Abstract
The perioperative period is characterized by profound changes in the body's homoeostatic processes. This review seeks to address whether epigenetic mechanisms may influence an individual's reaction to surgery and anaesthesia. Evidence from animal and human studies suggests that epigenetic mechanisms can explain many facets of susceptibility to acute and chronic pain, making them potential therapeutic targets. Modern pain management is still based upon opiates, and both the developmental expression of opioid receptors and opioid-induced hyperalgesia have been linked to epigenetic mechanisms. In general, opiates seem to increase global DNA methylation levels. This is in contrast to local anaesthetics, which have been ascribed a global demethylating effect. Even though no direct investigations have been carried out, the potential influence of epigenetics on the inflammatory response that follows surgery seems a promising area for research. There is a considerable body of evidence that supports the involvement of epigenetics in the complex process of wound healing. Epigenetics is an important emerging research topic in perioperative medicine, with a huge potential to positively influence patient outcome.
Linked Articles
This article is part of a themed section on Epigenetics and Therapy. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-11
Tables of Links
| TARGETS | LIGANDS |
|---|---|
| μ opioid receptor | Bupivacaine |
| DNA methyltransferase | Buprenorphine |
| Histone acetyltransferase | Chemokine CCL7 |
| Histone deacetylases | GABA |
| Histone deacetylase 7 | JQ1 |
| TRPA1 ion channel | Ketamine |
| Lidocaine | |
| Remifentanil |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,b,c).
Epigenetics is concerned with the stability and expression of information contained in human DNA. The three principal mechanisms that govern the transcription of genes are the modification of histone structure, methylation of DNA regions and nucleosome positioning (Portela and Esteller, 2010). In human disease, the epigenetic signature has been proposed both as a biomarker (Teschendorff et al., 2010) and as a therapeutic target (Navada et al., 2014). Epigenetics help explain why events early in life exert profound influences on how the body will react to a similar stimulus years or even decades later (Martin et al., 2010). Also, epigenetic traits are heritable, meaning that alterations in an epigenetic signature and the corresponding phenotype can sometimes be discerned generations later. One of the prototype scenarios is that of the Dutch Famine Winter of 1944–1945, a severe exogenous stressor that led to periconceptional epigenetic changes that are still detectable now, 60 years later (Heijmans et al., 2008). Therefore a strong, specific or persistent enough stressor has the potential to change epigenetic markers and influence body function for years, decades and potentially in coming generations.
The perioperative period in modern medicine is unique. Surgical and anaesthetic techniques, however refined they may be, still impose considerable stress on the human body. But patient monitoring is more comprehensive and provides more safeguards than ever before, while there are countless, continuous interventions to improve outcome. Antibiotics, pain killers, hypnotics, muscle relaxants, anti-emetics, haemostatic drugs, vasopressors, inotropes and local anaesthetics are examples of drugs given preoperatively, often simultaneously. Drugs are not only given in the operating room; many of these drugs are administered for days after surgery. Given the profound physiological changes elicited by surgery and anaesthesia, we will explore whether surgery or anaesthesia may influence epigenetic signatures and so modify short- and long-term outcomes, and whether epigenetic risk factors may, at least in part, determine an individual's reaction to surgery (Bain and Shaw, 2012; Naguib et al., 2012).
This review focuses on some research fields within epigenetics that relate to the perioperative period, including susceptibility to acute and chronic pain, the effects of opiates and local anaesthetics, and potential epigenetic roles in the stress response to surgery, wound healing, and cancer recurrence (Figure 1).
Figure 1.
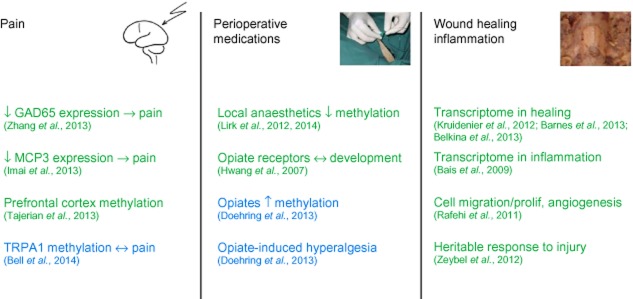
Summary of the main epigenetic mechanisms relevant for the perioperative period with green and blue colours designating experimental and human studies respectively. Chemokine CCL7; ; TRPA1 ion channel; prolif, proliferation.
