Abstract
Objective:
This study was conducted to evaluate the microshear bond strength (μSBS) and ultramorphological characterization of glass ionomer (GI) cements; conventional GI cement (Fuji IX, CGI), resin modified GI (Fuji II LC, RMGI) and nano-ionomer (Ketac N100, NI) to enamel, dentin and cementum substrates.
Materials and Methods:
Forty-five lower molars were sectioned above the cemento-enamel junction. The occlusal surfaces were ground flat to obtain enamel and dentin substrates, meanwhile the cervical one-third of the root portion were utilized to evaluate the bonding efficacy to cementum substrate. Each substrate received microcylinders from the three tested materials; which were applied according to manufacturer instructions. μSBS was assessed using a universal testing machine. The data were analyzed using two-way analysis of variance (ANOVA) and Tukey's post-hoc test. Modes of failure were examined using stereomicroscope at ×25 magnification. Interfacial analysis of the bonded specimens was carried out using environmental field emission scanning electron microscope.
Results:
Two-way ANOVA revealed that materials, substrates and their interaction had a statistically significant effect on the mean μSBS values at P values; ˂0.0001, 0.0108 and 0.0037 respectively. RMGI showed statistically significant the highest μSBS values to all examined tooth substrates. CGI and RMGI show substrate independent bonding efficiency, meanwhile; NI showed higher μSBS values to dentin and cementum compared to enamel.
Conclusion:
Despite technological development of GI materials, mainly the nano-particles use, better results have not been achieved for both investigations, when compared to RMGI, independent of tooth substrate.
Keywords: Cementum, conventional glass ionomer, dentin, enamel, microshear bond strength, nano-ionomer, resin-modified glass ionomer
INTRODUCTION
Since their introduction in 1972, glass ionomer (GI) cements have been widely used as dental restorative materials, in particular in restoration of cervical lesions.[1] Despite the advantages associated with the use of conventional GI (CGI) cements as; their chemical bonding efficacy to tooth substrate, long-term fluoride ion release, low coefficient of thermal expansion and relatively ease of use, yet they suffer from several problems as; moisture sensitivity, low mechanical strength and impaired translucency.[2]
In an attempt to overcome these problems, several innovations were done. In 1988 resin modified GI (RMGI) cements were introduced by adding polymerizable hydrophilic resin to CGI formulations. This material gained wider acceptance in the dental professions as adding light polymerizable (hydroxyethyl methacrylate [HEMA]) resin to the GI enhanced its handling characteristics, increased its working time and improved its esthetic properties.[3] However, RMGI cements exhibit shrinkage and substantial water sorption owing to the hydrophilic nature of its contents.[4] Recently a nanofilled RMGI restorative material (nano-ionomer [NI]) was launched into the market. Apart from user-friendliness, the major innovations of these materials involve incorporation of nanotechnology, which allowed a highly packed filler composition (approximately 69%) in the form of nano-sized fillers and nanoclusters.[5]
GI cements are the material of choice when dealing with root caries or noncarious cervical lesions.[6] In such areas, the operator is apt to deal with cementum substrate. The chemical composition of cementum is different from enamel and dentin. Cementum in the cervical area is an acellular extrinsic fibre cementum. This substratum is a nonuniform human cementum, which can be represented as a woven fabric-like material that provides tissue porosity and permeability. One of the surface features that distinguish cementum from dentin is the lack of patent tubule orifices, which may alter its bonding capability.[7,8]
Searching the literature, It was evident that almost all research work was concerned with bonding efficacy to enamel and dentin, however, the bonding efficacy to the cementum was scarcely addressed. Thus, this study was carried out to determine the bonding efficacy of the nanofilled RMGI (NI) restorative material to enamel, dentin, and cementum and compare it to conventional RMGI restorative material and packable conventional CGI cements. The null hypotheses examined that (1) there is no difference in the bonding performance between the three tested GI cements; CGI, RMGI, and NI when applied to enamel, dentin and cementum. (2) Each tested GI cement bond equally to enamel, dentin and cementum substrates.
