Abstract
Objective:
This study evaluated the effects of carbamide peroxide and hydrogen peroxide on the biaxial flexural strength and flexural modulus of bovine dentin.
Materials and Methods:
Thirty coronal dentin disks (0.5 mm thick × 6.0 mm diameter) were prepared from bovine teeth. The disks were randomly divided into three groups (n=10): A control group (unbleached), a group bleached with 10% carbamide peroxide (8 h at 37°C), and a group bleached with 38% hydrogen peroxide (three 10 min applications at 37°C). The specimens were tested in a biaxial flexural apparatus held in a universal testing machine at 1.27 mm/min until failure occurred, and the biaxial mechanical properties were calculated. For each test parameter, the data were statistically analyzed by Fisher's PLSD test (predetermined α = 0.05).
Results:
The group bleached with 38% hydrogen peroxide demonstrated significantly lower flexural strength than the unbleached control group. Hydrogen peroxide treatment resulted in a significantly lower flexural modulus compared with the control group and with carbamide peroxide bleaching.
Conclusion:
Exposure of dentin to hydrogen peroxide significantly reduced both the flexural strength and the flexural modulus compared with the no-treatment control, whereas exposure to carbamide peroxide did not significantly affect either parameter.
Keywords: Biaxial flexural strength, bleaching, carbamide peroxide, dentin, flexural modulus, hydrogen peroxide
INTRODUCTION
The use of hydrogen peroxide to treat discolored and stained teeth is older than the technique of night guard vital bleaching with 10% carbamide peroxide. For in-office treatment, the bleaching agent indicated is hydrogen peroxide at high concentrations (30–40%), which is a strong oxidizing agent. Hydrogen peroxide at high concentrations must not come into contact with soft oral tissues, and patients have reported both severe and mild tooth hypersensitivity during and after bleaching.[1,2,3,4]
Bleaching with 10% carbamide peroxide using a mouthguard was suggested by Heymann and Haywood in 1989.[5] The concentration of hydrogen peroxide in 10% carbamide peroxide is 10 times less than that in 35% hydrogen peroxide, resulting in low levels of hypersensitivity and lower surface morphology changes. In this technique, patients use a 10% carbamide peroxide gel for 3–8 h daily, whereas in the hydrogen peroxide technique, 35% hydrogen peroxide is applied to the buccal surfaces of the teeth for short periods of time. Nightguard vital bleaching with carbamide peroxide is considered an effective and safe technique for bleaching teeth.[1,2] In contrast, the use of high concentrations of hydrogen peroxide remains questionable, and some researchers have expressed concerns regarding the widespread use of this technique.[6,7,8,9,10,11,12,13,14,15,16,17]
Several studies have evaluated different types of peroxides at various concentrations, and have expressed concerns about such bleaching techniques and pointed out the limitations of those techniques. These concerns are related to changes in the enamel morphology and mineral content,[14,18,19,20,21] and to effects on the organic components of dentin and on the dentin–pulp complex.[15,16,17,22,23,24] However, few investigations have aimed at studying and comparing the effects of carbamide peroxide and hydrogen peroxide on the mechanical and physical properties of dentin, even though these effects are clinically important.[7,8,9,10,11,12,13] The purpose of this study was to compare the biaxial flexural strength and modulus of bovine dentin using dentin disks treated with hydrogen peroxide, disks treated with carbamide peroxide, and disks kept unbleached as a control group, simulating 1 day of a bleaching treatment. The null hypothesis tested was that the two peroxides would not affect the biaxial flexural strength or the flexural modulus of the dentin.
