INTRODUCTION
Lichen sclerosis (LS), also known as lichen sclerosus et atrophicus, is a chronic inflammatory dermatosis of anogenital area of unknown etiology. It is prevalent in females with bimodal onset in prepubertal and postmenopausal age group.[1] Extragenital lesions are rare and asymptomatic.[2] Diagnosis is mainly by clinical examination. However, in the early stages, diagnosis is difficult.[1,3] Dermoscopy is a simple, noninvasive skin diagnostic tool that unveils subtle surface as well as subsurface structures.[4] The use of dermoscopy in extragenital LS has not been extensively studied in the past. We observed a few characteristic dermoscopic patterns in three cases of extragenital LS that we believe aid in the clinical diagnosis of LS.
CASE REPORTS
Case 1
A 26-year-old male presented with multiple hypopigmented atrophic macules over the upper and lower limbs since one month [Figure 1]. Dermoscopy revealed whitish structureless areas (WSA), comedo like openings (CLO) [Figure 2] and dotted vessels arranged in a net-like pattern [Figure 3].
Figure 1.
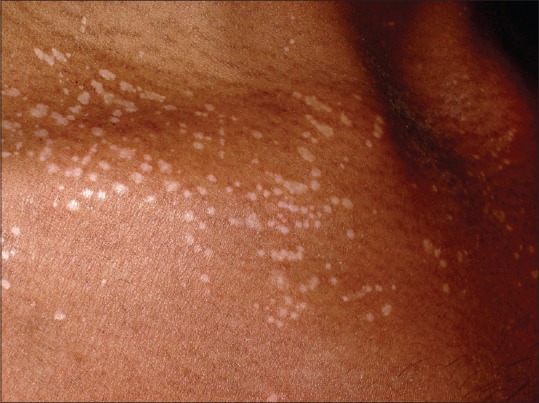
Multiple hypopigmented macules, papules distributed over chest with mild atrophy and kobnerization
Figure 2.
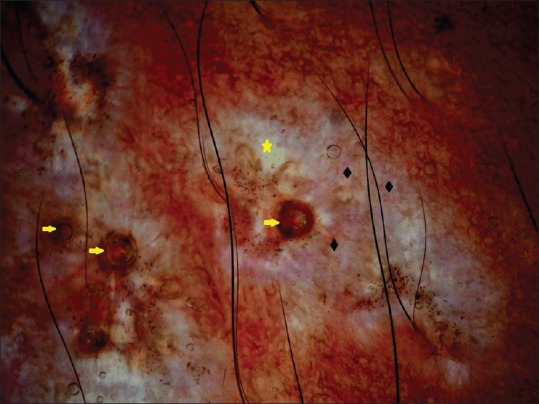
Dermoscopy showing white structureless areas (yellow star) and comedo like openings (yellow arrow) with telangiectasia of different lengths and calibers (black diamond). Brown dots indicate artifacts
Figure 3.
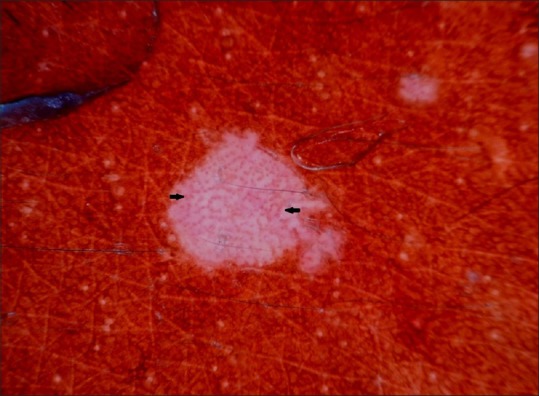
Dermoscopy showing dotted vessels arranged in a net like pattern (black arrow)
Case 2
A 9-year-old girl presented with asymptomatic papules over the face, back, upper and lower limbs since two months. Dermoscopy revealed WSA and telengiectasia [Figure 4].
Figure 4.