MATERIALS AND METHODS
Experimental design
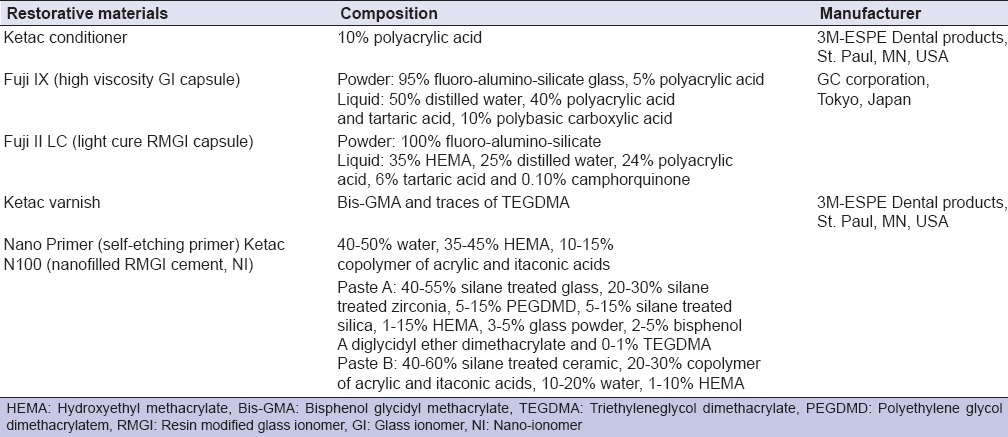
Forty-five extracted, noncarious lower first molars were collected, cleaned from debris and stored in distilled water with 0.5% thymol. Three GI - based restorative materials were tested: Conventional high viscosity GI cement (Fuji IX, GC Corporation, Tokyo, Japan), RMGI cement (Fuji II LC, GC Corporation, Tokyo, Japan) and NI cement (Ketac N 100, 3M ESPE, NI, St Paul, MN, USA). Material's composition and manufacturer are presented in Table 1. For each material, 10 molars were used to test their bond strength to different tooth substrates; enamel, dentin, and cementum while 5 teeth were utilized for the ultrastructural examination of the interface.
Table 1.
Material's composition and manufacturer
Microshear bond strength testing
Specimen preparation
Each molar was sectioned 1 mm coronal to the cervical line to separate the crown from the roots using a diamond disk (K6974, Komet, Germany) in a low-speed handpiece under copious water cooling. The roots were separated, and each root was embedded in self-cured resin (Rapid Repair, DeguDent GmbH, Hanau, Germany). The flat cementum regions from the mesial and distal surfaces of the mesial and distal roots respectively, at cervical root third, were used as a bonding substrate, after grinding with wet 600 grit silicon carbide paper. The presence of cementum was previously verified under a stereomicroscope, (Carl Zeiss, Germany) at ×25 magnification. The coronal portion of each tooth was embedded vertically in self-cured resin that the occlusal surface was used for testing. The occlusal enamel was then removed, and 1 mm of dentin was ground, thus exposing superficial occlusal dentin surrounded by an enamel rim. The thickness of the enamel should be at least 1.5 mm wide to be utilized for microshear bond strength (μSBS) testing.[9] A uniform smear layer was created by abrading the occlusal surface (enamel and dentin) with 600 grit silicon carbide paper.[10]
Application of the restorative materials
All materials were applied following the manufacturer's instructions. For CGI and RMGI, Ketac dentin conditioner was applied to enamel, dentin or cementum substrates for 20 s using a microbrush (Shofu, Japan). It was then rinsed thoroughly with air-water spray and the excess water was blotted with cotton pellet. For NI group, Ketac N100 Primer was applied for 15 s. The primer was then air-thinned with an air syringe for 10 s and light cured with a light curing unit (DB 686-1, COXO, China) at an intensity of 1200 mW/cm2 for 20 s.
After mixing of CGI and RMGI capsules using an amalgamator (Silamat Plus, Vivadent, Austria) and manual mixing of equal volumes of NI, the materials were injected into polyethylene tubes of 0.9 mm in diameter and 0.7 mm in height. For RMGI and NI group, the restoration was light-cured for 20 s. Light intensity was periodically checked using a radiometer (Kerr; Orange, CA, USA). Then, Ketac glaze was painted on the top of CGI and RMGI cylinders only and light-cured for 10 s. All polyethylene tubes were then removed, and the bonded specimens were stored in distilled water for 24 h. In coronal specimens, enamel and dentin substrates received four microcylinders for each. In each root specimen, each surface received two microcylinders (Each molar received four microcylinders for enamel, dentin, and cementum).