MATERIALS AND METHODS
Thirty sound bovine incisors free from structural cracks and defects were selected for this study. After pumicing, the incisors were stored in a 0.1% thymol solution at 4°C for 30 days. Bovine dentin disks (0.5 mm thick × 6.0 mm diameter) were obtained from the coronal buccal surfaces by use of a hole saw. The disks were polished with 1200- and 2000-grit silicon carbide paper (Norton, Guarulhos, SP, Brazil) and randomly divided into three groups (n = 10):
A control group (unbleached). The disks in this group were kept at 100% humidity for 8 h at 37°C
Dentin disks bleached with 10% carbamide peroxide (Opalescence PF, Ultradent Products Inc., Salt Lake City, UT, USA). Each disk in this group was immersed in 0.2 mL of peroxide gel for 8 h at 37°C. After bleaching, the disks were thoroughly rinsed with an air/water spray and then tested
Dentin disks bleached with 38% hydrogen peroxide (Opalescence Boost PF, Ultradent Products Inc., Salt Lake City, UT, USA). Each disk in this group was immersed in 0.2 mL of peroxide gel for 10 min at 37°C and then rinsed with hydrogen peroxide (10 volume). This protocol was performed three times and then, after the last repetition, the specimens were thoroughly rinsed with an air/water spray and tested.
Both of the protocols used in this study simulated only one session of bleaching treatment (either a mouthguard technique or an in-office technique). Composition and batch number of bleaching agents used in this study are listed in Table 1.
Table 1.
Characteristics of bleaching agents used in this study

The disks were individually placed in a custom-made testing jig and tested in biaxial flexure on a universal testing machine (Instron 5844, Instron Corp., Canton, MA, USA) at 1.27 mm/min until failure occurred.[25] The maximum load was recorded for each specimen, and the elastic modulus was determined from the linear portion of the stress/strain curve. The formula used for the biaxial flexural strength σ was:
σ = - 0.238×7P(X - Y)/b2,
where σ is the maximum tensile stress in the center (in mega Pascals), P is the total load at fracture (in Newtons),
X = (1 + ν) ln (r2 / r3)2 +[(1 − ν) / 2](r2 / r3)2,
Y = (1 + ν)[1 + ln (r1/r3)2] + [(1 − ν)(r1 / r3)2,
and b is the specimen thickness (in millimeters) at the origin of the fracture. In the above equations, ν is Poisson's ratio (a value of 0.25 was used), r1 is the radius of the support circle (in millimeters), r2 is the radius of the loaded area (in millimeters), and r3 is the radius of the specimen (in millimeters). The flexural strength and modulus were calculated using the SRS biaxial-testing software package (Instron Corp., Canton, MA, USA). The data for the flexural strength and modulus were normal and homoscedastic. For each test parameter, the data were analyzed statistically using Fisher's protected least significant difference test (with a predetermined α = 0.05).
RESULTS
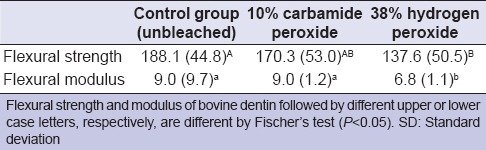
The values of the biaxial flexural strength and modulus are presented in Table 2. Statistically significant differences were found among the groups for the flexural strength (P = 0.0309) and for the modulus (P = 0.0003). The dentin disks treated with 38% hydrogen peroxide exhibited a lower flexural strength and modulus than did the control group (unbleached), whereas bleaching with 10% carbamide peroxide resulted in no significant difference in the flexural strength and modulus from the control group (P > 0.05). The 38% hydrogen peroxide produced a significantly lower flexural modulus than did bleaching with 10% carbamide peroxide (P = 0.0002), whereas no significant difference was found between the two bleaching materials for the flexural strength (P = 0.1517).
Table 2.
Biaxial flexural strength (in MPa) and modulus (in GPa) means (SD) of the bovine dentin
DISCUSSION
Some studies have reported changes in the fracture toughness, tensile strength, and flexural strength of human dentin and enamel after bleaching; however, the type of bleaching agent and the duration of the bleaching treatment were not standardized.[6,7,8,9,10,11,12,13] Although many techniques and bleaching agents were used, the authors of these studies agree that caution is necessary when peroxides are used for prolonged treatment times, since the action of hydrogen peroxide is not specific against only pigments that discolor or stain teeth. The current study was aimed at comparing the effects of 10% carbamide peroxide and 38% hydrogen peroxide on the mechanical properties of bovine dentin, simulating only one appointment or session.