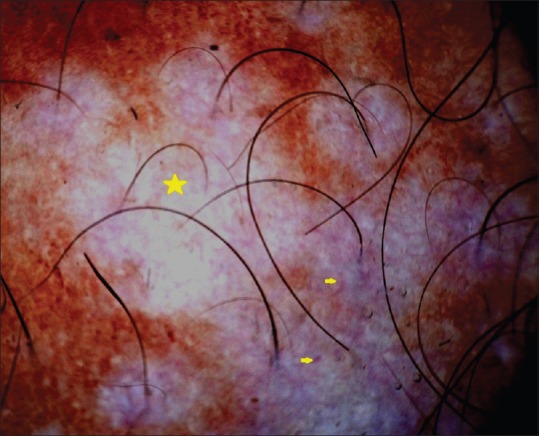
Dermoscopy showing white structureless areas (yellow star) and telangiectasia (yellow arrow)
Case 3
A 22-year-old male had atrophic, hypopigmented macules and papules over chest, trunk and lower limbs since 5 months. Dermoscopy revealed WSA, white chrysalis like structures (WCLA) and telangiectasia of different lengths and calibers [Figure 5 and 6].
Figure 5.
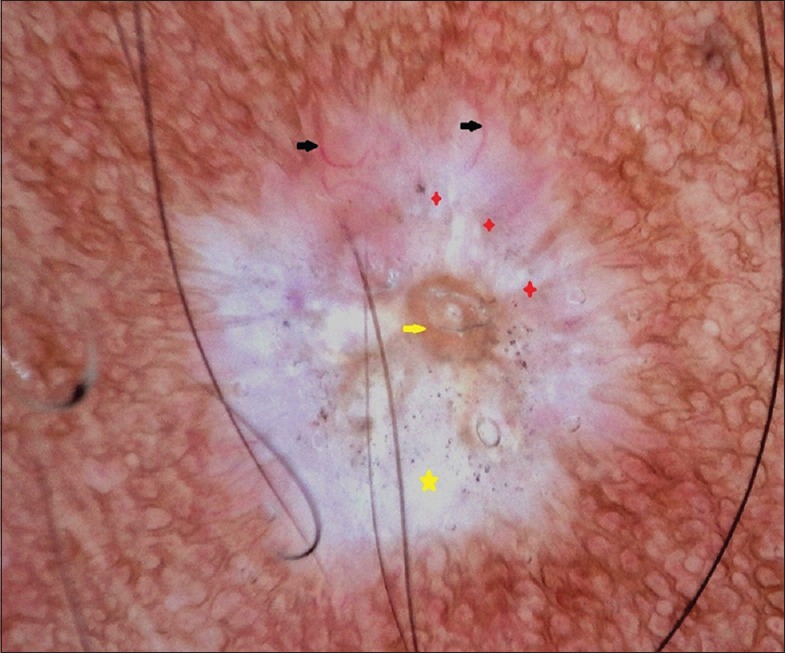
Polarized dermoscopy showing white structureless areas (yellow star) comedo like openings (yellow arrow), white chrysalis like structures (red star) and telangiectasia of different lengths and calibers (black arrow)
Figure 6.
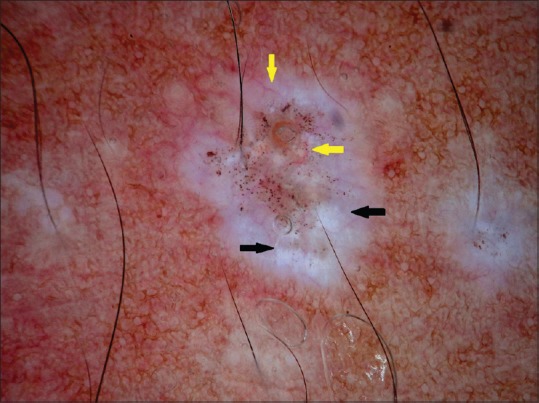
Polarized dermoscopy showing white chrysalis like structures (black arrows) and telangiectasia of different lengths and calibers (yellow arrows). Brown dots indicate artifacts
Histopathology of lesions showed atrophy of the epidermis, hyperkeratosis [Figure 7], and follicular plugging with basal cell degeneration. [Figure 8] The dermis showed edema and homogenization of collagen and interstitial and perivascular lymphocytic infiltration [Figure 9].
Figure 7.
