Abstract
The importance of genetic factors (e.g. sequence variation) in the absorption, distribution, metabolism, excretion (ADME) and overall efficacy of therapeutic agents is well established. Our ability to identify, interpret and utilize these factors is the subject of much clinical investigation and therapeutic development. However, drug ADME and efficacy are also heavily influenced by epigenetic factors such as DNA/histone methylation and non-coding RNAs [especially microRNAs (miRNAs)]. Results from studies using tools, such as in silico miRNA target prediction, in vitro functional assays, nucleic acid profiling/sequencing and high-throughput proteomics, are rapidly expanding our knowledge of these factors and their effects on drug metabolism. Although these studies reveal a complex regulation of drug ADME, an increased understanding of the molecular interplay between the genome, epigenome and transcriptome has the potential to provide practically useful strategies to facilitate drug development, optimize therapeutic efficacy, circumvent adverse effects, yield novel diagnostics and ultimately become an integral component of personalized medicine.
Linked Articles
This article is part of a themed section on Epigenetics and Therapy. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-11
Tables of Links
| TARGETS | |
|---|---|
| Enzymesa | Transportersc |
| CYP1A1 | ABCA1 |
| CYP1B1 | ABCB1, MDR1/P-gp |
| CYP2E1 | ABCC1 |
| CYP3A4 | ABCC2 |
| DNMT1, DNA methyltransferase 1 | ABCC3 |
| DNMT3A, DNA methyltransferase 3α | ABCC4 |
| HDAC | ABCC5 |
| Histone methyltransferase EZH2 | ABCC10 |
| Nuclear hormone receptorsb | ABCE1 |
| ESR1, oestrogen receptor α, NR3A1 | ABCG2 |
| Glucocorticoid receptor, NR3C1 | Cystine/glutamate exchanger, SLC7A11 |
| LXRA, liver X receptor α, NR1H3 | SERT, 5-HT transporter, SLC6A4 |
| PPARα, NR1C1 | SLC15 |
| PXR, pregnane X receptor | vGLUT1, vesicular glutamate transporter 1, SLC17A7 |
| RXR, retinoid receptors |
| LIGANDS | |
|---|---|
| 17β-oestradiol | Raloxifene |
| Acetaminophen, paracetamol | Tamoxifen |
| Cholesterol | Testosterone |
| Cisplatin | Topotecan |
| Cocaine | Vinblastine |
| Dexamethasone | |
| Dox, doxorubicin | |
| Fluoxetine | |
| Gemcitabine | |
| GSH, glutathione | |
| Halothane | |
| Isoflurane | |
| Methadone | |
| Mitoxantrone |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,b,c).
The proper control of absorption, distribution, metabolism and excretion (ADME) of xenobiotics is essential for living organisms to obtain energy, acquire necessary building blocks (e.g. essential amino acids) and maintain homeostasis in a complex chemical environment. Genes involved in ADME activities encode various receptor/transporters, biotransformation enzymes and accessory proteins (PharmaADME, http://pharmaadme.org/joomla/). These proteins include membrane transporters responsible for the absorption and excretion of specific molecules and enzymes to convert xenobiotics for excretion. To date, over 300 transporters and enzymes directly involved in ADME process have been described. This long list of components makes the study of ADME inherently complex, as transporters and enzymes work in concert to respond dynamically to diverse external factors. Despite the formidable complexity of the field, an understanding of ADME is critical for drug development in order to increase therapeutic efficacy and reduce adverse effects (Caldwell et al., 2009; Emoto et al., 2010; Bell and Wang, 2012).
It is well known that there are considerable inter-individual variations in response to drug treatment (Alomar, 2014). Besides polymorphisms within the sequence of therapeutic targets, genetic variations in ADME-related genes have been shown to affect therapeutic response through drug transportation and metabolism (Nakajima and Aoyama, 2000; Evrard and Mbatchi, 2012). However, genetic variations cannot completely explain the differences observed between individuals. Recent studies using high-throughput global molecular profiling technologies have shown that ADME activities have also been affected by a number of epigenetic factors, including DNA methylation, post-translational histone modifications and non-coding regulatory RNAs, which directly or indirectly alter the expression of ADME genes (Ivanov et al., 2012). For example, microRNAs (miRNAs) are short, non-coding regulatory RNAs involved in modulating ADME-related gene expression and protein translation levels (Liu and Pan, 2014). The study of epigenetic factors on drug metabolism, pharmacoepigenetics, is a rapidly growing field and plays an important role in therapeutic efficacy by affecting the pharmacokinetic (PK) and pharmacodynamic properties of drugs. In this review, we summarize the current understanding on the involvement of miRNAs in ADME.
MicroRNA
More than 60% of human genomic DNA is transcribed into RNA, yet protein-coding genes account for only less than 3% of the genome (Bernstein et al., 2012; Kellis et al., 2014). Non-coding RNAs (ncRNAs) are RNAs that are not translated into proteins. Some ncRNAs, such as ribosomal RNAs and transfer RNAs, have well-established cellular roles. However, there are other classes of ncRNAs that have been recently discovered (Esteller, 2011). Among them, miRNAs are probably the best studied due to their important roles in the post-transcriptional regulation of messenger RNAs (mRNAs). miRNAs were originally discovered in the nematode Caenorhabditis elegans as mediators of temporal pattern formation (Ambros, 2001; Lagos-Quintana et al., 2001). These small regulatory RNAs were found to be conserved in metazoan species and have also been observed in several virus families (Pfeffer et al., 2004). miRNAs are involved in a broad range of cellular activities, including proliferation, development, homeostasis, immunity, metabolism and apoptosis (Kloosterman and Plasterk, 2006; Stefani and Slack, 2008). Because of their important regulatory roles in these diverse biological processes, miRNAs display characteristic expression patterns in cells and tissues, different developmental stages and disease processes.
miRNA genes are found in diverse locations throughout the genome (Rodriguez et al., 2004) and often cluster together (Lagos-Quintana et al., 2001; Seitz et al., 2004). Most of the sequences that encode miRNAs are transcribed by RNA polymerase II (Lee et al., 2004). Many miRNAs may be transcribed on the same primary transcript (pri-miRNA). This pri-miRNA is then processed into a smaller hairpin structure (pre-miRNA) by the microprocessor complex, RNase III enzyme Drosha/DGCR8. Following nuclear export via the RanGTP-dependent double-strand RNA binding protein exportin-5, the pre-RNA is then cleaved by another RNase III enzyme, Dicer, to yield an approximately 22 nucleotide-long RNA duplex (Kim et al., 2009). Unwinding by an RNA helicase allows a mature miRNA strand to be loaded onto the protein Argonaute to form the RNA-induced silencing complex (RISC). The RISC typically interacts with the 3’ untranslated region (UTR) of mRNA targets, resulting in the translational repression or degradation of the transcript. Unlike other regulatory molecules, miRNA target recognition is relatively flexible, as the miRNA sequence itself is short and the interaction between miRNA and mRNA is based upon partial sequence complementarity (Bartel, 2009). This permits a single miRNA to interact with many mRNA targets. Additionally, a single mRNA can also contain many different miRNA binding sites. It has been estimated that the majority of the human mRNAs are targeted by one or more miRNAs (Friedman et al., 2009). These loose constraints governing miRNA–mRNA interactions increase the diversity of possible interactions and make miRNA-mediated regulatory networks often difficult to unravel.
At present, over 2500 human mature miRNAs have been deposited into the online repository miRBase (v20, www.mirbase.org) (Griffiths-Jones et al., 2008). To decipher the interactions between miRNA and mRNA, a number of informatic tools, such as TargetScan (www.targetscan.org) (Lewis et al., 2005) and miRanda (www.microrna.org) (Enright et al., 2003), have been developed to predict miRNA–mRNA interactions based upon sequence complementation, binding stability and degree of sequence conservation. A typical approach to evaluate the specificity of miRNA–target interactions is the use of gene reporter assays in cell culture. A specific miRNA of interest is transfected into a cell line expressing a transgenic construct containing a reporter gene (e.g. luciferase) and the 3′-UTR region of a putative mRNA target. However, this approach relies upon overexpressed components and may ultimately have limited physiological relevance. The strongest support for in vivo relevance is derived from a combination of experiential methods (reviewed in Thomson et al., 2011). Although thousands of miRNA–mRNA interactions have been predicted, few mRNA–miRNA interactions have been empirically validated. These validated interactions are archived in online databases, such as miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/), Tarbase (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=tarbase/index) and miRecords (http://mirecords.biolead.org/).
MicroRNAs regulate the expression of ADME phase I and II enzymes
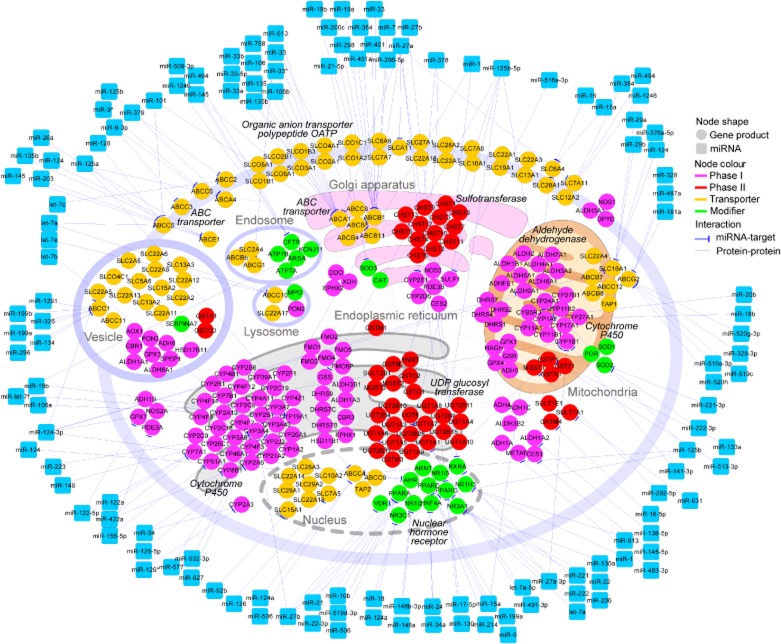
Increasing evidence has indicated that a substantial number of transporters and drug metabolizing enzymes are regulated by miRNAs (Yu, 2007; 2009,) (Table 1 and Figure 1). We collected all miRNA–ADME interactions from literature and constructed a miRNA-mediated regulatory network for ADME (Figure 1). The network contains 189 interactions among the 381 nodes, which includes 120 miRNAs and 261 ADME genes.
