Abstract
Objective:
The aim of this study was to evaluate the influence of oral contraceptive use on the silent period (SP) of anterior temporal and masseter muscles during the menstrual cycle.
Materials and Methods:
Totally, 28 women on reproductive age were selected including 15 nonusers of any hormone and 13 contraceptive users. All patients were dentate without muscular temporomandibular disorders. Electromyography (SP test) of the anterior temporal and masseter muscles was conducted every week during three consecutive menstrual cycles at 1st day of menstruation (P1), 7th day (P2), 14th day (P3) and 21st day (P4).
Results:
The SP values in the anterior temporal and masseter muscles were measured at both sides. The SP values of the right side (13.49 ms) at P2 were significantly different compared to the left side (12.28 ms). However, there was no significant difference on the interactions among the three factors.
Conclusion:
It can be concluded that the SP values in healthy women in reproductive age may not be influenced by the menstrual cycle with similar results for both muscles.
Keywords: Computerized dentistry, menstrual cycle, electromyography, silent period, sexual hormones
INTRODUCTION
Electromyography (EMG) allows evaluation of the masticatory system through record of muscular electrical current during resting, chewing and tooth clenching.[1] The silent period (SP) represents a reduced electromyographic activity that induces voluntary muscular activity through magnetic transcranial stimulation in the primary motor cortex analyzed by EMG.[2,3] In the elevator muscles of the mandible, the SP begins after functional dental contact or stimulus in the oral and periodontal region during muscular contraction.[4] Thus, the SP can be induced by the release of sudden stress in the muscle, percussion on the chin, voluntary and rhythmic dental contact, and electrical stimulus.[5,6]
The SP might be influenced by patient's age as well as deficient occlusion, tooth loss, and emotional behavior.[7] Some studies have suggested that the SP is an important factor for evaluation of neuropathies and motor deficiency.[8] The researchers demonstrated that the SP could be an auxiliary exam for diagnosis of functional disturbance as a correlation between period length and symptoms severity.[9] However, only one long SP should not be related to muscular pain.
Several etiological factors may be related to symptom of temporomandibular dysfunctions.[10,11] Some previous studies[12,13] also presented the influence of sexual hormones on the development of joint and muscular alterations. The effect of estrogen on the masticatory system depends on the amount of estrogen receptors present in muscles and joints.[12] In this sense, some studies showed a difference on pain modulation during the menstrual cycle[14] and in contraceptive users.[13] Although there is a study about the electrical performance of masticatory muscles during the menstrual cycle was found,[15] it did not mention SPs of the muscles.
Thus, the aim of this study was to evaluate the SP on masticatory cycle of two elevator muscles of the mandible during the menstrual cycle of dentate female patients using oral contraceptive (OC) or not. The research hypothesis assumed that the SP on masticatory cycles would be different regarding muscles, OCs users and nonusers, and menstrual period.
METHODS
Sample
Amongst 342 female students of the Araçatuba Dental School-São Paulo State University-Brazil, 30 women were selected after initial exam including anamnesis and clinical evaluation. Half of the sample was not taking any sexual hormone (G1) while the other half (G2) was using some type of hormone. Two women of G2 did not return to the appointments, totalizing 28 patients. The inclusion criteria assumed female patients in reproductive age between 18 and 32 years old and also users and nonusers of some OC (estrogen and/or progesterone hormones) to avoid pregnancy, as confirmed by the volunteer's physician. The G1 patients should present regular menstrual cycles (28 ± 3 days) without taking OCs for at least 3 months. The G2 patients were taking low-dose pills for at least 3 months in a cycle of 21 days for active medication followed by 7 days without active medication. The exclusion criteria were signs and symptoms of temporomandibular disorders (TMD), as confirmed by the Dworkin and Leresche Research Diagnostic Criteria for TMD;[16] presence of systemic pathology; pregnancy during the study; more than one tooth loss in each quadrant (excluding third molar); orthodontic treatment; presence of occlusal interference on the opposite side of mastication; symptoms on muscle palpation; and patients submitted to dental treatment including alteration of occlusion as well as prostheses and restorations.
The patients were informed about the experiment and signed an informed consent form in accordance with the recommendations of the Committee on Ethics in Human Research, which approved the study.
Menstrual cycles monitoring
All female students were observed every week during three consecutive menstrual cycles and the exams initiated on the 1st day of the cycle of each month. The menstrual cycle was divided into four distinct phases at 1st day of menstruation to 2 days after (P1), 7th day ± 2 days (P2), 14th day ± 2 days (P3) and 21st day ± 2 days (P4).[17] The patients were instructed to avoid any analgesic medication before exams. Women who were not on OC group predicted their ovulation using the ClearPlan ovulation prediction kit (ClearPlan Easy, Unipath Research, Princeton, NJ, US). The patients received enough kits for the first cycle.