Testing of specimens
Microshear bond strength testing was done using a universal testing machine (Model LRX-plus, Lloyd Instruments; Fareham, UK). Each specimen with its bonded microcylinders was secured with tightening screws to the lower fixed compartment of the universal testing machine. A loop of orthodontic stainless steel wire (0.014 inch in diameter) was wrapped around the bonded microcylinder as close to its base as possible and aligned with the loading axis of the upper movable compartment of the testing machine. The specimens were stressed in shear using a load cell of 5 KN at a crosshead speed of 0.5 mm/min. The shear force at failure was recorded and converted to shear stress in MPa units using computer software (Nexygen-MT Lloyd Instruments, Fareham, UK). For each molar, the four readings obtained from enamel, dentin and cementum substrates were averaged to obtain one reading for each substrate from each molar (n = 10).
Data were analyzed using two-way analysis of variance (ANOVA) to determine the effect of restorative material, tooth substrate and their interaction on mean μSBS values. Comparing between the three materials for each tooth substrate and between the three substrates for each restorative material was carried out using One way ANOVA followed by Tukey's post-hoc test. The significance level was set at P ≤ 0.05. Statistical analysis was performed with SPSS 16.0, Inc., (SPSS, Inc., Chicago, IL, USA) for Windows.
Fractographic analysis
Following microshear testing, specimens were examined using a stereomicroscope (Carl Zeiss, Germany) at ×25 magnification to evaluate the fracture mode. The mode of failure was identified as: Type I (adhesive failure at the interface), Type II (mixed adhesive failure at the interface + cohesive in the restorative material), Type III (cohesive in the restorative material).
Interfacial analysis of the bonded interface
Fifteen molar were utilized for ultrastructural analysis of the interface (five for each restorative material). The specimens were prepared in a similar manner for μSBS. The restorative materials (Fuji IX, Fuji II LC and Ketac N100) were applied to the different substrates as described before but in one layer of 2 mm in thickness. A transparent mylar strip (Moyco, USA) was placed on top of the restorative material until setting.
Teeth were then sectioned bucco-lingually using a diamond disc at a slow speed under copious water cooling. The sectioned specimens were finished with Soflex discs (3M-ESPE Dental products, St. Paul, MN, USA) of descending grits. The specimens were then placed into an ultrasonic cleaner (Elma-Transsonic 460 Hz, Germany) in distilled water for 30 min to remove the smear layer. Specimens were examined under low vacuum without gold sputtering using environmental field emission scanning electron microscopy (Quanta Field Emission Gun 250, Holland 115/230 V, Inspect SFEI, Phillips, Holland), and representative enviromental scanning photomicrographs of enamel-, dentin- and cementum-restoration interface were obtained at ×500 magnification.
RESULTS
Microshear bond strength
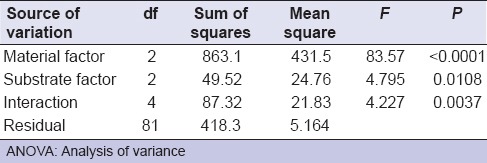
Two-way ANOVA revealed that restorative materials, substrates examined, and their interaction had a statistically significant effect on mean μSBS values at P values; <0.0001, 0.0108 and 0.0037 respectively [Table 2].
Table 2.
Two-way ANOVA
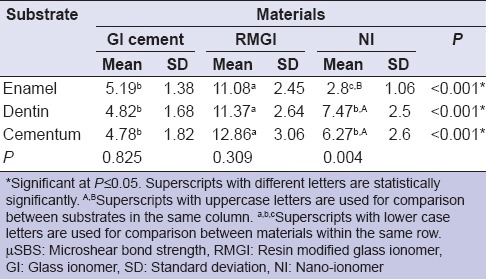
Mean and standard deviation of the tested groups are presented in Table 3. It was evident that RMGI showed the highest statistical significant μSBS to all tested substrates compared to CGI and NI. CGI and RMGI showed equal bonding performance to enamel, dentin, and cementum. Meanwhile, NI showed statistically significant lower μSBS values to enamel compared to dentin and cementum.
Table 3.
Means±SD of μSBS values in MPa of all tested groups
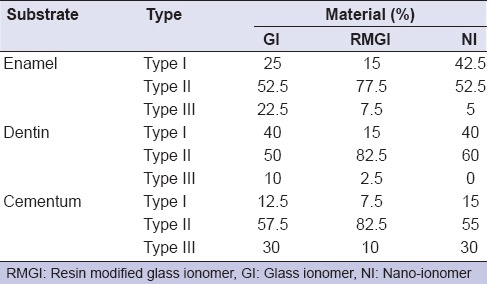
Fracture mode analysis results are presented in percentages in Table 4. It was evident that the highest percent of cohesive failure (Type III) was present in the CGI group.