In most previous studies, bar-shaped specimens were used and a three-point flexural test was performed to evaluate the flexural strength and modulus of the dentin. In contrast, the present study used a biaxial flexural test and disk-shaped specimens, and it seems to be the first investigation in which the biaxial flexural strength and modulus of dentin have been studied.[7,12] Our biaxial test has the advantage of utilizing small specimens, which allowed the peroxide to be in contact with the entire sample. Flexural strength is a mechanical property that indicates the maximum stress before fracture. When the stress exceeds the flexural strength, the loading may lead to dental fracture, which compromises the integrity of the tooth. The biaxial test also provided another important property, the flexural modulus, which is the ratio between stress and strain in the elastic regime: In other words, it tells us how much stress is required to deform the material before the proportional limit is reached.[25]
According to the results of this study, one session in which bovine dentin was exposed to 38% hydrogen peroxide (three 10 min applications at 37°C) was enough to significantly reduce the flexural strength and modulus compared with the unbleached control group, whereas exposure to carbamide peroxide did not change the mechanical properties of the dentin. Thus, the null hypothesis stating that the two peroxides (carbamide peroxide and hydrogen peroxide) would not affect the biaxial flexural strength and flexural modulus of dentin was rejected, since 38% hydrogen peroxide altered both of these mechanical properties.
Although 10% carbamide peroxide used for just 8 h did not change the biaxial flexural strength and modulus of bovine dentin, some other authors have shown that, depending on the bleaching time, carbamide peroxide may affect the strength of teeth as hydrogen peroxide does. Studies have shown that the flexural strength and modulus of dentin can be reduced after 2 weeks or prolonged use (2 months) of 10% carbamide peroxide applied directly to the dentin.[7,24] In those studies, when the carbamide peroxide application was on enamel, the mechanical properties of underlying dentin was not affect. Toledano et al.[25] found that mouthguard and in-office bleaching gels induced collagenolityc activity when applied to human dentin. In that study, 21 carbamide peroxide applications with duration of 8 h/day can induced higher collagen degradation than three applications of 38% hydrogen peroxide for 20 min each. However, Seghi and Denry[6] investigated the effects of 10% carbamide peroxide on the fracture toughness, hardness, and abrasion characteristics of enamel and reported that the abrasion resistance and toughness decreased after only one 12 h session of bleaching.
Hydrogen peroxide is a binary compound of hydrogen and oxygen that is a highly reactive oxygen species in aqueous solution.[3,4] The color of the dentin disks treated with 38% hydrogen peroxide changed to white, demonstrating the visual effect of the oxidizing effects of hydrogen peroxide. However, the carbamide peroxide bleaching did not modify the color of the dentin samples, nor did it change the mechanical properties measured. The dentin disks from the control (untreated) group showed higher values of the flexural modulus than the disks that were bleached with 38% hydrogen peroxide. A study reported that six applications during 3 weeks (1 h each application and 2 days/week) of 35% hydrogen peroxide did not cause a decrease in dentin flexural strength and modulus.[24] It used dentin bars (2 mm × 2 mm × 20 mm), while this study tested very thin and small dentin disks (0.5 mm thick × 6.0 mm diameter), which may be responsible, in part, for differences in the findings of studies. Thus, higher concentration of hydrogen peroxide and the dimension of dentin disks that facilitated the contact with entire sample and peroxide reaction caused the reduction of the two dentin mechanical properties.
Some authors have suggested that peroxides should not be applied over exposed dentin, in order to avoid the effects of bleaching on the mechanical properties of dentin.[7,10,11,13] The main hypothesis for why peroxides affect the mechanical properties of dentin is that peroxides can degrade or disorganize type I collagen fibrils, denature proteins, and cause loss of mineral components attached to the organic matrix.[25,26,27,28,29] Demineralization has been observed and measured by several different methods, such as scanning electron microscopy, polarized light microscopy, microhardness measurements, atomic absorption spectrometry, and Fourier transform Raman spectroscopy.[20,21,22,30] Jiang et al.[23] described the dissolution of dentin in hydrogen peroxide. According to these authors, the destruction of the organic components was due to the oxidizing ability of hydrogen peroxide while the changes in the mineral components were caused by its acidity.