Electromyography measurements
The EMG was measured every week according to the menstrual cycle phases using bipolar surface electrodes (Bio Research, Redmond, WA, US) with 10 mm in diameter. A 21 mm interelectrode distance was determined and the electrodes were connected to an amplifier by cables that were attached to a computer using the Bio Research “Bio EMG” software to capture the electrical signal and analyze the muscular electrical activity. The test was conducted at sampling rate of 1000 Hz during 15 s using 1X amplification. The electrodes were positioned based on palpation of the muscles at 1.5–2.0 cm superior to the zygomatic arch, immediately behind the frontal process of the zygomatic bone.[18] The evaluation was conducted bilaterally over dry skin parallel to the masseter and temporal muscle fibers. The center part of the masseter muscle was localized at the point equidistant to the upper and lower insertions of the muscle with teeth in occlusal contact. For the temporal muscle, the anterior border was located during mandibular movement to place the electrode perpendicular to the sagittal plane.
The SP values were obtained in open-close-clench-chewing cycles. This cycle is defined as a cycle when a subject firmly closes the mouth from the open mouth position with teeth in the habitual occlusal position during the chewing cycle. Masticatory performance was carried out using a raisin.[19] Each subject chewed the food for 35 cycles, which is very close to the moment of deglutition, and each cycle corresponded to the maximum bite chewing performed for each subject to grind the food. Eight subsequent cycles of SP were recorded for each subject among the 35 cycles.[20,21] SP measurements were obtained 3 times in each subject for each chewing cycle and the mean was calculated in milliseconds (ms). The SP length was measured using the double-zoom option with error about 1.2 ms. The measurement considered the peak of the last significant spike preceding the inhibition to the peak of the first significant spike being part of the ongoing muscle activity. In case of SP with two or three inhibitory pauses (double or triple SP) or depressed muscle activity after complete inhibition of motor neurons, the length of all inhibitory pauses was measured or added to the duration of a depressed activity after the last inhibitory pause.[4] During the SP records, the subjects were kept in a comfortable sitting position without headrest and with the Frankfort horizontal plane parallel to the floor.
Statistical analyses
All statistical analyses were conducted using the SPSS version 19.0 statistical software (SPSS Inc., Chicago, USA). The normality of SP values was assessed by Shapiro-Wilks’ W-test. A four-way repeated measures analysis of variance (ANOVA) was performed to verify any significant difference regarding muscle, side, group, and period. The means were compared by Tukey Honestly Significant Difference test (α =0.05) post hoc test.
RESULTS
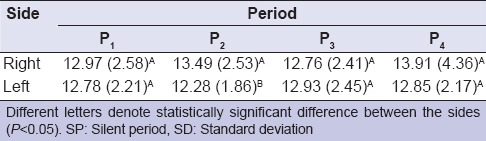
Table 1 shows the means and standard deviations of the SP values for each period of analysis. All groups exhibited similar SP value over time [Table 1]. Table 2 shows the results of four-way repeated measures ANOVA. Although side and period statistically affected the SP values (P = 0.03, ANOVA) [Table 2], there was no statistically significant difference on the interaction among the factors (muscle × side × group × period) (P > 0.05, ANOVA) [Table 2]. For the interaction between side and period, the SP values of the right side (13.49 ms) were different from the left side (12.28 ms) at P2, regardless group and muscle (P < 0.05) [Table 3].
Table 1.
Mean values (SDs) of SP (ms) of the sample
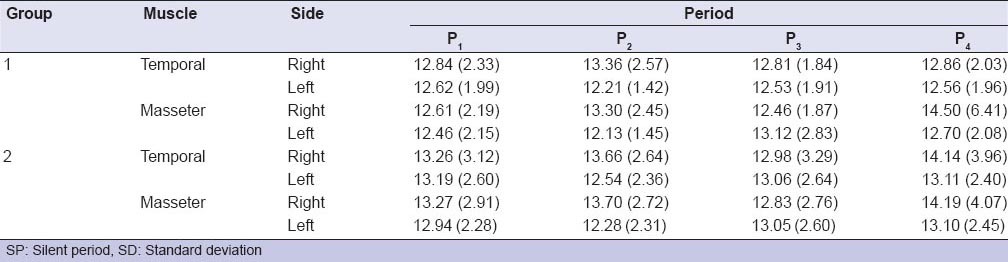
Table 2.