Table 4.
The percentages of fracture modes of all tested groups
Enviromental field emission scanning electron microscope
All tested groups showed a gap-free junction with the existence of a filler-free zone of variable thickness, except at the NI-enamel interface at which a gap was evident [Figure 1]. The greatest thickness of the filler-free zone was observed for the NI-enamel interface. Several cracks were seen propagating within the CGI cement.
Figure 1.
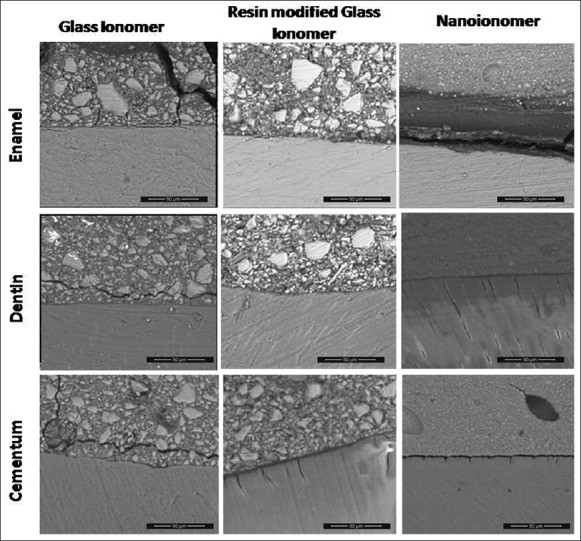
Environmental field emission scanning electron microscope photomicrograph representing tooth-restoration interface of all tested groups at ×500 magnification. It revealed the existence of a filler free zone at the interface of all tested groups. A gap is present at the nano-ionomer-enamel interface
DISCUSSION
This study was conducted to elaborate the bonding efficacy of GI cements to different tooth substrates (enamel, dentin, and cementum) via the use of μSBS test and environmental field emission scanning electron microscope (EFESEM). Three GI cements were evaluated; high viscosity chemical-cure CGI cement, RMGI, and NI. NI, Ketac N 100, is light curing RMGI modified using bonded nanofillers, and nanocluster fillers, along with fluoroaluminosilicate glass.[11] A RMGI (Fuji II LC) was chosen to elucidate the value of the latter modification. A CGI cement (Fuji IX) was compared as control.
In this study enamel, dentin and cementum specimens were obtained from the same tooth for more standardization of the chemical composition and the degree of mineralization when comparing the three substrates with each other. In the present study, μSBS test was used to evaluate the bonding efficacy. This test is considered a relatively simple test that permits efficient screening of adhesive systems, regional and depth profiling of a variety of substrates, with conservation of teeth.[12] Furthermore, it could be a viable test when evaluating brittle materials, having low modulus of elasticity as GI cements as it lowers the probability of having a crack opening relative to the load applied.[13] Fractographic analysis of the debonded specimens gives clues about some of the reasons of failure, the usage and limitations of each material.[14] In addition, evaluation of the interface was carried out using EFESEM. EFESEM photomicrographs may give clues about the bond quality at the interface, which can be a complementary tool for bond strength evaluation.[15] It is a more suitable tool for evaluation of GI cements restoratives which are sensitive to the vacuuming, processing and gold sputtering step which are mandatory in the conventional scanning electron microscope.[15,16]
Results of the present study revealed that the RMGI showed the highest statistically significant μSBS values compared to CGI and NI irrespective to substrate examined, thus the first null hypothesis is rejected. This result is in agreement with several authors whom found that the bond strength of RMGI was significantly higher than CGI cements to enamel.[17,18] and to dentin.[8,14,19] In addition, in agreement with Coutinho et al.,[20] whom found that RMGI showed significantly higher bond strength values compared to NI to both enamel and dentin substrates. However, the bonding of GI restoratives to cementum was not previously addressed in literature.