Pain
Despite many advances in pain research, outcome after surgery is still bad for patients in many acute and chronic categories (Kehlet et al., 2006; Correll et al., 2014). Especially after prototype surgeries such as amputation and thoracotomy, between 50 and 80% of patients suffer from chronic pain. The prevalence of phantom pain following surgical amputation is high. A recent study confined to patients undergoing lower limb amputation for peripheral vascular disease reported phantom limb pain in 79% of patients (Richardson et al., 2006). Other survey data describe figures of around 75% (Kern et al., 2009). For amputations incurred during wartime, reports from the U.S. Department of Veteran Affairs cite similar numbers (72–76%) (Reiber et al., 2010). The reason why some patients develop ongoing pain and in others the pain resolves is still unknown. However, part of this risk is very likely to be genetic. Acute pain thresholds, in contrast to chronic pain levels, are less genetically determined (with estimated heritability scores of 22–55%) (Norbury et al., 2007). In contrast, mono- versus di-zygotic twins studies show a heritable component to the risk of developing persistent pain of up to 60% (Nyman et al., 2011). Such data clearly suggest a genetic predisposition for those who develop chronic pain following a precipitating incident, such as limb amputation and attendant nerve damage, and some preliminary insights have been gained. For example, the incidence of phantom pain post-limb amputation in Israeli war veterans ranges from 56 to 78%, depending on the genotype of the patient at one single-nucleotide polymorphism (SNP) in KCNS1 (rs734784, P = 0.0001). In addition the haplotype of KCNS1 identified by this SNP was also associated with pain outcome in five out of six human populations tested (overall P value of association with higher pain risk = 1.14 E-08) (Costigan et al., 2010).
However, in addition to genetic factors, which may determine baseline risk, epigenetic factors are increasingly seen as important in the transition from acute to chronic pain (Buchheit et al., 2012). This field of research was initiated by animal studies demonstrating an association of chronic pain with the epigenetic modification of specific genes. Zhang and colleagues investigated epigenetic contributions to the development of chronic pain in complete Freund's adjuvant injection and spinal nerve ligation. Here it was shown that, after induction of persistent inflammatory and neuropathic pain, the expression of GAD65 was epigenetically suppressed by histone hypoacetylation, decreasing inhibition in the GABA synaptic pathway. Conversely, inhibition of histone deacetylase increased GAD65 activity, led to normalized GABA transmission, and ultimately resulted in less sensitivity to pain. This effect was absent in mice knocked out for the gene encoding GAD65 (Zhang et al., 2011). In a model of partial sciatic nerve ligation, Imai and colleagues demonstrated IL-6-dependent demethylation of the H3 histone at Lys27, resulting in an increased expression of the chemokine CCL7 (MCP3) in glial cells. They hypothesized that this interaction would promote neuron–glial interactions, determining chronic neuropathic pain (Imai et al., 2013).
A study by Tajerian and colleagues probably has the most far-reaching implications. They demonstrated altered DNA methylation in the prefrontal cortex, in a mouse model of peripheral nerve injury associated with persistent neuropathic pain (Tajerian et al., 2013). This study showed that the consequences of peripheral nerve injury reach far into the CNS. Notably, animals that received environmental enrichment therapy to alleviate pain showed a resolution of epigenetic alterations in parallel with decreases in pain behaviour. Another facet of epigenetic involvement in pain syndromes is the fact that epigenetic traits are heritable. In the case of stress-induced visceral hypersensitivity, the epigenetic methylation signature associated with this experimental irritable bowel disease could still be detected in offspring two generations later (van den Wijngaard et al., 2013).