Four-way repeated measures ANOVA
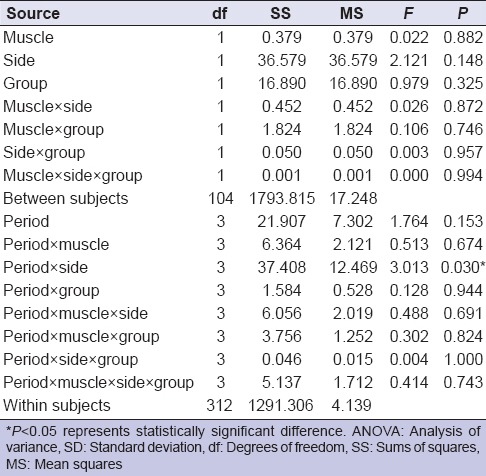
Table 3.
Mean values (SDs) of SP (ms) for each side and period, regardless group and muscle
DISCUSSION
Electromyography has been used to evaluate muscular alterations considering that SP represents the functional state of the neuromuscular system. The research hypothesis was partially accepted assuming the difference in SP between side and period of analysis. Celebic et al.[4] found mean SP values between 12.5 and 12.9 ms in healthy individuals during tooth clenching. McNamara et al.[22] showed mean SP from 9.21 to 11.5 ms in healthy patients during dental percussion and chewing while Watt et al.[23] reported higher SP during occlusion.
Chewing is a complex and rhythmic physiological process of mandible that requires interaction between receptors and nerves to control bone, joints and muscles of the stomatognathic system.[21] In the present study, the SP was evaluated through tooth contact during chewing considering the three steps of the chewing cycle (open-close-clench). It has been suggested that masseter presents the highest strength and electrical activity during food grinding.[24,25] On the other hand, the temporal muscle is effective for the most accurate mandibular movements, such as laterality.[22,23] However, the present study did not find a significant difference between those muscles since the structural composition and functional characteristics may change as an adaptation to functional requirements.[24] In this sense, some authors confirmed that strengthening and stretching exercises might cause alterations in muscular properties[20,26,27] because of higher tolerance to stretching or biomolecular structural alterations that may change with gender.[28]
It is important to emphasize that mastication may occur on both sides simultaneously or alternately, but it is important to consider that the majority of people chew more on one particular side that is, they have a preferred chewing side (PCS).[29,30,31] This preference may be influenced by occlusion, pain, hemispheric laterality and other factors.[32,33] Chewing is an exercise for the muscles of mastication, and the preference for one side may stimulate this side more intensively. Although in the present study, preferred side of chewing of the voluntaries has not been determined, we could speculate that the difference between sides at P2 could be probably due to a stimulus generated by periodontal or muscular receptors important for SP.[34,35] It should be necessary more studies to investigate this subject.
Furthermore, we can suppose that the hormonal alterations at P2 probably influenced the muscular activity during SP, the OCs did not affect the muscular function [Tables 2 and 3]. In addition, it is important to consider the direct influence of occlusion on the masticatory standard considering the alterations in muscular contraction during food grinding.[36]
For the OCs, previous studies[12,13,37] observed that hormonal alterations in women change muscular physiology. Although the level of sexual hormones reduces during the menstrual cycle, especially estrogen,[38] there was no significant difference between the group and the menstrual period [Table 2]. In addition, additional factors such as stress, structural alterations in muscles and joint, occlusion and parafunctional habits also influence the physiopathology of the stomatognathic system in female patients.
