50 mg/day to all patients above 12 years of age in the treatment group. In the control group, we had administered placebo pills supplied by the hospital pharmacy.
A follow up score of 0 was considered complete remission, a score of 1–6 marked improvement, 6–12 moderate improvement, and 13–18 poor/no improvement.
Statistical analysis revealed that the patients who had received azathioprine for the initial eight weeks had significantly better suppression of symptoms than did the placebo group; this was true throughout the follow-up period. Thus, azathioprine not only had immunomodulatory properties during the treatment period, but also had lasting therapeutic effect as well. There were some gastrointestinal side effects in the initial stages of study, but no incidence of hematological or biochemical disturbances.
CONCLUSION
Among the immunosuppressive agents that have so far been used for CU, azathioprine is a novel agent. There are no previous reports of case–control blinded studies regarding the usage of the drug for CU. Azathioprine was found to be effective in ASST positive CU in this study. It was more effective than placebo when given in conjunction with nonsedative antihistamines. The incidence of adverse effects was minimal. Thiopurine s-methyltransferase levels could not be estimated due to financial constraints. Other drawbacks of the study were a relatively small sample size, and low sensitivity and specificity of ASST (65–81% and 71–78% respectively)., It is imperative that azathioprine be put to more trials so as to confirm the results of the present study. It is hoped that it might eventually emerge as an effective drug in recalcitrant CU.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Grattan CH, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol. 2002;46:645–57. doi: 10.1067/mjd.2002.122759. [DOI] [PubMed] [Google Scholar]
- 2.Deacock SJ. An approach to the patient with urticaria. Clin Exp Immunol. 2008;153:151–61. doi: 10.1111/j.1365-2249.2008.03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greaves MW, Kaplan AP. Chronic urticaria: Autoimmune chronic urticaria and idiopathic chronic urticaria. In: Greaves MW, Kaplan AP, editors. Urticaria and angioedema. 1st ed. Heidelberg: Springer; 2004. pp. 321–41. [Google Scholar]
- 4.Bajaj AK, Saraswat A, Upadhyay A, Damisetty R, Dhar S. Autologous serum therapy in chronic urticaria: Old wine in a new bottle. Indian J Dermatol Venereol Leprol. 2008;74:109–13. doi: 10.4103/0378-6323.39691. [DOI] [PubMed] [Google Scholar]
- 5.Elion GB. The george hitchings and gertrude elion lecture. The pharmacology of azathioprine. Ann N Y Acad Sci. 1993;685:400–7. doi: 10.1111/j.1749-6632.1993.tb35897.x. [DOI] [PubMed] [Google Scholar]
- 6.Tan BB, Lear JT, Gawkrodger DJ, English JS. Azathioprine in dermatology: A survey of current practice in the U.K. Br J Dermatol. 1997;136:351–5. [PubMed] [Google Scholar]
- 7.Elion GB. Significance of azathioprine metabolites. Proc R Soc Med. 1972;65:257–60. doi: 10.1177/003591577206500315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournier C, Bach MA, Dardenne M, Bach JF. Selective action of azathioprine on T cells. Transplant Proc. 1973;5:523–6. [PubMed] [Google Scholar]
- 9.Patel AA, Swerlick RA, McCall CO. Azathioprine in dermatology: The past, the present, and the future. J Am Acad Dermatol. 2006;55:369–89. doi: 10.1016/j.jaad.2005.07.059. [DOI] [PubMed] [Google Scholar]