In humans, increased DNA methylation levels in peripheral blood leukocytes have been linked to the severity of chronic pain in patients on long-term treatment using potent opioids (Doehring et al., 2013). A recent investigation in monozygotic twins found that the degree of heat pain sensitivity correlated with the methylation status of the gene that encodes the TRPA-1 ion channel (Bell et al., 2014). This ion channel transforms thermal stimuli into heat pain (Julius, 2013). To explain the complex susceptibility and resilience to developing chronic pain, the concept of pain vulnerability has been proposed by Denk and co-authors, linking genetic and epigenetic risk factors (Denk et al., 2014).
In conclusion, epigenetic mechanisms seem to explain many facets of acute and chronic pain susceptibility. However, further work needs to be done to explain the detailed role of perioperative factors.
Perioperative opiates
Opiates are a fundamental component of perioperative analgesic regimens, and one of the oldest classes of drugs in existence. They can be subdivided into drugs with differential affinity for one of four major receptor families, that is μ, κ, δ or nociceptin receptors (Chiou et al., 2004; Pasternak, 2004), all belonging to the family of GPCRs.
The expression of μ opioid receptors is specific to cell type, localization and developmental stage (Pasternak and Pan, 2013). There are several regions in the CNS associated with high levels of μ opioid receptor mRNA, such as the periaquaeductal gray, locus coeruleus and the raphe magnus. Among the somatosensory regions, μ opioid receptor mRNA is abundant in the dorsal horn and dorsal root ganglia, the spinal trigeminal, ambiguus and tractus solitarii nuclei, and the thalamus (Pasternak and Pan, 2013). Hwang and colleagues have shown that epigenetic factors mediate the expression of μ opioid receptors according to developmental stage. Specifically, P19 embryonic carcinoma cells showed hypermethylation in the proximal promoter region of the gene encoding for this receptor, associated with gene silencing. In contrast, the promoter was unmethylated, and the gene stably expressed when differentiation into mature neuronal/astrocyte cells was induced with retinoic acid (Hwang et al., 2007). At the same time, differentiation led to decreased methyl-CpG-binding protein 2 (MeCP2) binding in the promoter region of the gene for μ opioid receptors (Hwang et al., 2007). MeCP2 binds to methylated DNA regions and causes histone deacetylation, with subsequent compacting of the histone complex and decreased transcription (Nan et al., 1998). Therefore, both DNA methylation and histone modification contribute to regulating μ-opioid receptor expression.
Clinically, μ opioid receptor agonists, with morphine as their prototype compound, have remained the most relevant subgroup of opiates. Opiates are indispensable in modern perioperative medicine, but recent research has shown that multimodal pain treatment using several different classes of drugs can decrease the amount of opiates administered, and adverse effects (McCarthy et al., 2010). The most important opiate side effects are nausea, vomiting, and bowel paralysis (Sharma and Jamal, 2013), suppression of the respiratory drive (Naso-Kaspar et al., 2013), and opioid-induced hyperalgesia (OIH) (Bekhit, 2010).
Hyperalgesia induced by opiates is a clinically relevant phenomenon seen after both short- and long-term administration of opiates. The risk of inducing chronic hyperalgesia seems to vary with the opiate used, and is assumed to be highest for remifentanil (van Gulik et al., 2012), while the partial μ receptor agonist and κ receptor antagonist, buprenorphine has even been associated with an antihyperalgesic effect (Pergolizzi et al., 2010). OIH is prevented by the administration of the NMDA receptor antagonist, ketamine (Laulin et al., 2002). Mechanisms conjectured in animal and human studies include NMDA receptor activation, cholecystokinin, NO and neuroinflammation, with the end result of a change in gene expression, and adaptations in nociceptive systems (Bekhit, 2010). Liang and colleagues were able to demonstrate in mice that inhibition of the histone acetyltransferase in the presence of morphine reduced signs of OIH, while histone deacetylase inhibition exaggerated OIH (Liang et al., 2013). In a recent clinical study, methylation analysis was undertaken in patients who had undergone methadone substitution following heroin addiction, patients receiving potent opiates for chronic pain, and age-matched controls. In the leukocytes of patients treated with various opioids, there was a global increase of DNA methylation at (LINE-1) and also a specific increase of methylation at the μ opioid receptor 1. Interestingly, a positive correlation was described between the methylation levels and pain intensity in patients treated with opiates, but not in patients in control groups receiving other pain medications (Doehring et al., 2013). It should be kept in mind, however, that the opiates constitute a heterogeneous group of drugs with distinct physicochemical properties, and the epigenetic effects of many opiates widely used perioperatively, such as tramadol (Scott and Perry, 2000), have not been investigated.