Table 1.
List of interactions between miRNA and drug metabolism-related genes
| Gene ID | Function | Interacting miRNA | References |
|---|---|---|---|
| CYP1A1 | Phase I enzyme | miR-18b, -20b | Wang et al., 2009; Glubb and Innocenti, 2011 |
| CYP1B1 | Phase I enzyme | miR-27b | Tsuchiya et al., 2006; Chuturgoon et al., 2014 |
| CYP2A3 | Phase I enzyme | miR-126*, -34 | Kalscheuer et al., 2008 |
| CYP2E1 | Phase I enzyme | miR-378 | Mohri et al., 2010; Takahashi et al., 2014 |
| CYP2J2 | Phase I enzyme | Let-7b | F Chen et al., 2012a |
| CYP3A4 | Phase I enzyme | miR-27b, -577, -1, -532-3p, -627, -223, -148 | Takagi et al., 2008; Pan et al., 2009a; Takahashi et al., 2014; Wei et al., 2014 |
| CYP7A1 | Phase I enzyme | miR-122a, miR-422a | Song et al., 2010 |
| CYP19A1 | Phase I enzyme | miR-19b, -106a, let-7f | Shibahara et al., 2012; Kumar et al., 2013 |
| CYP24A1 | Phase I enzyme | miR-125b | Komagata et al., 2009 |
| GSS | Phase I enzyme | miR-125b | Tili et al., 2012 |
| GPX7 | Phase I enzyme | miR-122 | Akinc et al., 2008 |
| SULF1 | Phase I Enzyme | miR-516a | Takei et al., 2011 |
| GSTP1 | Phase II enzyme | miR-133a, -513a-3p | Zhang et al., 2012; Uchida et al., 2013 |
| SULT1A1 | Phase II enzyme | miR-631 | Yu et al., 2010 |
| UGT1A | Phase II enzyme | miR-491-3p | Dluzen et al., 2014 |
| ABCA1 | Transporter | miR-33, -33*, -758, -106b, -613, -27a, -27b, -145, -101, -135b | Ramirez et al., 2011; Borel et al., 2012; Kim et al., 2012; Goedeke et al., 2013; Kang et al., 2013; Rottiers et al., 2013; Zhang et al., 2014; Zhao et al., 2014 |
| ABCB1/P-gp/MDR1 | Transporter | miR-451, -27a, -298, -354, -7, -200c, -19a/b | Kovalchuk et al., 2008; Zhu et al., 2008; Pogribny et al., 2010; Bitarte et al., 2011; Bao et al., 2012; J Chen et al., 2012b; F Wang et al., 2013a |
| ABCB11 | Transporter | miR-33 | Allen et al., 2012 |
| ABCC1/MRP-1 | Transporter | miR-134, -326, -1291, -199a/b, -296 | Guo et al., 2010; Liang et al., 2010; Borel et al., 2012; Pan et al., 2013 |
| ABCC2 | Transporter | miR-379, let-7c | Haenisch et al., 2011; Zhan et al., 2013 |
| ABCC3 | Transporter | miR-9* | Jeon et al., 2011 |
| ABCC4/MRP4 | Transporter | miR-124a, -506, -125a/b | Borel et al., 2012; Markova and Kroetz, 2014 |
| ABCC5 | Transporter | miR-128 | Zhu et al., 2011 |
| ABCC6 | Transporter | miR-9* | Jeon et al., 2011 |
| ABCC7/CFTR | Transporter | miR-145, -494, -1246, -509-3p | Gillen et al., 2011; Ramachandran et al., 2013 |
| ABCC10 | Transporter | Let-7a/e | Borel et al., 2012 |
| ABCE1 | Transporter | miR-124, -203, -26a, -135b, -145 | Furuta et al., 2010; Borel et al., 2012 |
| ABCG1 | Transporter | miR-33 | Marquart et al., 2010; Rayner et al., 2010 |
| ABCG2/BCRP | Transporter | miR-520h, -519c, -328, -487a, -181a | Liao et al., 2008; Pan et al., 2009b; To et al., 2009; Wang et al., 2010; Li et al., 2011; Padmanabhan et al., 2012; Jiao et al., 2013; Ma et al., 2013 |
| SLC6A4 | Transporter | miR-16, -15a | Baudry et al., 2010; Tamarapu Parthasarathy et al., 2012; Moya et al., 2013 |
| SLC7A5 | Transporter | miR-126 | Miko et al., 2011 |
| SLC7A11 | Transporter | miR-27a | Drayton et al., 2014 |
| SLC15A1/PEPT1 | Transporter | miR-92a | Dalmasso et al., 2011b |
| SLC12A2 | Transporter | miR-384, -494, -1246 | Gillen et al., 2011 |
| SLC16A1/MCT1 | Transporter | miR-124, -29a/b | KK Li et al., 2009b; Pullen et al., 2011 |
| NR1I2/PX receptor | Nuclear Receptor | miR-148a | Takagi et al., 2008 |
| NR3C1/GR | Nuclear Receptor | miR-18, -124a | Vreugdenhil et al., 2009 |
| NR2B1/RXRA | Nuclear Receptor | miR-27a, -27b | Ji et al., 2009; Komagata et al., 2009; Mohri et al., 2009 |
| NR1I1/VD receptor | Nuclear Receptor | miR-27b, -125b | Mohri et al., 2009; Pan et al., 2009a |
| NR1C1 | Nuclear Receptor | miR-10b, -506, -21, -27b | Zheng et al., 2010; Kida et al., 2011; Tong et al., 2011; Zhou et al., 2011 |
| NR1C3 | Nuclear Receptor | miR-130, -27b, -27a | Jennewein et al., 2010; Kim et al., 2010; Lee et al., 2012 |
| NR1C2 | Nuclear Receptor | miR-15a, -199a, -214, -9 | Yin et al., 2010; el Azzouzi et al., 2013; Thulin et al., 2013 |
| NR1H3/LXRA | Nuclear Receptor | miR-613, -206, -1 | Ou et al., 2011; Zhong et al., 2013; Vinod et al., 2014 |
| NR2A1/HNF4A | Nuclear Receptor | miR-24, -34a | Takagi et al., 2010 |
| NR3A1 | Nuclear Receptor | miR-221, -222, let-7a, miR-130a, -22, -206 | Iorio et al., 2005; Adams et al., 2007; Zhao et al., 2008; 2011b,; Pandey and Picard, 2009; Xiong et al., 2010; Tang et al., 2011 |
| RXRA | Modifier | miR-27a/b | Ji et al., 2009 |
Figure 1.
A hypothetical network view on the interactions between miRNA and ADME transcripts. Molecular interactions used were obtained from KEGG pathway and literature. The network contains 189 interactions between miRNA and its target genes, and 381 nodes including120 miRNAs (squares) and 261 ADME genes (circles). Different colours of circles represent different categories of ADME genes as indicated. The visualization of the network was carried our by Cytoscape.
The biotransformation of xenobiotics is generally separated into two phases: phase I includes the addition or exposure of chemically reactive functional groups such as amine (–NH2), hydroxyl (–OH), sulfhydryl (–SH) or carboxyl (–COOH) group by enzymes, in preparation for phase II, where the phase I products are further modified with water-soluble or charged chemical motif(s), converting them into water-soluble structures for excretion. Cytochrome P450 (CYP450, a class of monooxygenases) is the largest group of phase I enzymes. In humans, there are 57 functional CYP450 genes, which can be grouped into 18 different families based upon sequence similarity (Lewis, 2004). A number of miRNAs have been shown to be involved in the post-transcriptional regulation of cytochrome P450 genes (Table 1 and Figure 1). For example, the expression level of CYP1A1 was correlated with the levels of miR-18b and miR-20b (Wang et al., 2009; Glubb and Innocenti, 2011). CYP1B1 and CYP3A4 were found to be regulated by miR-27b (Tsuchiya et al., 2006; Pan et al., 2009a), and CYP2E1 by miR-378 (Mohri et al., 2010).
A few phase I enzymes have gained more attention due to their involvement in metabolizing important therapeutic agents. CYP3A4, a member of the CYP3 family predominantly expressed in liver and intestine, metabolizes more than 50% of the therapeutic drugs currently on the market (Plant and Gibson, 2003). It is also involved in the synthesis and metabolism of a number of endogenous molecules, including cholesterol, steroid hormones (e.g. testosterone), bile acids and vitamin D (Wang et al., 2013c). Because of its important role in drug metabolism, changes in CYP3A4 activity (either through altered gene expression or inhibition of enzyme activity) cause a number of adverse effects associated with drug usage. CYP3A4 can be induced by ligands that bind and activate the pregnane X (PX) receptor (Lehmann et al., 1998). Experimental data also indicate that the level of CYP3A4 is affected by epigenetic factors, including histone acetylation and methylation (Dannenberg and Edenberg, 2006; Kacevska et al., 2012) and also miRNAs.
A recent study based upon several different miRNA target prediction programmes identified 105 miRNAs that may potentially interact with CYP3A4 mRNA (Wei et al., 2014). Among the 105 miRNA candidates, 14 of them were experimentally verified to interact directly with the 3′-UTR of CYP3A4 transcript in HEK293T cells. Analysis of the miRNAs identified in vitro and CYP3A4 transcript and protein levels in human liver samples suggested that four of these miRNAs (miR-1, -532-3p, -577 and -627) attenuate the translation of CYP3A4 in vivo. Besides showing that miRNAs affect both transcript and protein levels in vivo, results from this study also illustrated our limited understanding of how miRNAs select their targets in cells, since only about 10% of the predicted interactions could be verified experimentally.