High concentrations of hydrogen peroxide, such as 30 or 40%, can cause alterations in the surface morphology of human enamel and promote an increase in the permeability of dentin.[14,16,18,19] Furthermore, as reported by Costa et al.,[17] bleaching with 38% hydrogen peroxide for 45 min may lead to irreversible pulp damage in the lower incisors, because of the high permeability of dentin to hydrogen peroxide. However, despite the changes in the properties of dentin induced by hydrogen peroxide, is important to note that in the present study the bleaching agents were applied directly to dentin, whereas in clinical use they should be applied to dental enamel. Carbamide peroxide seems to produce less severe side effects than hydrogen peroxide, and this must be considered in relation to clinical indications for the use of bleaching techniques.
CONCLUSION
The exposure of bovine dentin to 38% hydrogen peroxide significantly reduced both the flexural strength and the flexural modulus compared with the no-treatment control, whereas exposure to 10% carbamide peroxide did not significantly affect either of these parameters.
ACKNOWLEDGMENT
This study was supported by grants from CAPES (3110/2010), CNPq (#305777/2010-6), Brazil and Georgia Regents University, Augusta, GA, USA.
Footnotes
Source of Support: This study was supported by grants from CAPES (3110/2010), CNPq (#305777/2010-6), Brazil and Georgia Regents University, Augusta, GA, USA.
Conflict of Interest: None declared
REFERENCES
- 1.Moghadam FV, Majidinia S, Chasteen J, Ghavamnasiri M. The degree of color change, rebound effect and sensitivity of bleached teeth associated with at-home and power bleaching techniques: A randomized clinical trial. Eur J Dent. 2013;7:405–11. doi: 10.4103/1305-7456.120655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandrino L, Gomes Y, Alves E, Costi H, Rogez H, Silva C. Effects of a bleaching agent with calcium on bovine enamel. Eur J Dent. 2014;8:320–5. doi: 10.4103/1305-7456.137634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joiner A. The bleaching of teeth: A review of literature. J Dent. 2006;34:412–9. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Kihn PW. Vital tooth whitening. Dent Clin North Am. 2007;51:319–31. doi: 10.1016/j.cden.2006.12.001. viii. [DOI] [PubMed] [Google Scholar]
- 5.Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989;20:173–6. [PubMed] [Google Scholar]
- 6.Seghi RR, Denry I. Effects of external bleaching on indentation and abrasion characteristics of human enamel in vitro. J Dent Res. 1992;71:1340–4. doi: 10.1177/00220345920710061201. [DOI] [PubMed] [Google Scholar]
- 7.Tam LE, Abdool R, El-Badrawy W. Flexural strength and modulus properties of carbamide peroxide-treated bovine dentin. J Esthet Restor Dent. 2005;17:359–67. doi: 10.1111/j.1708-8240.2005.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 8.da Silva AP, de Oliveira R, Cavalli V, Arrais CA, Giannini M, de Carvalho RM. Effect of peroxide-based bleaching agents on enamel ultimate tensile strength. Oper Dent. 2005;30:318–24. [PubMed] [Google Scholar]
- 9.Piemjai M, Surakompontorn J. Effect of tooth-bleaching on the tensile strength and staining by caries detector solution on bovine enamel and dentin. Am J Dent. 2006;19:387–92. [PubMed] [Google Scholar]
- 10.Tam LE, Kuo VY, Noroozi A. Effect of prolonged direct and indirect peroxide bleaching on fracture toughness of human dentin. J Esthet Restor Dent. 2007;19:100–9. doi: 10.1111/j.1708-8240.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- 11.Tam LE, Noroozi A. Effects of direct and indirect bleach on dentin fracture toughness. J Dent Res. 2007;86:1193–7. doi: 10.1177/154405910708601210. [DOI] [PubMed] [Google Scholar]
- 12.Ghavamnasiri M, Abedini S, Mehdizadeh Tazangi A. Effect of different time periods of vital bleaching on flexural strength of the bovine enamel and dentin complex. J Contemp Dent Pract. 2007;8:21–8. [PubMed] [Google Scholar]