In conclusion, opiates remain of paramount importance in perioperative pain treatment. Both the developmental expression of the μ opioid receptor, and pathological hyperalgesia are mediated, at least in part, by epigenetic mechanisms. In general, opiates seem to increase global DNA methylation levels. This is in contrast to another group of perioperative drugs – the local anaesthetics.
Local anaesthetics
Just like the opiates, local anaesthetics are integral to perioperative medicine. They are a heterogeneous group of compounds characterized by the ability to block voltage-gated sodium channels, leading to distal anaesthesia and/or analgesia. For more than a hundred years, local anaesthetics have been used to provide neuraxial or peripheral anaesthesia and analgesia. However, recent research has shown that a substantial share of the effects of regional anaesthesia is due to systemic effects secondary to resorption from the site of injection (Lirk and Hollmann, 2013), and i.v. administration of, for example, lidocaine can replicate some effects of epidural anaesthesia (Herroeder et al., 2007). Specifically, some trials with local anaesthetics have demonstrated antinociceptive properties (Koppert et al., 2004), anti-inflammatory effects suppressing virtually every step of the inflammatory cascade (Hollmann and Durieux, 2000) and a reduction in perioperative hypercoagulability without inducing a clinically relevant bleeding risk (Hollmann et al., 2001).
Two studies have demonstrated the epigenetic effects of local anaesthetics. In breast cancer cells in vitro, the prototype local anaesthetic, lidocaine, induced demethylation of DNA as shown by MethyLight assays in a time- and concentration-dependent manner (Lirk et al., 2012). These effects were more prominent in BT-20 oestrogen receptor-negative cells than in MCF-7 oestrogen-receptor positive cells, as BT-20 cells featured baseline methylation levels 100-fold higher than MCF-7 cells, and were observed at clinically relevant concentrations as seen during epidural anaesthesia (Fukuda et al., 2003) or i.v. administration of lidocaine (Sun et al., 2012). In a subsequent study, it was found that these effects could also be observed with another local anaesthetic ropivacaine, but not with bupivacaine. Also, there were no supra-additive effects when treatment was combined with the classic demethylating drug, 5-aza-2′-deoxycytidine (Lirk et al., 2014). The mechanism of demethylation remains unclear for contemporary amide-type local anaesthetics such as lidocaine, but research on ester-type local anaesthetics, a related group of drugs, showed that procaine had an inhibitory effect on DNA methyltransferase 1, the enzyme responsible for the maintenance methyltransferase activity of the cell (Castellano et al., 2008).
These results are of potential interest for the development of chronic pain and cancer medicine. While opiates lead to increased methylation correlating with increased levels of pain suffering (Doehring et al., 2011), local anaesthetics have the potential to induce global DNA demethylation (Lirk et al., 2012), and may thereby protect against the development of hyperalgesia. Clinically, some studies have described an antinociceptive effect of local anaesthetics (Koppert et al., 2004), but others have found no clinically relevant effects (Herroeder et al., 2007; Martin et al., 2008). Whether perioperative anaesthetic technique can influence cancer recurrence after surgery remains fiercely debated, and most long-term studies are projected to last until the end of the decade because of long follow-up periods (Snyder and Greenberg, 2010). In this context, the therapeutic-demethylating levels of local anaesthetics in the perioperative setting may be of potential benefit, but the direct clinical relevance remains to be tested.
In conclusion, local anaesthetics are frequently administered perioperatively, and in contrast to opiates, they have a global DNA demethylating effect. This effect is of potential interest in the development of chronic pain and perioperative cancer medicine.