CYP2E1 is involved in the metabolism of anaesthetics, such as isoflurane and halothane, organic solvents, such as ethanol and benzene, acetaminophen (paracetamol) and other compounds. Computational analysis predicted a potential miR-378 binding site within the 3′-UTR of CYP2E1 (Mohri et al., 2010), the functionality of which was verified by in vitro reporter assays. In addition, an inverse correlation between CYP2E1 protein levels and miR-378 abundances was observed in a panel of 25 human liver specimens, providing further support for the possible significance of this interaction in vivo (Mohri et al., 2010).
CYP1B1 is overexpressed in diverse cancers (e.g. breast, colon, lung, oesophagus, skin, lymph node, brain and testis) relative to their corresponding normal tissues (Murray et al., 1997) and is known to activate various pro-carcinogens (Shimada et al., 1996). In addition, CYP1B1 enhances the conversion of 17β-oestradiol into 4-hydroxyestradiol (Han and Liehr, 1994; Newbold and Liehr, 2000), a metabolite known to cause DNA damage through free radical production and which may promote the development of oestrogen-dependent cancers. Examination of the 3′-UTR of CYP1B1 transcript revealed a high-quality target site for miR-27b (Tsuchiya et al., 2006), which was confirmed using luciferase reporter assays. Ablation of miR-27b targeting via an antisense oligonucleotide increased the abundance and activity of endogenous CYP1B1 in MCF-7 cells. In addition, the amount of miR-27b was inversely correlated with CYP1B1 protein level in most of the oestrogen receptor/progesterone receptor positive breast cancer samples examined (Tsuchiya et al., 2006).
GSH is synthesized from glutamylcysteine and glycine by glutathione synthetase (GSS) in an ATP-dependent process (Snoke and Bloch, 1955). GSH has many cellular functions, including the scavenging of peroxides and free radical species, and the detoxification of xenobiotics (Pastore et al., 2003). Overexpression of miR-125b in chronic lymphocytic leukaemia-derived cell lines reduced GSS protein abundance and led to a decrease of GSH activity (Tili et al., 2012). Using a luciferase reporter assay, miR-125b was verified to directly interact with the 3′-UTR of the GSS transcript.
Glutathione peroxidase 7 (GPX7) counters oxidative stress generated from polyunsaturated fatty acid metabolism (Utomo et al., 2004) and can neutralize hydrogen peroxide in the absence of glutathione (Peng et al., 2012). Knockdown of GPX7 expression resulted in an increase in reactive oxygen species, DNA damage and apoptosis in oesophageal squamous epithelial cells upon exposure to bile acids in vitro. GPX7 level was found to be up-regulated in the livers of mice treated with antagomiR-122 (a miR-122 antagonist). This suggests a possible interaction between miR-122 and GPX7 transcript (Krutzfeldt et al., 2005; Akinc et al., 2008).
UDP-glucuronosyltransferases (UGTs) are responsible for the phase II processing of many endogenous (e.g. bilirubin, steroid hormones) (Bosma et al., 1994; Belanger et al., 2003) and exogenous (e.g. drugs, chemotherapeutics and carcinogens) compounds (recent review in Rowland et al., 2013). This family of enzymes is composed of two subfamilies (UGT1A and 2B) and catalyses the addition of carbohydrate groups (e.g. glucuronide) to its substrates for excretion (Mackenzie et al., 2005; Nagar and Remmel, 2006). The UGT1A family has nine alternatively spliced protein isoforms sharing a common 3′-UTR. In silico analysis of the UGT1A 3′-UTR identified a potential miR-491-3p target sequence (Dluzen et al., 2014). Introducing a miR-491-3p mimic into HuH-7 cells reduced UGT1A1 mRNA abundance by 48% relative to non-targeting control mimics and direct interaction between miR-491-3p and UGT1A1 3′-UTR was also confirmed by reporter assays. This reduction in UGT1A1 mRNA was complemented with a significant reduction in UGTA1 enzymatic activity, as measured by the conversion of raloxifene into glucuronidated metabolites (raloxifene-6-glucuronide and -4′-glucuronide). Knockdown of the endogenous miR-491-3p level in HepG2 cells increased the abundance of these metabolites, although without reducing UGT1A1 mRNA levels (Dluzen et al., 2014). An inverse correlation between miR-491-3p abundance and the quantity of UGTA3 and A6 isoforms (sharing the same 3′-UTR as UGT1A1) was also observed in samples of normal human liver (Dluzen et al., 2014).
MicroRNAs regulate the expression of transporter proteins
miRNAs have also been observed to regulate two major classes of drug transporters: ATP-binding cassette (ABC) and solute carrier (SLC) proteins (Table 1 and Figure 1). As these membrane transporters facilitate the uptake and excretion of many endo- and xenobiotics, alterations in their levels may affect the PK properties of drugs.
A recent study reported that the levels of a number of ABC transporters were increased in hepatocellular carcinoma and that the increase is associated with the decrease of several miRNAs (Borel et al., 2012). Using luciferase reporter assays, Borel et al. confirmed the interactions of some ABC transporter transcripts and miRNAs including ABCA1 (interaction with miR-101 and miR-135b), ABCC1 (miR-199a/b and miR-296), ABCC4 (miR-125a/b), ABCC5 (miR-101, -125a and let-7a), ABCC10 (let-7a/e) and ABCE1 (miR-26a, -135b and -145) (Borel et al., 2012). In addition to miR-101 and miR-135b, experimental data also suggest an interaction between ABCA1 and miR-33, since administration of a locked nucleic acid miRNA antagonist (antimiR) of miR-33 into a non-human primate model resulted in functional de-repression of the ABCA1 level. This finding demonstrates the possibility of modulating the interaction between specific miRNA and mRNA using antimiR, and provides proof of principle for this antimiR-based therapeutic modality (Rottiers et al., 2013).
Besides miR-199a/b and miR-296, ABCC1 transcript is also targeted by miR-326. Overexpressing miR-326 reversed the multidrug resistance phenotype, sensitizing the drug-resistant MCF-7/VP cells to doxorubicin (DOX) (Liang et al., 2010). The ABCC1 transcript was also observed to be targeted by miR-134 and the ABCC1 protein level is decreased by the increased level of miR-134 in drug-resistant H69AR cells (Guo et al., 2010).
Other miRNA–ABC transporter interactions have also been reported. For example, miR-379 interacts with the 3′-UTR of ABCC2 and suppresses ABCC2 transcript level in HepG2 cells (Haenisch et al., 2011). miR-298 overexpression in vitro down-regulated the level of ABCB1, also known as the drug transporter multidrug resistance protein 1/P-glycoprotein (MDR1/P-gp), which leads to breast cancer cell sensitivity to DOX (i.e. decreased the efflux of DOX from cells). miR-298 was found to directly interact with 3′-UTR of ABCB1 transcript (Bao et al., 2011). MiR-451 and miR-27a have also been observed to suppress the expression of ABCB1 and sensitize breast cancer cells to DOX (Kovalchuk et al., 2008). In a display of the complexity of miRNA–mRNA regulatory networks, overexpression of miR-19a/b has been shown to increase the abundance of the ABCB1 transcript, which is likely to be mediated through indirect interactions with other gene expression regulatory factors (Wang et al., 2013a).
Cancer cells can become resistant to chemotherapy by evading miRNA-based control of ADME genes. For example, ABCG2 plays an important role in cellular transport of anti-cancer drugs, such as mitoxantrone, doxorubicin and topotecan, and has been observed to acquire a truncated 3′-UTR in drug-resistant cells relative to the parental cell line (To et al., 2008). The truncated mRNA omits a miR-519c binding site, which mediates a decrease in ABCG2 expression level by miR-519c. This adaptive truncation of the 3′-UTR was later found to be present in several ABCG2-overexpressing cell lines (To et al., 2009). In addition to miR-519c, other miRNAs such as miR-520h and miR-328 have also been found to interact with ABCG2 transcript. The levels of miR-520h and miR-328 are lower in cells expressing ABCG2 relative to ABCG2-negative cells (Wang et al., 2010). Results from a comparative study indicated stronger inhibition of ABCG2 expression in human breast cancer cells by miR-519c and miR-328 than miR-520h (Li et al., 2011).
miRNAs have also been found to affect the absorption and excretion of small molecules through SLC proteins. Folate, nucleoside and amino acid transporters all belong to the SLC transporter family and mediate the uptake of hydrophilic drugs such as gemcitabine and other nucleoside analogues (Candelaria et al., 2010). These levels of membrane transporters are also regulated by miRNA. For example, the expression of SLC15 was found to inversely correlate with miR-92b, which was then demonstrated to reduce SLC15 mRNA, protein and transport activity levels in Caco2-BBE cells (Dalmasso et al., 2011b). Another SLC transporter, serotonin transporter 1 (SERT1, SLC6A4), has been found to be suppressed by miR-16 (Baudry et al., 2010). In silico target prediction identified miR-16 as a potential regulator of SLC6A4. Overexpression of miR-16 in 1C11 cells reduced the SLC6A4 level. Reduction of miR-16 by an anti-miR-16 oligonucleotide resulted in an increase of SLC6A4 level. This interaction has also been demonstrated in vivo: in mouse studies, administration of the selective serotonin reuptake inhibitor fluoxetine was found to increase miR-16 abundance in serotonergic raphe nuclei with a reduced SLC6A4 level (Baudry et al., 2010).
miRNA profiling data identified miR-27a as down-regulated in cisplatin-resistant bladder cancer cell lines, relative to the cisplatin-sensitive lines from which they were derived (Drayton et al., 2014). MiR-27a was found to directly target the cystine/glutamate exchanger – SLC7A11, which forms a heterodimer with SLC3A2 to assemble the xc-cystine-glutamate transporter. This protein complex imports cysteine (as cystine) for the synthesis of GSH, which enables cisplatin detoxification. miR-27a expression was found to inversely correlate with SLC7A11 level in patient samples. Low miR-27a and high SLC7A11 were found to correlate with poor patient prognosis.