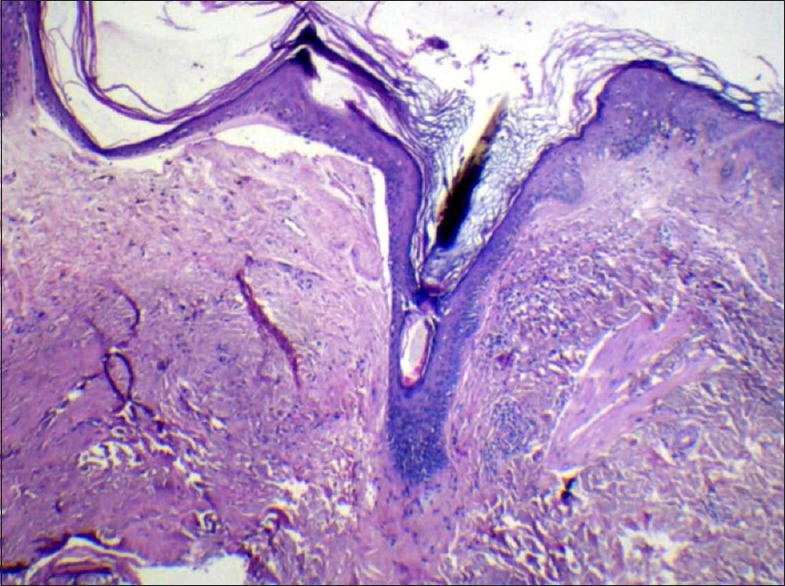
Histopathology showing hyperkeratosis and follicular plugging (H and E, ×10)
Figure 8.
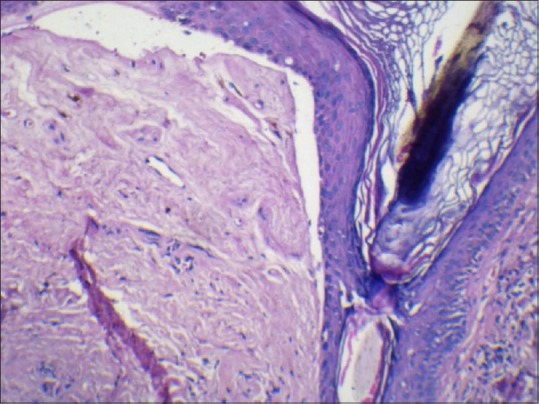
Histopathology showing follicular plugging and basal layer vacuolar degeneration (H and E, ×40)
Figure 9.
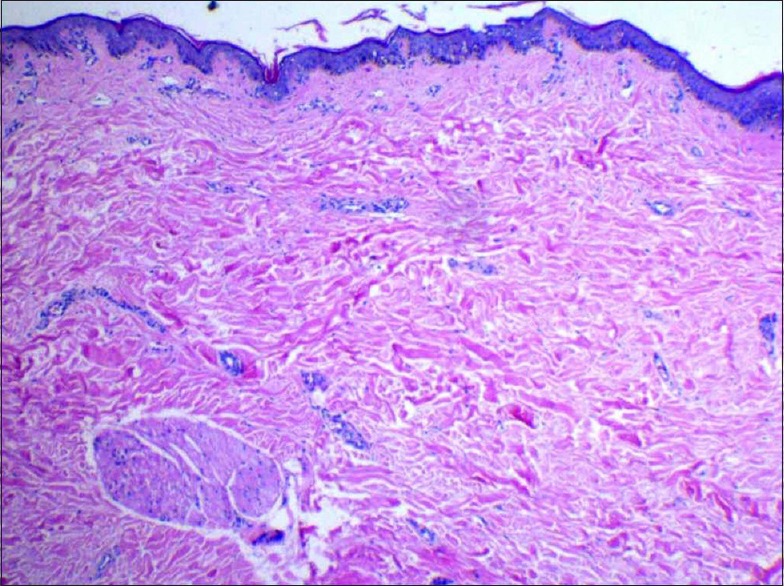
Histopathology showing homogenization of collagen and edema with mild perivascular, interstitial lymphocytic infiltration in dermis (H and E, ×10)
Hair, nail, oral, genital mucosa, systemic examination, and blood investigations were within normal limits in all three patients. A diagnosis of extragenital LS was made in all three cases based on clinical features and histopathology.
DISCUSSION
Early diagnosis and prompt treatment are mandatory as LS is a chronically relapsing disease with the potential for atrophy and scarring. Diagnosis of LS is mainly by clinical examination but in inconclusive cases histopathology can help confirm the diagnosis. Nevertheless, in the early stages of the disease, both clinical and histopathological features are uncharacteristic.[5] Edmonds et al., reported nonspecific histopathological changes in one-third of men with characteristic signs of LS.[6] Dermoscopy, which visualizes the color patterns in the epidermis, dermo-epidermal junction and papillary dermis can be utilized in this context.