- 13.Woo JM, Ho S, Tam LE. The effect of bleaching time on dentin fracture toughness in vitro. J Esthet Restor Dent. 2010;22:179–84. doi: 10.1111/j.1708-8240.2010.00333.x. [DOI] [PubMed] [Google Scholar]
- 14.Titley K, Torneck CD, Smith D. The effect of concentrated hydrogen peroxide solutions on the surface morphology of human tooth enamel. J Endod. 1998;14:69–74. doi: 10.1016/S0099-2399(88)80004-9. [DOI] [PubMed] [Google Scholar]
- 15.Gökay O, Müjdeci A, Algin E. In vitro peroxide penetration into the pulp chamber from newer bleaching products. Int Endod J. 2005;38:516–20. doi: 10.1111/j.1365-2591.2005.00979.x. [DOI] [PubMed] [Google Scholar]
- 16.Camargo SE, Valera MC, Camargo CH, Gasparoto Mancini MN, Menezes MM. Penetration of 38% hydrogen peroxide into the pulp chamber in bovine and human teeth submitted to office bleach technique. J Endod. 2007;33:1074–7. doi: 10.1016/j.joen.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e59–64. doi: 10.1016/j.tripleo.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 18.McGuckin RS, Babin JF, Meyer BJ. Alterations in human enamel surface morphology following vital bleaching. J Prosthet Dent. 1992;68:754–60. doi: 10.1016/0022-3913(92)90197-i. [DOI] [PubMed] [Google Scholar]
- 19.Pinto CF, Oliveira Rd, Cavalli V, Giannini M. Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz Oral Res. 2004;18:306–11. doi: 10.1590/s1806-83242004000400006. [DOI] [PubMed] [Google Scholar]
- 20.Berger SB, Cavalli V, Ambrosano GM, Giannini M. Changes in surface morphology and mineralization level of human enamel following in-office bleaching with 35% hydrogen peroxide and light irradiation. Gen Dent. 2010;58:e74–9. [PubMed] [Google Scholar]
- 21.Berger SB, Cavalli V, Martin AA, Soares LE, Arruda MA, Brancalion ML, et al. Effects of combined use of light irradiation and 35% hydrogen peroxide for dental bleaching on human enamel mineral content. Photomed Laser Surg. 2010;28:533–8. doi: 10.1089/pho.2009.2506. [DOI] [PubMed] [Google Scholar]
- 22.Dadoun MP, Bartlett DW. The microhardness of bleached dentine and its bond strength to a dentine bonding agent. Eur J Prosthodont Restor Dent. 2007;15:131–4. [PubMed] [Google Scholar]
- 23.Jiang T, Ma X, Wang Y, Zhu Z, Tong H, Hu J. Effects of hydrogen peroxide on human dentin structure. J Dent Res. 2007;86:1040–5. doi: 10.1177/154405910708601104. [DOI] [PubMed] [Google Scholar]
- 24.Tam LE, Lim M, Khanna S. Effect of direct peroxide bleach application to bovine dentin on flexural strength and modulus in vitro. J Dent. 2005;33:451–8. doi: 10.1016/j.jdent.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Toledano M, Yamauti M, Osorio E, Osorio R. Bleaching agents increase metalloproteinases-mediated collagen degradation in dentin. J Endod. 2011;37:1668–72. doi: 10.1016/j.joen.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Giannini M, Mettenburg D, Arrais CA, Rueggeberg FA. The effect of filler addition on biaxial flexure strength and modulus of commercial dentin bonding systems. Quintessence Int. 2011;42:e39–43. [PubMed] [Google Scholar]
- 27.Kim BM, Eichler J, Reiser KM, Rubenchik AM, Da Silva LB. Collagen structure and nonlinear susceptibility: Effects of heat, glycation, and enzymatic cleavage on second harmonic signal intensity. Lasers Surg Med. 2000;27:329–35. doi: 10.1002/1096-9101(2000)27:4<329::aid-lsm5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 28.Lin PY, Lyu HC, Hsu CY, Chang CS, Kao FJ. Imaging carious dental tissues with multiphoton fluorescence lifetime imaging microscopy. Biomed Opt Express. 2010;2:149–58. doi: 10.1364/BOE.2.000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, et al. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–81. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 30.White DJ, Duschner H, Götz H. Effects of hydrogen peroxide bleaching strips on root surfaces in vitro. J Clin Dent. 2007;18:87–90. [PubMed] [Google Scholar]