MicroRNAs regulate the expression of nuclear receptors
Nuclear receptors play important roles in cellular responses towards environmental stimulation by activating or inactivating the expression of genes, including those that encode drug transporters and biotransformation enzymes. For instance, the retinoid X receptor (RXR) heterodimerizes with the steroid family of orphan nuclear receptors, the constitutive androstane receptor (CAR) or the pregnane X receptor (PXR) to participate in the xenobiotic-mediated transcriptional activation of CYP2B and CYP3A. This type of interaction increases the complexity of the role of miRNAs in regulating ADME activities, since miRNAs may affect the expression of ADME genes via indirect targeting the 3′-UTRs of nuclear receptors (Table 1 and Figure 1). For example, the interactions between miR-27b and miR-125b with the vitamin D (VD) receptor/RXRA alter CYP3A4 expression (Komagata et al., 2009; Mohri et al., 2009). The level of CYP3A4 is also affected by miR-148 through its interaction with PXRs (Takagi et al., 2008).
The PPARα (NR1C1) regulates the expression of a number of ADME genes and its abundance is affected by the levels of miR-21or miR-27b (Kida et al., 2011). An independent study showed that the overexpression of miR-506 also suppresses NR1C1expression, which leads to hydroxycamptothecin resistance in a colon cancer cell line (Tong et al., 2011). Another miRNA-regulated nuclear receptor is liver X receptor α (LXRA/NR1H3), which is closely related to RXR and PPAR, and can be targeted by miR-613. The activation of LXRA resulted in an increased level of miR-613 (Ou et al., 2011). This type of feedback loop probably plays an important part in maintaining proper ADME activity, which is critical for endo- and xenobiotic transport and metabolism.
The oestrogen receptor α (ESR1) and the glucocorticoid receptor (NR3C1) are known to affect the expression of CYP3A4 and ABCG2. MiR-221 and -222 inhibit ESR1 expression. Overexpression of miR-221/222 in a breast cancer cell line resulted in the cells becoming resistant to tamoxifen (an antagonist of the oestrogen receptor) treatment, while knocking down the level of miR-221/222 sensitized the cells (Zhao et al., 2008). A similar effect was also observed with let-7b and let-7i (Y Xhao et al., 2011b). The ESR1 transcript has also been observed to be targeted by miR-206, and ESR1 mRNA abundance negatively correlates with the miR-206 level (Iorio et al., 2005; Adams et al., 2007). To further understand the interaction of miRNA and ESR1 transcript, Picard et al. performed a study on 14 miRNAs, which might be involved in ESR1expression and found that miR-22 exerted the strongest inhibition of oestrogen signalling through targeting ESR mRNA (Pandey and Picard, 2009).
Effects of epigenetic factors on miRNA expression
Even though numerous studies showed the effects of specific miRNAs on ADME-related transcripts, the levels of individual miRNA sequences are affected by intrinsic and extrinsic factors. Like other transcripts, the expression of miRNAs can be affected by epigenetic factors, such as DNA methylation. For example, rapid changes to miRNA levels have been observed upon treating cells with histone deacetylase (HDAC) inhibitors (Scott et al., 2006). Alterations to miRNA promoter methylation status are associated with disease conditions. For example, the promoter of the tumour suppressor miR-125b is hyper-methylated in human breast cancer, leading to a lower level of miR-125b and the de-repression of its target transcription factor, ETS1 (V-Ets avian erythroblastosis virus E26 oncogene homologue 1) (Zhang et al., 2011). In addition, the promoter of the metastasis suppressor miR-355 is also hyper-methylated in breast cancer (Png et al., 2011). Hypo-methylation has also been observed to alter miRNA expression, as let-7a-3 has been found to be heavily methylated in normal human tissue, but hypo-methylated in some human lung cancers (Brueckner et al., 2007).
In a display of further complexity, miRNAs can reciprocally influence epigenetic states by targeting transcripts that encode enzymes involved in DNA methylation and histone modifications (Table 2). Several HDACs have been observed by reporter assays to be regulated by different miRNAs (Scott et al., 2006; H Li et al., 2009a; Sato et al., 2011; Wang et al., 2013b). In addition, some polycomb proteins that are involved in the remodelling of chromatin structure are also regulated by miRNAs. For example, the expression of the histone methyltransferase EZH2 is repressed by miR-101 (Varambally et al., 2008).
Table 2.
List of microRNAs affecting enzymes involved in epigenetic modifications
| Gene ID | Gene description | Interacting miRNA | References |
|---|---|---|---|
| DNMT1 | DNA (cytosine-5-)-methyltransferase 1 | miR-126, -152, -185, -148, -140, -342 | Wang et al., 2011; S Zhao et al., 2011a; Takata et al., 2013; Azizi et al., 2014; Xiang et al., 2014 |
| DNMT3A | DNA (cytosine-5-)-methyltransferase 3 alpha | miR-29b, -143, -199a, -370 | Fabbri et al., 2007; Garzon et al., 2009; Ng et al., 2009; 2014,; Qi et al., 2013; Chen et al., 2014 |
| DNMT3B | DNA (cytosine-5-)-methyltransferase 3 beta | miR-29, -148, -495 | Fabbri et al., 2007; Duursma et al., 2008; Garzon et al., 2009; Yang et al., 2014 |
| HDAC1 | Histone deacetylase 1 | miR-34, -449, -520h | Noonan et al., 2009; Zhao et al., 2013; Shen et al., 2014 |
| HDAC2 | Histone deacetylase 2 | miR-145 | Noh et al., 2013 |
| HDAC4 | Histone deacetylase 4 | miR-1, -155, -365 | Chen et al., 2006; Guan et al., 2011; Sandhu et al., 2012 |
| HDAC5 | Histone deacetylase 5 | miR-2861 | H Li et al., 2009a |
| HDAC6 | Histone deacetylase 6 | miR-433 | Simon et al., 2010 |
| EZH2 | Enhancer of zeste homologue 2 | miR-26a, -26b, -101, -214, -124, -181a, -210, -424, -138 | Sander et al., 2008; Varambally et al., 2008; Juan et al., 2009; Zhang et al., 2013; Overhoff et al., 2014; Xie et al., 2014 |
| BMI1 | B lymphoma Mo-MLV insertion region 1 homologue | miR-200c, -203 -183, -128 | Godlewski et al., 2008; Wellner et al., 2009 |
Influence of xenobiotic drugs on miRNA expression
miRNA expression also can be affected by exposure to xenobiotics. For example, the abundances of a number of miRNAs (miR-27a, -148a, -124a and miR-451) in MCF-7, Caco2, SH-SY5Y and BE(2)-M17 cell lines were affected by exposing the cells to common pharmacological agents, including dexamethasone, vinblastine, bilobalide and cocaine (Rodrigues et al., 2011). As miRNAs regulate drug metabolizing enzymes and transporters, this might lead to considerable changes in the PK properties of the drug itself.
Suppression of miRNAs that control drug metabolism and disposition may explain changes in the expression of efflux transporters (Yu, 2009). For instance, the induction of CYP3A4 and ABCB1 by dexamethasone functions at least partially through the suppression of miR-27b, -451 and -148a, which may interact with CYP3A4 and ABCB1 transcripts (Rodrigues et al., 2011). Vinblastine reduces the levels of miR-27a/b, -324-3p, -328, -148a and -451, which may cause the increase of ABCC1 expression in MCF-7 cells (Schrenk et al., 2001), ABCB1 in LS-180 cells (Harmsen et al., 2010) and CYP3A4 in HepG2 cells (Smith et al., 2010). Another example is the brain-specific miR-124a, a major regulator of neuronal identity (Conaco et al., 2006; Maisel et al., 2010). The abundance of this miRNA was reduced by psychoactive drugs (cocaine, methadone and fluoxetine), which might present a mechanism of neuroplasticity in response to xenobiotic agents.
Xenobiotic drugs may act on other proteins responsible for miRNA processing, leading to an altered expression of mature miRNAs (Shan et al., 2008; Melo et al., 2011). For example, enoxacin, a broad-spectrum antibacterial fluoroquinolone, enhanced endogenous miRNA production, including tumour suppressor miRNAs, by targeting the pre-miRNA processing protein TAR RNA-binding protein 2 (Melo et al., 2011).
Host miRNA expression changes in response to the gut microbiome
The gastrointestinal tract contains a broad spectrum of microbial species that modulate the utilization of xenobiotics beyond the capability encoded in the host genome (Kau et al., 2011; Haiser and Turnbaugh, 2013). Alterations to the microbiome population can influence the therapeutic efficacy of drugs (Viaud et al., 2013). In addition, microbes can also contribute to dose-limiting toxicity of other agents, an undesirable effect that can be relieved by inhibition of bacterial β-glucuronidases (Wallace et al., 2010).
The gut microbiome can also affect host miRNA expression, which may modulate the levels of various ADME genes and affect the ADME system. Comparison of the colon miRNA profiles of germ-free and control mice identified several dys-regulated miRNA species, including miR-128, -200C*, -342-5p, -465c-5p, -466d-3p, -466d-5p, -665 and -683 (Dalmasso et al., 2011a). Using various experimental tools including luciferase assays and immunoblotting, it has been shown that the ABCC3, a cell surface transporter, is a direct target of miR-665. Microbiome inoculation down-regulated miR-665 level, which, in turn, increased the level of ABCC3 in enterocytes at both the mRNA and the protein levels (Dalmasso et al., 2011a). Although the exact effects of the changes of ABCC3 level are yet to be determined, the biliary transport and excretion of organic anions are most likely to be affected. This finding demonstrates the complex interactions between gut microbiome and xenobiotic metabolism. The interaction is not just directly at chemical levels but also involves complex gene/protein networks through regulatory factors such as miRNA and other epigenetic factors.
Conclusions and future prospects
Variation between individuals in response to therapeutic agents is often attributed to the differences in host genetic factors. However, our knowledge of other contributors including epigenetic factors and microbiome dimensions is rapidly increasing. miRNA plays an important role in various physiopathological processes and substantial evidence is accumulating for its involvement in ADME. However, miRNA-mediated regulatory process is complex; although a number of computational algorithms have been developed to predict miRNA–mRNA interactions (Enright et al., 2003; Lewis et al., 2005), the accuracy of these predicted interactions is limited. In addition, most biological studies have been performed in vitro with only limited in vivo complementation. The precise physiologically relevant effects of miRNAs on ADME remains unclear and further study is required to generate detailed, highly substantiated empirical interaction networks to realize their diagnostic and therapeutic potential.