Some dermoscopic patterns are observed consistently and characteristically in certain diseases and could be used for their diagnosis.[7] Dermoscopy is traditionally employed in pigmented lesions; however, its usefulness in inflammatory skin conditions is less-explored.[4]
Studies on the use of dermoscopy in LS are sparse. Shim et al., concluded that WSA and CLO were statistically significant in LS, whereas fibrotic bands were significant in morphea. Nevertheless, comma shaped vessels, hairpin like vessels and dotted vessels were exclusively seen in LS. They correlated dermoscopic patterns with histopathology: WSA representing epidermal atrophy and CLO representing follicular plugging in histopathology.[8] Similar dermoscopic patterns were observed in our patients.
Identical dermoscopic patterns were described in four women with extragenital LS with predominant CLO and WSA in the early lesions. Conversely, these were less prominent in late lesions.[9] This may be due to lessened hyperkeratosis and destruction of follicles in the late stages.
Telangiectasia of different lengths and calibers and WCLA were reported in a histopathologically proven case of LS coexisting with lichen planus and morphea in addition to above mentioned dermoscopic patterns.[10] WCLA are described as shiny, bright white, parallel or orthogonal or disordered linear streaks, seen only by polarized dermoscopy. They are usually seen in dermatofibroma, basal cell carcinoma, Spitz nevus and melanoma and are due to excessive collagen in dermis.[11] WCLA was observed in one patient with longer duration of lesions in this study.
CONCLUSION
In this study, WCLA suggested homogenization of collagen in the dermis and seen only in late lesions. CLO was predominant in early lesions, indicating follicular plugging. WSA indicates hyperkeratosis and epidermal atrophy; telangiectasia and dotted vessels represent atrophic epidermis with dilated blood vessels. The latter were observed in both early and late lesions. Thus, dermoscopic patterns correlate well with histopathological features and aid in confirmation of the diagnosis. Authors recommend studies with a large sample size for further evaluation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Goodfield MJ, Jones SK, Veale DJ. The connective tissue diseases. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. West Sussex: Wiley-Blackwell; 2010. pp. 51.112–8. [Google Scholar]
- 2.Khatu S, Vasani R. Isolated, localized extragenital bulbous lichen sclerosus et atrophicus: A rare entity. Am J Clin Dermatol. 2013;14:27–47. doi: 10.4103/0019-5154.117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683–4. doi: 10.1016/j.jaad.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Micali G. Introduction. In: Micali G, Lacarubbba F’s, editors. Dermatoscopy in clinical practice- Beyond pigmented lesions. 1st ed. London: Informa Healthcare; 2010. pp. 1–2. [Google Scholar]
- 5.Carlson JA, Lamb P, Malfetano J, Ambros RA, Mihm MC., Jr Clinicopathologic comparison of vulvar and extragenital lichen sclerosus: Histologic variants, evolving lesions, and etiology of 141 cases. Mod Pathol. 1998;11:844–54. [PubMed] [Google Scholar]
- 6.Edmonds EV, Oyama N, Chan I, Francis N, McGrath JA, Bunker CB. Extracellular matrix protein 1 autoantibodies in male genital lichen sclerosus. Br J Dermatol. 2011;165:218–9. doi: 10.1111/j.1365-2133.2011.10326.x. [DOI] [PubMed] [Google Scholar]
- 7.Nischal KC, Khopkar U. Dermoscope. Indian J Dermatol Venereol Leprol. 2005;71:300–3. doi: 10.4103/0378-6323.16633. [DOI] [PubMed] [Google Scholar]
- 8.Shim WH, Jwa SW, Song M, Kim HS, Ko HC, Kim MB, et al. Diagnostic usefulness of dermatoscopy in differentiating lichen sclerous et atrophicus from morphea. J Am Acad Dermatol. 2012;66:690–1. doi: 10.1016/j.jaad.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Garrido-Ríos AA, Alvarez-Garrido H, Sanz-Muñoz C, Aragoneses-Fraile H, Manchado-López P, Miranda-Romero A. Dermoscopy of extragenital lichen sclerosus. Arch Dermatol. 2009;145:1468. doi: 10.1001/archdermatol.2009.261. [DOI] [PubMed] [Google Scholar]
- 10.Tiodorovic-Zivkovic D, Argenziano G, Popovic D, Zalaudek I. Clinical and dermoscopic findings of a patient with co-existing lichen planus, lichen sclerosus and morphea. Eur J Dermatol. 2012;22:143–4. doi: 10.1684/ejd.2011.1585. [DOI] [PubMed] [Google Scholar]
- 11.Marghoob AA, Cowell L, Kopf AW, Scope A. Observation of chrysalis structures with polarized dermoscopy. Arch Dermatol. 2009;145:618. doi: 10.1001/archdermatol.2009.28. [DOI] [PubMed] [Google Scholar]