The better bonding performance of the RMGI compared to CGI could be attributed to several theories acting simultaneously; first; to the difference in the rates of adsorption on the calcified tissue surface and crosslinking in the bulk regions of the material.[21] Light-curing of RMGI causes an initial increase in the ionization rate due to the production of acid (via the photoinitiator), and this results in a very strong adsorbed layer. At the same time, internal cross-linking reactions result from free-radical polymerization processes. Thus, the unsaturated methacrylate groups will polymerize and co-polymerize with the modified polyacrylic acid, forming a polyacrylic acid network entangled within the collagen web.[22] Second; to the resinous component in RMGI cements that can form a covalently bonded matrix, allowing the material to have greater fracture strengths than CGI cements.[17] Fractographic analysis showed that the highest percentage of cohesive failure was present in CGI. Furthermore, FESEM photos revealed existence of multiple cracks within the CGI matrix. Third, this might be attributed to the carboxylic acid copolymers contained in the cement, which provide a more reactive and acidic liquid with increased number of carboxyl groups per chain unit thus increasing its chemical bonding potential.[8] Forth; to the high HEMA concentration in RMGI cement, which improves its wetting ability and results in better bonding.[19] The HEMA concentration in the RMGI cement used is 35%; meanwhile, it is completely absent in CGI cement.
Nano-ionomer primer contains the same HEMA concentration as RMGI, but it showed lower bonding performance with all tooth substrates, despite the existence of a thick filler-free zone with gap-free junction with dentin and cementum substrates. This observation is in line with Coutinho et al.,[20] whom attributed this to the difference in the technique of application and the conditioning step. In RMGI cement polyacrylic acid conditioning is applied and rinsed prior to the material application which allows for partial demineralization and removal of the smear layer, giving the opportunity for the HEMA component to enhance the wetting of the surface and the production of microporosity in the different tooth substrates. This could contribute to either increased surface area for chemical bonding with residual hydroxyl apatite or micromechanical bonding through micromechanical interlocking. Meanwhile, with NI, the polyacrylic acid is incorporated in the primer which is applied onto the smear layer and is not rinsed away. In addition, NI primer has a relatively higher pH (3) than polyacrylic acid pH (1.5). This difference could be reflected in the ability of polyacrylic acid to remove the smear layer resulting in distinct performance.[11,19]
Coutinho et al.,[20] reported that the the filler-free zone at the NI interface most likely represented remnants of the primer that did not polymerize well due to the presence of oxygen, and therefore only showed a nonhomogeneous layer which might have not contributed to the bonding effectiveness. Although the existence of filler free zone is important to act as a stress breaking layer, the increase in its thickness above a certain level appears to be a negating factor similar to the scenario of the undesired increase in the hybrid/adhesive layer thickness.[23]
However, this FESEM observation is in contrary to El-Askary and Nassif,[5] whom reported the existence of a gap between the NI and the dentin substrate, when the material was applied according to the manufacturer instructions. This contradiction could be attributed to the differences in the technique adopted for specimen preparations for FESEM examination.
The results also revealed that there was no statistically significant difference between enamel, dentin and cementum substrates with both CGI and RMGI cements. Meanwhile, NI showed significantly lower bond strength values to enamel compared to dentin and cementum. Thus, the second null hypothesis is partially accepted. This result is in partial agreement with Fritz et al.,[24] and Coutinho et al.,[20] whom found no statistically significant difference between enamel and dentin substrates. On the other hand, μSBS values revealed that NI showed significantly lower bond strength values to enamel compared to dentin and cementum. This result is in line with FESEM photomicrograph, which revealed the existence a gap at NI-enamel interface. This result is in agreement with Uysal et al.,[25] who found a significant decrease in bond strength NI was bonded to the enamel. Compared to the low organic content in the enamel (1% by weight),[26] the organic content in dentin comprises 20% by weight while cementum comprises 33% by weight.[7] This might explain the low bonding capacity of NI to enamel. As the high pH of Ketac primer, which acts as a mild self-etch adhesive, make it sensitive to degree of tissue mineralization.[22] These results are in contrary to Korkmaz et al.,[27] whom found that there was no significant difference observed between enamel and dentin at the NI group.
In the present investigation, no direct relationship between the shear bond strength and the mode of failure observed. This substantiates other results in which high bond strength values were not necessarily related to a cohesive type of fracture. This is in agreement with Sidhu et al.,[14] air voids, cracks, defects or geometric features act as stress raisers when an interface is loaded, which lead to rupture of the “joint” at stresses very much lower than that which should theoretically occur. This was confirmed by the FESEM micrograph which showed many cracks in the CGI extending to the interface which was also observed with several authors.[14,28,29,30] The presence of cracks in GI cement might reflect the high percentage of Type III failure present in this group. This would suggest that the bonding configuration surpassed the inherent strength of the GI strength, denoting that the weak point in this interface is the mechanical properties of the material.