The recent realization of the complexity of the gut microbiome and its capacity to manipulate xenobiotics provides a new front in the study of drug metabolism and its effects on miRNA expression. Even though germ-free animal models provide some important insights on the effect of gut microbiome on host gene and miRNA expression, the host–microbiome interaction is complex and remains to be deciphered. Systems biology seeks to integrate results from different high-throughput profiling technologies to understand the dynamic changes of a biological system and predict its responses to various inputs. Using this approach to study the effects of epigenetic factors and microbiome on drug metabolism would provide a more comprehensive view on how different parts of the system interact with each other. A better understanding of factors affecting drug metabolism will be integral to personalized medicine, as it would provide guidance on drug development, increase the therapeutic efficacy, tailor specific treatment strategies for individuals and reduce adverse effects.
Acknowledgments
This study was supported by grants from the National Science Foundation of China 81201223 (Y. H.), Science and Technology Program of Guangdong 2013B051000080 (Y. H.), International Science & Technology Cooperation Program of Dongguan 2013508152005 (Y. H.), Guangdong Medical College Research Startup Fund B2013001 (Y. H.) and Guangdong Medical College Integration of Industry, Education and Research C2013004 (Y. H.), research contracts from US Department of Defense W911NF-10-2-0111 and HDTRA1-13-C-0055 and Center for Systems Biology of the National Institutes of Health P50GM076547 (K. W., T. K. K. and J. R. C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- ABC
ATP-binding cassette
- ABCB1/MDR1/P-gp
multidrug resistance protein 1/P-glycoprotein
- ADME
absorption, distribution, metabolism, excretion
- antimiR
miRNA antagonist
- CYP450
cytochrome P450
- DOX
doxorubicin
- ESR1
oestrogen receptor alpha
- ETS1
V-Ets avian erythroblastosis virus E26 oncogene homologue 1
- GPX7
glutathione peroxidase 7
- GSS
glutathione synthetase
- HDAC
histone deacetylase
- LXRA
liver X receptor α
- miRNA
microRNA
- mRNA
messenger RNA
- ncRNA
non-coding RNA
- NR1C1
PPARα
- PK
pharmacokinetic
- PXR
pregnane X receptor
- RISC
RNA-induced silencing complex
- RXR
retinoid X receptor
- SLC
solute carrier
- UGT
UDP-glucuronosyltransferase
- UTR
untranslated region
- VD receptor
vitamin D receptor
Conflict of interest
The authors declare no conflict of interest.
References
- Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21:1132–1147. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013a;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol. 2013b;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013c;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RM, Marquart TJ, Albert CJ, Suchy FJ, Wang DQ, Ananthanarayanan M, et al. miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol Med. 2012;4:882–895. doi: 10.1002/emmm.201201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomar MJ. Factors affecting the development of adverse drug reactions (Review article) Saudi Pharm J. 2014;22:83–94. doi: 10.1016/j.jsps.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- Azizi M, Teimoori-Toolabi L, Arzanani MK, Azadmanesh K, Fard-Esfahani P, Zeinali S. MicroRNA-148b and microRNA-152 reactivate tumor suppressor genes through suppression of DNA methyltransferase-1 gene in pancreatic cancer cell lines. Cancer Biol Ther. 2014;15:419–427. doi: 10.4161/cbt.27630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Azzouzi H, Leptidis S, Dirkx E, Hoeks J, van Bree B, Brand K, et al. The hypoxia-inducible microRNA cluster miR-199a∼214 targets myocardial PPARδ and impairs mitochondrial fatty acid oxidation. Cell Metab. 2013;18:341–354. doi: 10.1016/j.cmet.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Bao L, Haque A, Jackson K, Hazari S, Moroz K, Jetly R, et al. Increased expression of P-glycoprotein is associated with doxorubicin chemoresistance in the metastatic 4T1 breast cancer model. Am J Pathol. 2011;178:838–852. doi: 10.1016/j.ajpath.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Hazari S, Mehra S, Kaushal D, Moroz K, Dash S. Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. Am J Pathol. 2012;180:2490–2503. doi: 10.1016/j.ajpath.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Belanger A, Pelletier G, Labrie F, Barbier O, Chouinard S. Inactivation of androgens by UDP-glucuronosyltransferase enzymes in humans. Trends Endocrinol Metab. 2003;14:473–479. doi: 10.1016/j.tem.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Bell LC, Wang J. Probe ADME and test hypotheses: a PATH beyond clearance in vitro–in vivo correlations in early drug discovery. Expert Opin Drug Metab Toxicol. 2012;8:1131–1155. doi: 10.1517/17425255.2012.695346. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitarte N, Bandres E, Boni V, Zarate R, Rodriguez J, Gonzalez-Huarriz M, et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells. 2011;29:1661–1671. doi: 10.1002/stem.741. [DOI] [PubMed] [Google Scholar]
- Borel F, Han R, Visser A, Petry H, van Deventer SJ, Jansen PL, et al. Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology. 2012;55:821–832. doi: 10.1002/hep.24682. [DOI] [PubMed] [Google Scholar]
- Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, et al. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem. 1994;269:17960–17964. [PubMed] [Google Scholar]
- Brueckner B, Stresemann C, Kuner R, Mund C, Musch T, Meister M, et al. The human let-7a-3 locus contains an epigenetically regulated microRNA gene with oncogenic function. Cancer Res. 2007;67:1419–1423. doi: 10.1158/0008-5472.CAN-06-4074. [DOI] [PubMed] [Google Scholar]
- Caldwell GW, Yan Z, Tang W, Dasgupta M, Hasting B. ADME optimization and toxicity assessment in early- and late-phase drug discovery. Curr Top Med Chem. 2009;9:965–980. doi: 10.2174/156802609789630929. [DOI] [PubMed] [Google Scholar]
- Candelaria M, de la Cruz-Hernandez E, Perez-Cardenas E, Trejo-Becerril C, Gutierrez-Hernandez O, Duenas-Gonzalez A. Pharmacogenetics and pharmacoepigenetics of gemcitabine. Med Oncol. 2010;27:1133–1143. doi: 10.1007/s12032-009-9349-y. [DOI] [PubMed] [Google Scholar]
- Chen BF, Gu S, Suen YK, Li L, Chan WY. microRNA-199a-3p, DNMT3A, and aberrant DNA methylation in testicular cancer. Epigenetics. 2014;9:119–128. doi: 10.4161/epi.25799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Chen C, Yang S, Gong W, Wang Y, Cianflone K, et al. Let-7b inhibits human cancer phenotype by targeting cytochrome P450 epoxygenase 2J2. PLoS ONE. 2012a;7:e39197. doi: 10.1371/journal.pone.0039197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tian W, Cai H, He H, Deng Y. Down-regulation of microRNA-200c is associated with drug resistance in human breast cancer. Med Oncol. 2012b;29:2527–2534. doi: 10.1007/s12032-011-0117-4. [DOI] [PubMed] [Google Scholar]
- Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuturgoon AA, Phulukdaree A, Moodley D. Fumonisin B(1) modulates expression of human cytochrome P450 1b1 in human hepatoma (Hepg2) cells by repressing Mir-27b. Toxicol Lett. 2014;227:50–55. doi: 10.1016/j.toxlet.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Ayyadurai S, et al. Microbiota modulate host gene expression via microRNAs. PLoS ONE. 2011a;6:e19293. doi: 10.1371/journal.pone.0019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Obertone TS, et al. MicroRNA-92b regulates expression of the oligopeptide transporter PepT1 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011b;300:G52–G59. doi: 10.1152/ajpgi.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg LO, Edenberg HJ. Epigenetics of gene expression in human hepatoma cells: expression profiling the response to inhibition of DNA methylation and histone deacetylation. BMC Genomics. 2006;7:181. doi: 10.1186/1471-2164-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DF, Sun D, Salzberg AC, Jones N, Bushey RT, Robertson GP, et al. Regulation of UDP-glucuronosyltransferase 1A1 expression and activity by microRNA 491-3p. J Pharmacol Exp Ther. 2014;348:465–477. doi: 10.1124/jpet.113.210658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton RM, Peter S, Myers K, Miah S, Dudziec E, Bryant HE, et al. MicroRNA-99a and 100 mediated upregulation of FOXA1 in bladder cancer. Oncotarget. 2014;5:6375–6386. doi: 10.18632/oncotarget.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duursma AM, Kedde M, Schrier M, le Sage C, Agami R. miR-148 targets human DNMT3b protein coding region. RNA. 2008;14:872–877. doi: 10.1261/rna.972008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto C, Murayama N, Rostami-Hodjegan A, Yamazaki H. Methodologies for investigating drug metabolism at the early drug discovery stage: prediction of hepatic drug clearance and P450 contribution. Curr Drug Metab. 2010;11:678–685. doi: 10.2174/138920010794233503. [DOI] [PubMed] [Google Scholar]
- Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Evrard A, Mbatchi L. Genetic polymorphisms of drug metabolizing enzymes and transporters: the long way from bench to bedside. Curr Top Med Chem. 2012;12:1720–1729. doi: 10.2174/156802612803531388. [DOI] [PubMed] [Google Scholar]
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Kozaki KI, Tanaka S, Arii S, Imoto I, Inazawa J. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2010;31:766–776. doi: 10.1093/carcin/bgp250. [DOI] [PubMed] [Google Scholar]
- Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen AE, Gosalia N, Leir SH, Harris A. MicroRNA regulation of expression of the cystic fibrosis transmembrane conductance regulator gene. Biochem J. 2011;438:25–32. doi: 10.1042/BJ20110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glubb DM, Innocenti F. Mechanisms of genetic regulation in gene expression: examples from drug metabolizing enzymes and transporters. Wiley Interdiscip Rev Syst Biol Med. 2011;3:299–313. doi: 10.1002/wsbm.125. [DOI] [PubMed] [Google Scholar]
- Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, et al. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68:9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- Goedeke L, Vales-Lara FM, Fenstermaker M, Cirera-Salinas D, Chamorro-Jorganes A, Ramirez CM, et al. A regulatory role for microRNA 33* in controlling lipid metabolism gene expression. Mol Cell Biol. 2013;33:2339–2352. doi: 10.1128/MCB.01714-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36(Database issue):D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan YJ, Yang X, Wei L, Chen Q. MiR-365: a mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J. 2011;25:4457–4466. doi: 10.1096/fj.11-185132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Liu Y, Bai Y, Sun Y, Xiao F, Guo Y. Gene expression profiling of drug-resistant small cell lung cancer cells by combining microRNA and cDNA expression analysis. Eur J Cancer. 2010;46:1692–1702. doi: 10.1016/j.ejca.2010.02.043. [DOI] [PubMed] [Google Scholar]
- Haenisch S, Laechelt S, Bruckmueller H, Werk A, Noack A, Bruhn O, et al. Down-regulation of ATP-binding cassette C2 protein expression in HepG2 cells after rifampicin treatment is mediated by microRNA-379. Mol Pharmacol. 2011;80:314–320. doi: 10.1124/mol.110.070714. [DOI] [PubMed] [Google Scholar]
- Haiser HJ, Turnbaugh PJ. Developing a metagenomic view of xenobiotic metabolism. Pharmacol Res. 2013;69:21–31. doi: 10.1016/j.phrs.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Liehr JG. DNA single-strand breaks in kidneys of Syrian hamsters treated with steroidal estrogens: hormone-induced free radical damage preceding renal malignancy. Carcinogenesis. 1994;15:997–1000. doi: 10.1093/carcin/15.5.997. [DOI] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Febus CL, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemother Pharmacol. 2010;66:765–771. doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- Ivanov M, Kacevska M, Ingelman-Sundberg M. Epigenomics and interindividual differences in drug response. Clin Pharmacol Ther. 2012;92:727–736. doi: 10.1038/clpt.2012.152. [DOI] [PubMed] [Google Scholar]
- Jennewein C, von Knethen A, Schmid T, Brune B. MicroRNA-27b contributes to lipopolysaccharide-mediated peroxisome proliferator-activated receptor gamma (PPARgamma) mRNA destabilization. J Biol Chem. 2010;285:11846–11853. doi: 10.1074/jbc.M109.066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HM, Sohn YW, Oh SY, Kim SH, Beck S, Kim S, et al. ID4 imparts chemoresistance and cancer stemness to glioma cells by derepressing miR-9*-mediated suppression of SOX2. Cancer Res. 2011;71:3410–3421. doi: 10.1158/0008-5472.CAN-10-3340. [DOI] [PubMed] [Google Scholar]
- Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583:759–766. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Jiao X, Zhao L, Ma M, Bai X, He M, Yan Y, et al. MiR-181a enhances drug sensitivity in mitoxantone-resistant breast cancer cells by targeting breast cancer resistance protein (BCRP/ABCG2) Breast Cancer Res Treat. 2013;139:717–730. doi: 10.1007/s10549-013-2607-x. [DOI] [PubMed] [Google Scholar]
- Juan AH, Kumar RM, Marx JG, Young RA, Sartorelli V. Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells. Mol Cell. 2009;36:61–74. doi: 10.1016/j.molcel.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacevska M, Ivanov M, Wyss A, Kasela S, Milani L, Rane A, et al. DNA methylation dynamics in the hepatic CYP3A4 gene promoter. Biochimie. 2012;94:2338–2344. doi: 10.1016/j.biochi.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Kalscheuer S, Zhang X, Zeng Y, Upadhyaya P. Differential expression of microRNAs in early-stage neoplastic transformation in the lungs of F344 rats chronically treated with the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 2008;29:2394–2399. doi: 10.1093/carcin/bgn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, Zhang LH, Wijesekara N, de Haan W, Butland S, Bhattacharjee A, et al. Regulation of ABCA1 protein expression and function in hepatic and pancreatic islet cells by miR-145. Arterioscler Thromb Vasc Biol. 2013;33:2724–2732. doi: 10.1161/ATVBAHA.113.302004. [DOI] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellis M, Wold B, Snyder MP, Bernstein BE, Kundaje A, Marinov GK, et al. Defining functional DNA elements in the human genome. Proc Natl Acad Sci U S A. 2014;111:6131–6138. doi: 10.1073/pnas.1318948111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida K, Nakajima M, Mohri T, Oda Y, Takagi S, Fukami T, et al. PPARα is regulated by miR-21 and miR-27b in human liver. Pharm Res. 2011;28:2467–2476. doi: 10.1007/s11095-011-0473-y. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon H, Ramirez CM, Lee SM, Hoe HS, Fernandez-Hernando C. MiR-106b impairs cholesterol efflux and increases Aβ levels by repressing ABCA1 expression. Exp Neurol. 2012;235:476–483. doi: 10.1016/j.expneurol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, et al. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun. 2010;392:323–328. doi: 10.1016/j.bbrc.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nature reviews. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Komagata S, Nakajima M, Takagi S, Mohri T, Taniya T, Yokoi T. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol Pharmacol. 2009;76:702–709. doi: 10.1124/mol.109.056986. [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, et al. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Kumar P, Luo Y, Tudela C, Alexander JM, Mendelson CR. The c-Myc-regulated microRNA-17∼92 (miR-17∼92) and miR-106a∼363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol Cell Biol. 2013;33:1782–1796. doi: 10.1128/MCB.01228-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Drakaki A, Iliopoulos D, Struhl K. MiR-27b targets PPARγ to inhibit growth, tumor progression and the inflammatory response in neuroblastoma cells. Oncogene. 2012;31:3818–3825. doi: 10.1038/onc.2011.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lewis DF. 57 varieties: the human cytochromes P450. Pharmacogenomics. 2004;5:305–318. doi: 10.1517/phgs.5.3.305.29827. [DOI] [PubMed] [Google Scholar]
- Li H, Xie H, Liu W, Hu R, Huang B, Tan YF, et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest. 2009a;119:3666–3677. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KK, Pang JC, Ching AK, Wong CK, Kong X, Wang Y, et al. miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum Pathol. 2009b;40:1234–1243. doi: 10.1016/j.humpath.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, Yu AM. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328, -519c and -520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol. 2011;81:783–792. doi: 10.1016/j.bcp.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K, et al. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol. 2010;79:817–824. doi: 10.1016/j.bcp.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Liao R, Sun J, Zhang L, Lou G, Chen M, Zhou D, et al. MicroRNAs play a role in the development of human hematopoietic stem cells. J Cell Biochem. 2008;104:805–817. doi: 10.1002/jcb.21668. [DOI] [PubMed] [Google Scholar]
- Liu N, Pan T. RNA epigenetics. Transl Res. 2014 doi: 10.1016/j.trsl.2014.04.003. doi: 10.1016/j.trsl.2014.04.003; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma MT, He M, Wang Y, Jiao XY, Zhao L, Bai XF, et al. MiR-487a resensitizes mitoxantrone (MX)-resistant breast cancer cells (MCF-7/MX) to MX by targeting breast cancer resistance protein (BCRP/ABCG2) Cancer Lett. 2013;339:107–115. doi: 10.1016/j.canlet.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, et al. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15:677–685. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- Maisel M, Habisch HJ, Royer L, Herr A, Milosevic J, Hermann A, et al. Genome-wide expression profiling and functional network analysis upon neuroectodermal conversion of human mesenchymal stem cells suggest HIF-1 and miR-124a as important regulators. Exp Cell Res. 2010;316:2760–2778. doi: 10.1016/j.yexcr.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Markova SM, Kroetz DL. ABCC4 is regulated by microRNA-124a and microRNA-506. Biochem Pharmacol. 2014;87:515–522. doi: 10.1016/j.bcp.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo S, Villanueva A, Moutinho C, Davalos V, Spizzo R, Ivan C, et al. Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing. Proc Natl Acad Sci U S A. 2011;108:4394–4399. doi: 10.1073/pnas.1014720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miko E, Margitai Z, Czimmerer Z, Varkonyi I, Dezso B, Lanyi A, et al. miR-126 inhibits proliferation of small cell lung cancer cells by targeting SLC7A5. FEBS Lett. 2011;585:1191–1196. doi: 10.1016/j.febslet.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Mohri T, Nakajima M, Takagi S, Komagata S, Yokoi T. MicroRNA regulates human vitamin D receptor. Int J Cancer. 2009;125:1328–1333. doi: 10.1002/ijc.24459. [DOI] [PubMed] [Google Scholar]
- Mohri T, Nakajima M, Fukami T, Takamiya M, Aoki Y, Yokoi T. Human CYP2E1 is regulated by miR-378. Biochem Pharmacol. 2010;79:1045–1052. doi: 10.1016/j.bcp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Moya PR, Wendland JR, Salemme J, Fried RL, Murphy DL. miR-15a and miR-16 regulate serotonin transporter expression in human placental and rat brain raphe cells. Int J Neuropsychopharmacol. 2013;16:621–629. doi: 10.1017/S1461145712000454. [DOI] [PubMed] [Google Scholar]
- Murray GI, Taylor MC, McFadyen MC, McKay JA, Greenlee WF, Burke MD, et al. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997;57:3026–3031. [PubMed] [Google Scholar]
- Nagar S, Remmel RP. Uridine diphosphoglucuronosyltransferase pharmacogenetics and cancer. Oncogene. 2006;25:1659–1672. doi: 10.1038/sj.onc.1209375. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Aoyama T. Polymorphism of drug-metabolizing enzymes in relation to individual susceptibility to industrial chemicals. Ind Health. 2000;38:143–152. doi: 10.2486/indhealth.38.143. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–237. [PubMed] [Google Scholar]