CONCLUSION
According to the results of the present study, it was concluded that the physiological, hormonal alterations during the menstrual cycle in OC users and nonusers do not affect the SP in healthy patients. There was no significant difference in the SP on masticatory cycle of muscles in contraceptive users and nonusers during the menstrual cycle of healthy patients.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Kemsley EK, Defernez M, Sprunt JC, Smith AC. Electromyographic responses to prescribed mastication. J Electromyogr Kinesiol. 2003;13:197–207. doi: 10.1016/s1050-6411(02)00065-2. [DOI] [PubMed] [Google Scholar]
- 2.Corsi FM, Fausti S, Serrao M, Casali C, Parisi L, Piazza G. Electromyographic mixed nerve and cutaneous silent period in evaluating the A-delta fibres in a patient with hereditary sensory-autonomic neuropathy. Funct Neurol. 2002;17:31–4. [PubMed] [Google Scholar]
- 3.Jaberzadeh S, Sakuma S, Zoghi M, Miles TS, Nordstrom MA. Focal transcranial magnetic stimulation of motor cortex evokes bilateral and symmetrical silent periods in human masseter muscles. Clin Neurophysiol. 2008;119:693–703. doi: 10.1016/j.clinph.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Celebic A, Valentic-Peruzovic M, Alajbeg IZ, Mehulic K, Knezovic-Zlataric D. Jaw elevator silent periods in complete denture wearers and dentate individuals. J Electromyogr Kinesiol. 2008;18:947–54. doi: 10.1016/j.jelekin.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Lund JP, Olsson KA. The importance of reflex and their control during jaw movement. Trends Neurosci. 1983;6:458–63. [Google Scholar]
- 6.Miyawaki S, Ohkochi N, Kawakami T, Sugimura M. Changes in masticatory muscle activity according to food size in experimental human mastication. J Oral Rehabil. 2001;28:778–84. doi: 10.1046/j.1365-2842.2001.00702.x. [DOI] [PubMed] [Google Scholar]
- 7.Goiato MC, Haddad MF, dos Santos DM, Garcia AR, Zuim PR, Zavanelli AC. Silent period-dentate, edentulous, and patients with craniomandibular dysfunction. J Craniofac Surg. 2010;21:1641–5. doi: 10.1097/SCS.0b013e3181ec69ee. [DOI] [PubMed] [Google Scholar]
- 8.McCall WD, Uthman AA, Mohl ND. TMJ symptom severity and EMG silent periods. J Dent Res. 1978;57:709–14. doi: 10.1177/00220345780570051001. [DOI] [PubMed] [Google Scholar]
- 9.Bäumer T, Hidding U, Hamel W, Buhmann C, Moll CK, Gerloff C, et al. Effects of DBS, premotor rTMS, and levodopa on motor function and silent period in advanced Parkinson's disease. Mov Disord. 2009;24:672–6. doi: 10.1002/mds.22417. [DOI] [PubMed] [Google Scholar]
- 10.Sener S, Akgunlu F. Correlation between the condyle position and intra-extraarticular clinical findings of temporomandibular dysfunction. Eur J Dent. 2011;5:354–60. [PMC free article] [PubMed] [Google Scholar]
- 11.Drobek W, Schoenaers J, De Laat A. Hormone-dependent fluctuations of pressure pain threshold and tactile threshold of the temporalis and masseter muscle. J Oral Rehabil. 2002;29:1042–51. doi: 10.1046/j.1365-2842.2002.00988.x. [DOI] [PubMed] [Google Scholar]
- 12.Alkan A, Bulut E, Arici S, Sato S. Evaluation of treatments in patients with nocturnal bruxism on bite force and occlusal contact area: A preliminary report. Eur J Dent. 2008;2:276–82. [PMC free article] [PubMed] [Google Scholar]
- 13.Vignolo V, Vedolin GM, de Araujo Cdos R, Rodrigues Conti PC. Influence of the menstrual cycle on the pressure pain threshold of masticatory muscles in patients with masticatory myofascial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:308–15. doi: 10.1016/j.tripleo.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 14.Rezaii T, Hirschberg AL, Carlström K, Ernberg M. The influence of menstrual phases on pain modulation in healthy women. J Pain. 2012;13:646–55. doi: 10.1016/j.jpain.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Turcio KH, Garcia AR, Zuim PR, Sundefeld ML, Goiato MC, dos Santos DM. Electrical activities and pressure pain threshold in oral contraceptives users and nonusers. Pain Stud Treat. 2014;2:99–105. [Google Scholar]
- 16.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- 17.Cimino R, Farella M, Michelotti A, Pugliese R, Martina R. Does the ovarian cycle influence the pressure-pain threshold of the masticatory muscles in symptom-free women? J Orofac Pain. 2000;14:105–11. [PubMed] [Google Scholar]
- 18.Goiato MC, Garcia AR, dos Santos DM. Electromyographic activity of the mandible muscles at the beginning and end of masticatory cycles in patients with complete dentures. Gerontology. 2008;54:138–43. doi: 10.1159/000118099. [DOI] [PubMed] [Google Scholar]