Further studies should be carried out to test the effect of the complex oral environmental conditions on the mechanical and chemical adhesion of different GI cement categories to the different tooth substrates.
CONCLUSION
Under the conditions of the present study, the following conclusions could be derived:
CGI and RMGI cement showed equal performance to the different tooth substrates, meanwhile NI showed lower bonding performance to enamel compared to dentin and cementum
RMGI cement showed prompt bonding to enamel, dentin and cementum compared to CGI and NI.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Inoue S, Van Meerbeek B, Abe Y, Yoshida Y, Lambrechts P, Vanherle G, et al. Effect of remaining dentin thickness and the use of conditioner on micro-tensile bond strength of a glass-ionomer adhesive. Dent Mater. 2001;17:445–55. doi: 10.1016/s0109-5641(01)00003-3. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari M, Davidson CL. Interdiffusion of a traditional glass ionomer cement into conditioned dentin. Am J Dent. 1997;10:295–7. [PubMed] [Google Scholar]
- 3.Sidhu SK, Watson TF. Resin-modified glass-ionomer materials. Part 1: Properties. Dent Update. 1995;22:429–32. [PubMed] [Google Scholar]
- 4.Khoroushi M, Karvandi TM, Sadeghi R. Effect of prewarming and/or delayed light activation on resin-modified glass ionomer bond strength to tooth structures. Oper Dent. 2012;37:54–62. doi: 10.2341/11-137-L. [DOI] [PubMed] [Google Scholar]
- 5.El-Askary FS, Nassif MS. The effect of the pre-conditioning step on the shear bond strength of nano-filled resin-modified glass-ionomer to dentin. Eur J Dent. 2011;5:150–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson CL. Advances in glass-ionomer cements. J Appl Oral Sci. 2006;14(Suppl):3–9. doi: 10.1590/s1678-77572006000700002. [DOI] [PubMed] [Google Scholar]
- 7.Sterrett JD, Gerard DA, Johnston K, Torres MA. “Burnishing” demineralization of root surfaces: Ultrastructural surface characteristics. J Adhes Dent. 2006;8:161–7. [PubMed] [Google Scholar]
- 8.Tanumiharja M, Burrow MF, Tyas MJ. Microtensile bond strengths of glass ionomer (polyalkenoate) cements to dentine using four conditioners. J Dent. 2000;28:361–6. doi: 10.1016/s0300-5712(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 9.Yousry MM. Effect of re-etching oxalate-occluded dentin and enamel on bonding effectiveness of etch-and-rinse adhesives. J Adhes Dent. 2012;14:31–8. doi: 10.3290/j.jad.a22744. [DOI] [PubMed] [Google Scholar]
- 10.Magni E, Ferrari M, Hickel R, Ilie N. Evaluation of the mechanical properties of dental adhesives and glass-ionomer cements. Clin Oral Investig. 2010;14:79–87. doi: 10.1007/s00784-009-0259-3. [DOI] [PubMed] [Google Scholar]
- 11.El-Askary FS, Nassif MS, Fawzy AS. Shear bond strength of glass. ionomer adhesive to dentin: Effect of smear layer thickness and different dentin conditioners. J Adhes Dent. 2008;10:471–9. [PubMed] [Google Scholar]
- 12.Elkassas DW, Fawzi EM, El Zohairy A. The effect of cavity disinfectants on the micro-shear bond strength of dentin adhesives. Eur J Dent. 2014;8:184–90. doi: 10.4103/1305-7456.130596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonough WG, Antonucci JM, He J, Shimada Y, Chiang MY, Schumacher GE, et al. A microshear test to measure bond strengths of dentin-polymer interfaces. Biomaterials. 2002;23:3603–8. doi: 10.1016/s0142-9612(02)00089-3. [DOI] [PubMed] [Google Scholar]
- 14.Sidhu SK, Sherriff M, Watson TF. Failure of resin-modified glass-ionomers subjected to shear loading. J Dent. 1999;27:373–81. doi: 10.1016/s0300-5712(98)00057-8. [DOI] [PubMed] [Google Scholar]