- Ng EK, Tsang WP, Ng SS, Jin HC, Yu J, Li JJ, et al. MicroRNA-143 targets DNA methyltransferases 3A in colorectal cancer. Br J Cancer. 2009;101:699–706. doi: 10.1038/sj.bjc.6605195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng EK, Li R, Shin VY, Siu JM, Ma ES, Kwong A. MicroRNA-143 is downregulated in breast cancer and regulates DNA methyltransferases 3A in breast cancer cells. Tumour Biol. 2014;35:2591–2598. doi: 10.1007/s13277-013-1341-7. [DOI] [PubMed] [Google Scholar]
- Noh JH, Chang YG, Kim MG, Jung KH, Kim JK, Bae HJ, et al. MiR-145 functions as a tumor suppressor by directly targeting histone deacetylase 2 in liver cancer. Cancer Lett. 2013;335:455–462. doi: 10.1016/j.canlet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Pookot D, Basak S, Whitson JM, Hirata H, et al. miR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene. 2009;28:1714–1724. doi: 10.1038/onc.2009.19. [DOI] [PubMed] [Google Scholar]
- Ou Z, Wada T, Gramignoli R, Li S, Strom SC, Huang M, et al. MicroRNA hsa-miR-613 targets the human LXRα gene and mediates a feedback loop of LXRα autoregulation. Mol Endocrinol. 2011;25:584–596. doi: 10.1210/me.2010-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overhoff MG, Garbe JC, Koh J, Stampfer MR, Beach DH, Bishop CL. Cellular senescence mediated by p16INK4A-coupled miRNA pathways. Nucleic Acids Res. 2014;42:1606–1618. doi: 10.1093/nar/gkt1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan R, Chen KG, Gillet JP, Handley M, Mallon BS, Hamilton RS, et al. Regulation and expression of the ATP-binding cassette transporter ABCG2 in human embryonic stem cells. Stem Cells. 2012;30:2175–2187. doi: 10.1002/stem.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, Gao W, Yu AM. MicroRNAs regulate CYP3A4 expression via direct and indirect targeting. Drug Metab Dispos. 2009a;37:2112–2117. doi: 10.1124/dmd.109.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol. 2009b;75:1374–1379. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, Zhou A, Hu Z, Yu AM. Small nucleolar RNA-derived microRNA hsa-miR-1291 modulates cellular drug disposition through direct targeting of ABC transporter ABCC1. Drug Metab Dispos. 2013;41:1744–1751. doi: 10.1124/dmd.113.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey DP, Picard D. miR-22 inhibits estrogen signaling by directly targeting the estrogen receptor alpha mRNA. Mol Cell Biol. 2009;29:3783–3790. doi: 10.1128/MCB.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333:19–39. doi: 10.1016/s0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Belkhiri A, Hu T, Chaturvedi R, Asim M, Wilson KT, et al. Glutathione peroxidase 7 protects against oxidative DNA damage in oesophageal cells. Gut. 2012;61:1250–1260. doi: 10.1136/gutjnl-2011-301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, et al. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Plant NJ, Gibson GG. Evaluation of the toxicological relevance of CYP3A4 induction. Curr Opin Drug Discov Devel. 2003;6:50–56. [PubMed] [Google Scholar]
- Png KJ, Yoshida M, Zhang XH, Shu W, Lee H, Rimner A, et al. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev. 2011;25:226–231. doi: 10.1101/gad.1974211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogribny IP, Filkowski JN, Tryndyak VP, Golubov A, Shpyleva SI, Kovalchuk O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer. 2010;127:1785–1794. doi: 10.1002/ijc.25191. [DOI] [PubMed] [Google Scholar]
- Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA. miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of monocarboxylate transporter 1 (Mct1) Mol Cell Biol. 2011;31:3182–3194. doi: 10.1128/MCB.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Hongjuan H, Ning G, Zhengbin H, Yanjiang X, Tiebo Z, et al. miR-370 is stage-specifically expressed during mouse embryonic development and regulates Dnmt3a. FEBS Lett. 2013;587:775–781. doi: 10.1016/j.febslet.2013.01.070. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Karp PH, Osterhaus SR, Jiang P, Wohlford-Lenane C, Lennox KA, et al. Post-transcriptional regulation of cystic fibrosis transmembrane conductance regulator expression and function by microRNAs. Am J Respir Cell Mol Biol. 2013;49:544–551. doi: 10.1165/rcmb.2012-0430OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez CM, Davalos A, Goedeke L, Salerno AG, Warrier N, Cirera-Salinas D, et al. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler Thromb Vasc Biol. 2011;31:2707–2714. doi: 10.1161/ATVBAHA.111.232066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AC, Li X, Radecki L, Pan YZ, Winter JC, Huang M, et al. MicroRNA expression is differentially altered by xenobiotic drugs in different human cell lines. Biopharm Drug Dispos. 2011;32:355–367. doi: 10.1002/bdd.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14(10A):1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers V, Obad S, Petri A, McGarrah R, Lindholm MW, Black JC, et al. Pharmacological inhibition of a microRNA family in nonhuman primates by a seed-targeting 8-mer antimiR. Sci Transl Med. 2013;5:212ra162. doi: 10.1126/scitranslmed.3006840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland A, Miners JO, Mackenzie PI. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol. 2013;45:1121–1132. doi: 10.1016/j.biocel.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TF, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112:4202–4212. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Volinia S, Costinean S, Galasso M, Neinast R, Santhanam R, et al. miR-155 targets histone deacetylase 4 (HDAC4) and impairs transcriptional activity of B-cell lymphoma 6 (BCL6) in the Emu-miR-155 transgenic mouse model. Proc Natl Acad Sci U S A. 2012;109:20047–20052. doi: 10.1073/pnas.1213764109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- Schrenk D, Baus PR, Ermel N, Klein C, Vorderstemann B, Kauffmann HM. Up-regulation of transporters of the MRP family by drugs and toxins. Toxicol Lett. 2001;120:51–57. doi: 10.1016/s0378-4274(01)00306-x. [DOI] [PubMed] [Google Scholar]
- Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 2006;66:1277–1281. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- Seitz H, Royo H, Bortolin ML, Lin SP, Ferguson-Smith AC, Cavaille J. A large imprinted microRNA gene cluster at the mouse Dlk1-Gtl2 domain. Genome Res. 2004;14:1741–1748. doi: 10.1101/gr.2743304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan G, Li Y, Zhang J, Li W, Szulwach KE, Duan R, et al. A small molecule enhances RNA interference and promotes microRNA processing. Nat Biotechnol. 2008;26:933–940. doi: 10.1038/nbt.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Yao Q, Sun J, Feng L, Lu H, Ma Y, et al. Downregulation of histone deacetylase 1 by microRNA-520h contributes to the chemotherapeutic effect of doxorubicin. FEBS Lett. 2014;588:184–191. doi: 10.1016/j.febslet.2013.11.034. [DOI] [PubMed] [Google Scholar]
- Shibahara Y, Miki Y, Onodera Y, Hata S, Chan MS, Yiu CC, et al. Aromatase inhibitor treatment of breast cancer cells increases the expression of let-7f, a microRNA targeting CYP19A1. J Pathol. 2012;227:357–366. doi: 10.1002/path.4019. [DOI] [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, et al. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- Simon D, Laloo B, Barillot M, Barnetche T, Blanchard C, Rooryck C, et al. A mutation in the 3'-UTR of the HDAC6 gene abolishing the post-transcriptional regulation mediated by hsa-miR-433 is linked to a new form of dominant X-linked chondrodysplasia. Hum Mol Genet. 2010;19:2015–2027. doi: 10.1093/hmg/ddq083. [DOI] [PubMed] [Google Scholar]
- Smith NF, Mani S, Schuetz EG, Yasuda K, Sissung TM, Bates SE, et al. Induction of CYP3A4 by vinblastine: role of the nuclear receptor NR1I2. Ann Pharmacother. 2010;44:1709–1717. doi: 10.1345/aph.1P354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoke JE, Bloch K. Studies on the mechanism of action of glutathione synthetase. J Biol Chem. 1955;213:825–835. [PubMed] [Google Scholar]
- Song KH, Li T, Owsley E, Chiang JY. A putative role of micro RNA in regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes. J Lipid Res. 2010;51:2223–2233. doi: 10.1194/jlr.M004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nature reviews. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- Takagi S, Nakajima M, Mohri T, Yokoi T. Post-transcriptional regulation of human pregnane X receptor by micro-RNA affects the expression of cytochrome P450 3A4. J Biol Chem. 2008;283:9674–9680. doi: 10.1074/jbc.M709382200. [DOI] [PubMed] [Google Scholar]
- Takagi S, Nakajima M, Kida K, Yamaura Y, Fukami T, Yokoi T. MicroRNAs regulate human hepatocyte nuclear factor 4alpha, modulating the expression of metabolic enzymes and cell cycle. J Biol Chem. 2010;285:4415–4422. doi: 10.1074/jbc.M109.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Oda Y, Toyoda Y, Fukami T, Yokoi T, Nakajima M. Regulation of cytochrome b5 expression by miR-223 in human liver: effects on cytochrome P450 activities. Pharm Res. 2014;31:780–794. doi: 10.1007/s11095-013-1200-7. [DOI] [PubMed] [Google Scholar]
- Takata A, Otsuka M, Yoshikawa T, Kishikawa T, Hikiba Y, Obi S, et al. MicroRNA-140 acts as a liver tumor suppressor by controlling NF-κB activity by directly targeting DNA methyltransferase 1 (Dnmt1) expression. Hepatology. 2013;57:162–170. doi: 10.1002/hep.26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Takigahira M, Mihara K, Tarumi Y, Yanagihara K. The metastasis-associated microRNA miR-516a-3p is a novel therapeutic target for inhibiting peritoneal dissemination of human scirrhous gastric cancer. Cancer Res. 2011;71:1442–1453. doi: 10.1158/0008-5472.CAN-10-2530. [DOI] [PubMed] [Google Scholar]