- 19.Slagter AP, van der Glas HW, Bosman F, Olthoff LW. Force-deformation properties of artificial and natural foods for testing chewing efficiency. J Prosthet Dent. 1992;68:790–9. doi: 10.1016/0022-3913(92)90204-n. [DOI] [PubMed] [Google Scholar]
- 20.Mottram CJ, Hunter SK, Rochette L, Anderson MK, Enoka RM. Time to task failure varies with the gain of the feedback signal for women, but not for men. Exp Brain Res. 2006;174:575–87. doi: 10.1007/s00221-006-0498-3. [DOI] [PubMed] [Google Scholar]
- 21.Woda A, Mishellany A, Peyron MA. The regulation of masticatory function and food bolus formation. J Oral Rehabil. 2006;33:840–9. doi: 10.1111/j.1365-2842.2006.01626.x. [DOI] [PubMed] [Google Scholar]
- 22.McNamara DC, Crane PF, McCall WD, Jr, Ash MM., Jr Duration of the electromyographic silent period following the jaw-jerk reflex in human subjects. J Dent Res. 1977;56:660–4. doi: 10.1177/00220345770560061501. [DOI] [PubMed] [Google Scholar]
- 23.Watt DM, Turnbull JR, Saberi M, Beyli MS, Aguilar E. The influence of percussion, occlusion and mastication on the occurrence of silent periods in masseter muscle activity. J Oral Rehabil. 1976;3:371–85. doi: 10.1111/j.1365-2842.1976.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 24.Hugger S, Schindler HJ, Kordass B, Hugger A. Clinical relevance of surface EMG of the masticatory muscles. (Part 1): Resting activity, maximal and submaximal voluntary contraction, symmetry of EMG activity. Int J Comput Dent. 2012;15:297–314. [PubMed] [Google Scholar]
- 25.Hugger S, Schindler HJ, Kordass B, Hugger A. Surface EMG of the masticatory muscles (Part 3): Impact of changes to the dynamic occlusion. Int J Comput Dent. 2013;16:119–23. [PubMed] [Google Scholar]
- 26.Oliveira AS, Gonçalves M, Cardozo AC, Barbosa FS. Electromyographic fatigue threshold of the biceps brachii muscle during dynamic contraction. Electromyogr Clin Neurophysiol. 2005;45:167–75. [PubMed] [Google Scholar]
- 27.Yoon T, Schlinder Delap B, Griffith EE, Hunter SK. Mechanisms of fatigue differ after low- and high-force fatiguing contractions in men and women. Muscle Nerve. 2007;36:515–24. doi: 10.1002/mus.20844. [DOI] [PubMed] [Google Scholar]
- 28.Clark BC, Manini TM, Thé DJ, Doldo NA, Ploutz-Snyder LL. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J Appl Physiol(1985) 2003;94:2263–72. doi: 10.1152/japplphysiol.00926.2002. [DOI] [PubMed] [Google Scholar]
- 29.Christensen LV, Radue JT. Lateral preference in mastication: A feasibility study. J Oral Rehabil. 1985;12:421–7. doi: 10.1111/j.1365-2842.1985.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 30.Paphangkorakit J, Thothongkam N, Supanont N. Chewing-side determination of three food textures. J Oral Rehabil. 2006;33:2–7. doi: 10.1111/j.1365-2842.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Gomis J, Lujan-Climent M, Palau S, Bizar J, Salsench J, Peraire M. Relationship between chewing side preference and handedness and lateral asymmetry of peripheral factors. Arch Oral Biol. 2009;54:101–7. doi: 10.1016/j.archoralbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Nissan J, Gross MD, Shifman A, Tzadok L, Assif D. Chewing side preference as a type of hemispheric laterality. J Oral Rehabil. 2004;31:412–6. doi: 10.1111/j.1365-2842.2004.01256.x. [DOI] [PubMed] [Google Scholar]
- 33.Diernberger S, Bernhardt O, Schwahn C, Kordass B. Self-reported chewing side preference and its associations with occlusal, temporomandibular and prosthodontic factors: Results from the population-based Study of Health in Pomerania (SHIP-0) J Oral Rehabil. 2008;35:613–20. doi: 10.1111/j.1365-2842.2007.01790.x. [DOI] [PubMed] [Google Scholar]
- 34.Türker KS, Sowman PF, Tuncer M, Tucker KJ, Brinkworth RS. The role of periodontal mechanoreceptors in mastication. Arch Oral Biol. 2007;52:361–4. doi: 10.1016/j.archoralbio.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Sowman PF, Flavel SC, McShane CL, Miles TS, Nordstrom MA. Transcranial magnetic stimulation reduces masseter motoneuron pool excitability throughout the cortical silent period. Clin Neurophysiol. 2008;119:1119–29. doi: 10.1016/j.clinph.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 36.van der Bilt A, Engelen L, Pereira LJ, van der Glas HW, Abbink JH. Oral physiology and mastication. Physiol Behav. 2006;89:22–7. doi: 10.1016/j.physbeh.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: A discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–94. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 38.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–61. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]