Perioperative inflammation
As part of an ancient evolutionary response, surgery is accompanied by a complex reaction referred to as ‘surgical stress response’. This response shares substantial similarities with traumatic or burn injury, and is primarily mediated by the hypothalamic–pituitary axis leading to the release of stress hormones such as cortisol, growth hormone and vasopressin (Finnerty et al., 2013). It encompasses a wide variety of inflammatory, immune, hormonal and genomic responses (Finnerty et al., 2013). The expression of inflammatory genes is regulated by epigenetic factors such as histone acetylation (Barnes, 2013). This type of histone modification can be recognized by the highly conserved bromodomain motif of several epigenetic proteins. Low MW inhibitors of these bromodomains, such as JQ1, can inhibit the transcription of cytokines from mouse macrophages and protect against lipopolysaccharide-induced death (Belkina et al., 2013). Not only histone acetylation, but also histone methylation regulates the expression of inflammatory genes. Thus, histone methylation can reduce TNF release from human macrophages in vitro. The classic local anaesthetic, lidocaine, which has been shown to demethylate DNA, has very strong anti-inflammatory properties (Herroeder et al., 2007), but any causal role by epigenetic regulatory mechanisms has not yet been directly investigated.
Even though no direct investigation has been carried out, the potential influence of epigenetics on the inflammatory response that follows surgery seems a promising research area.
Wound healing
The repair of surgical wounds is a complex adaptive process where initial fibrogenesis leads to the formation of a scar, which is subsequently replaced by healthy and appropriate cells (Mann and Mann, 2013). Using bone healing as experimental paradigm, Bais and colleagues demonstrated the complex temporal sequence of transcriptome changes during healing, involving more than 300 genes (Bais et al., 2009). This confirms previous investigations that demonstrate the involvement of epigenetic mechanisms in the induction of wound healing (Shaw and Martin, 2009). Also, the migration of human cells, critical for wound healing, is regulated by epigenetic mechanisms, including the histone deacetylase 7 (Zheng et al., 2012). Epigenetics provides a basis to explain how certain disease states can negatively influence wound healing. For example, long-standing diabetes can impair physiological wound healing by decreasing the expression of the enzyme dicer, a pro-angiogenetic molecule, in a mouse model of type 2 diabetes. Similarly, the proliferation of keratinocytes, key to re-epithelialization of wound surfaces, is regulated by epigenetic factors (Rafehi et al., 2011). Moreover, alcoholics have a higher risk of non-union after bone fractures, and this has been linked to epigenetic changes affecting cell migration, proliferation and differentiation (Sampson et al., 2011). Zeybel and co-workers investigated whether these epigenetic traits might be handed down through generations, and in a rat model of liver damage, they found that the fibrogenic component of liver damage was still alleviated in the first- and second- generation offspring after ancestral chronic liver injury induced by carbon tetrachloride (Zeybel et al., 2012). Lastly, epigenetics provides a therapeutic approach to regulating wound healing, for example by selectively targeting the promoter activity of relevant genes using oligodeoxynucleotides (Cutroneo and Chiu, 2000).
In conclusion, epigenetic mechanisms govern physiological wound healing, while the epigenetic side effects of diseases such as diabetes and alcoholism may explain erroneous wound healing. Therefore, epigenetics has been suggested as a therapeutic strategy to control wound healing.
Perioperative epigenetics and tumour recurrence
The perioperative period has long been recognized as a vulnerable time frame during which tumour progression and metastasis is often accelerated (Coffey et al., 2003). Indeed, some small and retrospective trials have demonstrated improved patient survival and disease-free intervals when regional anaesthesia was used for cancer surgery (Schlagenhauff et al., 2000; Exadaktylos et al., 2006). Several experimental studies support this assumption. In a landmark experiment, growth in a tumour cell line was halted in vitro when incubated with serum taken from patients undergoing surgery under regional anaesthesia, but no effect was observed when serum from patients given general anaesthesia was applied (Deegan et al., 2009). At this moment, several large-scale, multicentre, randomized and controlled trials are under way to investigate any beneficial effects of regional anaesthesia in tumour surgery, but they are projected to last until the end of the decade because of long follow-up times (Snyder and Greenberg, 2010).