- Tamarapu Parthasarathy P, Galam L, Huynh B, Yunus A, Abuelenen T, Castillo A, et al. MicroRNA 16 modulates epithelial sodium channel in human alveolar epithelial cells. Biochem Biophys Res Commun. 2012;426:203–208. doi: 10.1016/j.bbrc.2012.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Pu Y, Wong DK, Liu T, Tang H, Xiang T, et al. The hepatitis B virus-associated estrogen receptor alpha (ERalpha) was regulated by microRNA-130a in HepG2.2.15 human hepatocellular carcinoma cells. Acta Biochim Biophys Sin (Shanghai) 2011;43:640–646. doi: 10.1093/abbs/gmr051. [DOI] [PubMed] [Google Scholar]
- Thomson DW, Bracken CP, Goodall GJ. Experimental strategies for microRNA target identification. Nucleic Acids Res. 2011;39:6845–6853. doi: 10.1093/nar/gkr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulin P, Wei T, Werngren O, Cheung L, Fisher RM, Grander D, et al. MicroRNA-9 regulates the expression of peroxisome proliferator-activated receptor δ in human monocytes during the inflammatory response. Int J Mol Med. 2013;31:1003–1010. doi: 10.3892/ijmm.2013.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tili E, Michaille JJ, Luo Z, Volinia S, Rassenti LZ, Kipps TJ, et al. The down-regulation of miR-125b in chronic lymphocytic leukemias leads to metabolic adaptation of cells to a transformed state. Blood. 2012;120:2631–2638. doi: 10.1182/blood-2012-03-415737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 expression at the 3’ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol Cell Biol. 2008;28:5147–5161. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK, Robey RW, Knutsen T, Zhan Z, Ried T, Bates SE. Escape from hsa-miR-519c enables drug-resistant cells to maintain high expression of ABCG2. Mol Cancer Ther. 2009;8:2959–2968. doi: 10.1158/1535-7163.MCT-09-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong JL, Zhang CP, Nie F, Xu XT, Zhu MM, Xiao SD, et al. MicroRNA 506 regulates expression of PPAR alpha in hydroxycamptothecin-resistant human colon cancer cells. FEBS Lett. 2011;585:3560–3568. doi: 10.1016/j.febslet.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakajima M, Takagi S, Taniya T, Yokoi T. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006;66:9090–9098. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Chiyomaru T, Enokida H, Kawakami K, Tatarano S, Kawahara K, et al. MiR-133a induces apoptosis through direct regulation of GSTP1 in bladder cancer cell lines. Urol Oncol. 2013;31:115–123. doi: 10.1016/j.urolonc.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Utomo A, Jiang X, Furuta S, Yun J, Levin DS, Wang YC, et al. Identification of a novel putative non-selenocysteine containing phospholipid hydroperoxide glutathione peroxidase (NPGPx) essential for alleviating oxidative stress generated from polyunsaturated fatty acids in breast cancer cells. J Biol Chem. 2004;279:43522–43529. doi: 10.1074/jbc.M407141200. [DOI] [PubMed] [Google Scholar]
- Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod M, Chennamsetty I, Colin S, Belloy L, De Paoli F, Schaider H, et al. miR-206 controls LXRα expression and promotes LXR-mediated cholesterol efflux in macrophages. Biochim Biophys Acta. 2014;1841:827–835. doi: 10.1016/j.bbalip.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil E, Verissimo CS, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, et al. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology. 2009;150:2220–2228. doi: 10.1210/en.2008-1335. [DOI] [PubMed] [Google Scholar]
- Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Xue X, Wei J, An Y, Yao J, Cai H, et al. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103:567–574. doi: 10.1038/sj.bjc.6605724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, et al. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun. 2013a;434:688–694. doi: 10.1016/j.bbrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Wang H, Wu J, Meng X, Ying X, Zuo Y, Liu R, et al. MicroRNA-342 inhibits colorectal cancer cell proliferation and invasion by directly targeting DNA methyltransferase 1. Carcinogenesis. 2011;32:1033–1042. doi: 10.1093/carcin/bgr081. [DOI] [PubMed] [Google Scholar]
- Wang JH, Shih KS, Wu YW, Wang AW, Yang CR. Histone deacetylase inhibitors increase microRNA-146a expression and enhance negative regulation of interleukin-1beta signaling in osteoarthritis fibroblast-like synoviocytes. Osteoarthritis Cartilage. 2013b;21:1987–1996. doi: 10.1016/j.joca.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Wang L, Oberg AL, Asmann YW, Sicotte H, McDonnell SK, Riska SM, et al. Genome-wide transcriptional profiling reveals microRNA-correlated genes and biological processes in human lymphoblastoid cell lines. PLoS ONE. 2009;4:e5878. doi: 10.1371/journal.pone.0005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Schuetz EG, Xu Y, Thummel KE. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J Steroid Biochem Mol Biol. 2013c;136:54–58. doi: 10.1016/j.jsbmb.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Jiang S, Zhang Y, Wang X, Peng X, Meng C, et al. The effect of microRNAs in the regulation of human CYP3A4: a systematic study using a mathematical model. Sci Rep. 2014;4:4283. doi: 10.1038/srep04283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Ma N, Wang D, Zhang Y, Zhou J, Wu G, et al. MiR-152 and miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity by targeting DNMT1 directly: a novel epigenetic therapy independent of decitabine. Oncogene. 2014;33:378–386. doi: 10.1038/onc.2012.575. [DOI] [PubMed] [Google Scholar]
- Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie Y, et al. microRNA-124 inhibits proliferation and induces apoptosis by directly repressing EZH2 in gastric cancer. Mol Cell Biochem. 2014;392:153–159. doi: 10.1007/s11010-014-2028-0. [DOI] [PubMed] [Google Scholar]
- Xiong J, Yu D, Wei N, Fu H, Cai T, Huang Y, et al. An estrogen receptor alpha suppressor, microRNA-22, is downregulated in estrogen receptor alpha-positive human breast cancer cell lines and clinical samples. FEBS J. 2010;277:1684–1694. doi: 10.1111/j.1742-4658.2010.07594.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Wang G, Zhu S, Liu Q, Wei T, Leng Y, et al. MiR-495 suppresses mesendoderm differentiation of mouse embryonic stem cells via the direct targeting of Dnmt3a. Stem Cell Res. 2014;12:550–561. doi: 10.1016/j.scr.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, et al. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 2010;30:6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AM. Small interfering RNA in drug metabolism and transport. Curr Drug Metab. 2007;8:700–708. doi: 10.2174/138920007782109751. [DOI] [PubMed] [Google Scholar]
- Yu AM. Role of microRNAs in the regulation of drug metabolism and disposition. Expert Opin Drug Metab Toxicol. 2009;5:1513–1528. doi: 10.1517/17425250903307448. [DOI] [PubMed] [Google Scholar]
- Yu X, Dhakal IB, Beggs M, Edavana VK, Williams S, Zhang X, et al. Functional genetic variants in the 3'-untranslated region of sulfotransferase isoform 1A1 (SULT1A1) and their effect on enzymatic activity. Toxicol Sci. 2010;118:391–403. doi: 10.1093/toxsci/kfq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan M, Qu Q, Wang G, Zhou H. Let-7c sensitizes acquired cisplatin-resistant A549 cells by targeting ABCC2 and Bcl-XL. Pharmazie. 2013;68:955–961. [PubMed] [Google Scholar]
- Zhang H, Zhao M, Lv Z, Zhang X, Qin X, Wang H, et al. MiR-138 inhibits tumor growth through repression of EZH2 in non-small cell lung cancer. Cell Physiol Biochem. 2013;31:56–65. doi: 10.1159/000343349. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wu JF, Chen WJ, Tang SL, Mo ZC, Tang YY, et al. MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis. 2014;234:54–64. doi: 10.1016/j.atherosclerosis.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu J, Xing R, Tie Y, Fu H, Zheng X, et al. miR-513a-3p sensitizes human lung adenocarcinoma cells to chemotherapy by targeting GSTP1. Lung Cancer. 2012;77:488–494. doi: 10.1016/j.lungcan.2012.05.107. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan LX, Wu QN, Du ZM, Chen J, Liao DZ, et al. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011;71:3552–3562. doi: 10.1158/0008-5472.CAN-10-2435. [DOI] [PubMed] [Google Scholar]
- Zhao J, Lammers P, Torrance CJ, Bader AG. TP53-independent function of miR-34a via HDAC1 and p21(CIP1/WAF1.) Mol Ther. 2013;21:1678–1686. doi: 10.1038/mt.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, et al. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008;283:31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao R, Feng J, He G. miR-613 regulates cholesterol efflux by targeting LXRα and ABCA1 in PPARγ activated THP-1 macrophages. Biochem Biophys Res Commun. 2014;448:329–334. doi: 10.1016/j.bbrc.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Zhao S, Wang Y, Liang Y, Zhao M, Long H, Ding S, et al. MicroRNA-126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum. 2011a;63:1376–1386. doi: 10.1002/art.30196. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Deng C, Lu W, Xiao J, Ma D, Guo M, et al. let-7 microRNAs induce tamoxifen sensitivity by downregulation of estrogen receptor alpha signaling in breast cancer. Mol Med. 2011b;17:1233–1241. doi: 10.2119/molmed.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Lv GC, Sheng J, Yang YD. Effect of miRNA-10b in regulating cellular steatosis level by targeting PPAR-alpha expression, a novel mechanism for the pathogenesis of NAFLD. J Gastroenterol Hepatol. 2010;25:156–163. doi: 10.1111/j.1440-1746.2009.05949.x. [DOI] [PubMed] [Google Scholar]
- Zhong D, Zhang Y, Zeng YJ, Gao M, Wu GZ, Hu CJ, et al. MicroRNA-613 represses lipogenesis in HepG2 cells by downregulating LXRα. Lipids Health Dis. 2013;12:32. doi: 10.1186/1476-511X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, et al. Role of microRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582–588. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yu F, Jiao Y, Feng J, Tang W, Yao H, et al. Reduced miR-128 in breast tumor-initiating cells induces chemotherapeutic resistance via Bmi-1 and ABCC5. Clin Cancer Res. 2011;17:7105–7115. doi: 10.1158/1078-0432.CCR-11-0071. [DOI] [PubMed] [Google Scholar]