- 15.Coutinho E, Yoshida Y, Inoue S, Fukuda R, Snauwaert J, Nakayama Y, et al. Gel phase formation at resin-modified glass-ionomer/tooth interfaces. J Dent Res. 2007;86:656–61. doi: 10.1177/154405910708600714. [DOI] [PubMed] [Google Scholar]
- 16.Tay FR, Sidhu SK, Watson TF, Pashley DH. Water-dependent interfacial transition zone in resin-modified glass-ionomer cement/dentin interfaces. J Dent Res. 2004;83:644–9. doi: 10.1177/154405910408300812. [DOI] [PubMed] [Google Scholar]
- 17.Glasspoole EA, Erickson RL, Davidson CL. Effect of surface treatments on the bond strength of glass ionomers to enamel. Dent Mater. 2002;18:454–62. doi: 10.1016/s0109-5641(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 18.Rao KS, Reddy TP, Yugandhar G, Kumar BS, Reddy SN, Babu DA. Comparison of shear bond strength of resin reinforced chemical cure glass ionomer, conventional chemical cure glass ionomer and chemical cure composite resin in direct bonding systems: An in vitro study. J Contemp Dent Pract. 2013;14:21–5. doi: 10.5005/jp-journals-10024-1263. [DOI] [PubMed] [Google Scholar]
- 19.Pereira LC, Nunes MC, Dibb RG, Powers JM, Roulet JF, Navarro MF. Mechanical properties and bond strength of glass-ionomer cements. J Adhes Dent. 2002;4:73–80. [PubMed] [Google Scholar]
- 20.Coutinho E, Cardoso MV, De Munck J, Neves AA, Van Landuyt KL, Poitevin A, et al. Bonding effectiveness and interfacial characterization of a nano-filled resin-modified glass-ionomer. Dent Mater. 2009;25:1347–57. doi: 10.1016/j.dental.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Lin A, McIntyre NS, Davidson RD. Studies on the adhesion of glass-ionomer cements to dentin. J Dent Res. 1992;71:1836–41. doi: 10.1177/00220345920710111401. [DOI] [PubMed] [Google Scholar]
- 22.Gordan VV, Boyer D, Söderholm KJ. Enamel and dentine shear bond strength of two resin modified glass ionomers and two resin based adhesives. J Dent. 1998;26:497–503. doi: 10.1016/s0300-5712(98)00010-4. [DOI] [PubMed] [Google Scholar]
- 23.Cho BH, Dickens SH. Effects of the acetone content of single solution dentin bonding agents on the adhesive layer thickness and the microtensile bond strength. Dent Mater. 2004;20:107–15. doi: 10.1016/s0109-5641(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 24.Fritz UB, Finger WJ, Uno S. Resin-modified glass ionomer cements: Bonding to enamel and dentin. Dent Mater. 1996;12:161–6. doi: 10.1016/s0109-5641(96)80015-7. [DOI] [PubMed] [Google Scholar]
- 25.Uysal T, Yagci A, Uysal B, Akdogan G. Are nano-composites and nano-ionomers suitable for orthodontic bracket bonding? Eur J Orthod. 2010;32:78–82. doi: 10.1093/ejo/cjp012. [DOI] [PubMed] [Google Scholar]
- 26.Baldassarri M, Margolis HC, Beniash E. Compositional determinants of mechanical properties of enamel. J Dent Res. 2008;87:645–9. doi: 10.1177/154405910808700711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korkmaz Y, Ozel E, Attar N, Ozge Bicer C. Influence of different conditioning methods on the shear bond strength of novel light-curing nano-ionomer restorative to enamel and dentin. Lasers Med Sci. 2010;25:861–6. doi: 10.1007/s10103-009-0718-8. [DOI] [PubMed] [Google Scholar]
- 28.Ngo H, Mount GJ, Peters MC. A study of glass-ionomer cement and its interface with enamel and dentin using a low-temperature, high-resolution scanning electron microscopic technique. Quintessence Int. 1997;28:63–9. [PubMed] [Google Scholar]
- 29.Yip HK, Tay FR, Ngo HC, Smales RJ, Pashley DH. Bonding of contemporary glass ionomer cements to dentin. Dent Mater. 2001;17:456–70. doi: 10.1016/s0109-5641(01)00007-0. [DOI] [PubMed] [Google Scholar]
- 30.Zoergiebel J, Ilie N. An in vitro study on the maturation of conventional glass ionomer cements and their interface to dentin. Acta Biomater. 2013;9:9529–37. doi: 10.1016/j.actbio.2013.08.010. [DOI] [PubMed] [Google Scholar]