The potential mechanisms for anti-metastatic effects from regional anaesthesia are either direct or indirect. Direct effects could include the interference of local anaesthetics with tumour-promoting pathways (Votta-Velis et al., 2013), direct toxic effects when used for local infiltration (Schlagenhauff et al., 2000) and changes in the epigenetic signature of tumour cells (Lirk et al., 2012). Indirect effects could result from reduction of the perioperative stress response and the preservation of the immune response (Dong et al., 2012). As many of these effects can be duplicated using i.v. local anaesthetics (Herroeder et al., 2007), a new avenue of research will be the employment of systemic local anaesthetics in the perioperative period of tumour surgery. Notably, local anaesthetics, at clinically relevant doses, demethylate DNA in breast cancer cells in vitro, opening up the possibility that epigenetic mechanisms could potentially explain why some anaesthetic techniques may have an anti-metastatic effect.
In conclusion, there is limited evidence suggesting that perioperative interventions may influence the recurrence of cancer after surgery. Epigenetics has been suggested as one potential pathway of interest.
Future directions
Research is needed to define whether perioperative surgical and anaesthetic interventions have the potential to alter epigenetic signatures and thereby potentially influence long-term patient outcome. In particular, despite the involvement of pain pathways, the ‘epigenetic potentials’ of widely used opiates, of NMDA antagonists and of non-opioid pain medications are unknown. In line with corresponding genetic research, epigenetic factors that render individual patients susceptible to development of chronic pain should be identified, and the concept of pain vulnerability (Denk et al., 2014) should be further developed and refined. The ultimate aim would be to predict, based on the type of intervention (Kehlet et al., 2006), patient history (Kalkman et al., 2003) and genetic and epigenetic factors (Denk et al., 2014), which patients are at risk of experiencing acute and chronic pain, and to tailor the anaesthetic and surgical plan accordingly, allocating analgesic resources to patients who need it most. For example, this may influence the choice of administering regional anaesthesia or multimodal analgesia, or both (Lirk and Hollmann, 2013).
Epigenetic factors in the perioperative period are only beginning to be understood and have major therapeutic and diagnostic potential.
Acknowledgments
The authors gratefully acknowledge the expert language editing assistance of Mrs Senay Boztas, Amsterdam, The Netherlands.
Glossary
- GAD
glutamic acid decarboxylase
- MeCP2
methyl-CpG-binding protein 2
- OIH
opiate-induced hyperalgesia
- SNP
single-nucleotide polymorphism
Author contributions
P.L. wrote the manuscript and serves as the corresponding author. H.F. assisted in writing the manuscript and approved the final version. N.C.W. assisted in writing the manuscript and approved the final version. M.W.H. wrote the manuscript and is the senior author.
Conflict of interest
None declared.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain CR, Shaw AD. Genetics and epigenetics in perioperative medicine. Curr Opin Crit Care. 2012;18:548–554. doi: 10.1097/MCC.0b013e328357af6d. [DOI] [PubMed] [Google Scholar]
- Bais M, McLean J, Sebastiani P, Young M, Wigner N, Smith T, et al. Transcriptional analysis of fracture healing and the induction of embryonic stem cell-related genes. PLoS ONE. 2009;4:e5393. doi: 10.1371/journal.pone.0005393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12:543–559. doi: 10.1038/nrd4025. [DOI] [PubMed] [Google Scholar]
- Bekhit MH. Opioid-induced hyperalgesia and tolerance. Am J Ther. 2010;17:498–510. doi: 10.1097/MJT.0b013e3181ed83a0. [DOI] [PubMed] [Google Scholar]
- Belkina AC, Nikolajczyk BS, Denis GV. BET protein function is required for inflammation: Brd2 genetic disruption and BET inhibitor JQ1 impair mouse macrophage inflammatory responses. J Immunol. 2013;190:3670–3678. doi: 10.4049/jimmunol.1202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JT, Loomis AK, Butcher LM, Gao F, Zhang B, Hyde CL, et al. Differential methylation of the TRPA1 promoter in pain sensitivity. Nat Commun. 2014;5:2978. doi: 10.1038/ncomms3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheit T, Van de Ven T, Shaw A. Epigenetics and the transition from acute to chronic pain. Pain Med. 2012;13:1474–1490. doi: 10.1111/j.1526-4637.2012.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano S, Kuck D, Sala M, Novellino E, Lyko F, Sbardella G. Constrained analogues of procaine as novel small molecule inhibitors of DNA methyltransferase-1. J Med Chem. 2008;51:2321–2325. doi: 10.1021/jm7015705. [DOI] [PubMed] [Google Scholar]
- Chiou LC, Fan SH, Chuang KC, Liao YY, Lee SZ. Pharmacological characterization of nociceptin/orphanin FQ receptors, a novel opioid receptor family, in the midbrain periaqueductal gray. Ann N Y Acad Sci. 2004;1025:398–403. doi: 10.1196/annals.1316.049. [DOI] [PubMed] [Google Scholar]
- Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760–768. doi: 10.1016/s1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- Correll DJ, Vlassakov KV, Kissin I. No evidence of real progress in treatment of acute pain, 1993–2012: scientometric analysis. J Pain Res. 2014;7:199–210. doi: 10.2147/JPR.S60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519–2527. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutroneo KR, Chiu JF. Comparison and evaluation of gene therapy and epigenetic approaches for wound healing. Wound Repair Regen. 2000;8:494–502. doi: 10.1046/j.1524-475x.2000.00494.x. [DOI] [PubMed] [Google Scholar]
- Deegan CA, Murray D, Doran P, Ecimovic P, Moriarty DC, Buggy DJ. Effect of anaesthetic technique on oestrogen receptor-negative breast cancer cell function in vitro. Br J Anaesth. 2009;103:685–690. doi: 10.1093/bja/aep261. [DOI] [PubMed] [Google Scholar]
- Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- Doehring A, Geisslinger G, Lotsch J. Epigenetics in pain and analgesia: an imminent research field. Eur J Pain. 2011;15:11–16. doi: 10.1016/j.ejpain.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Doehring A, Oertel BG, Sittl R, Lotsch J. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain. 2013;154:15–23. doi: 10.1016/j.pain.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang Y, Xi H. The effects of epidural anaesthesia and analgesia on natural killer cell cytotoxicity and cytokine response in patients with epithelial ovarian cancer undergoing radical resection. J Int Med Res. 2012;40:1822–1829. doi: 10.1177/030006051204000520. [DOI] [PubMed] [Google Scholar]
- Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr. 2013;37:21S–29S. doi: 10.1177/0148607113496117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Kakiuchi Y, Miyabe M, Kihara S, Kohda Y, Toyooka H. Free lidocaine concentrations during continuous epidural anesthesia in geriatric patients. Reg Anesth Pain Med. 2003;28:215–220. doi: 10.1053/rapm.2003.50131. [DOI] [PubMed] [Google Scholar]
- van Gulik L, Ahlers SJ, van de Garde EM, Bruins P, van Boven WJ, Tibboel D, et al. Remifentanil during cardiac surgery is associated with chronic thoracic pain 1 yr after sternotomy. Br J Anaesth. 2012;109:616–622. doi: 10.1093/bja/aes247. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herroeder S, Pecher S, Schonherr ME, Kaulitz G, Hahnenkamp K, Friess H, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007;246:192–200. doi: 10.1097/SLA.0b013e31805dac11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93:858–875. doi: 10.1097/00000542-200009000-00038. [DOI] [PubMed] [Google Scholar]
- Hollmann MW, Wieczorek KS, Smart M, Durieux ME. Epidural anesthesia prevents hypercoagulation in patients undergoing major orthopedic surgery. Reg Anesth Pain Med. 2001;26:215–222. doi: 10.1053/rapm.2001.23209. [DOI] [PubMed] [Google Scholar]
- Hwang CK, Song KY, Kim CS, Choi HS, Guo XH, Law PY, et al. Evidence of endogenous mu opioid receptor regulation by epigenetic control of the promoters. Mol Cell Biol. 2007;27:4720–4736. doi: 10.1128/MCB.00073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Ikegami D, Yamashita A, Shimizu T, Narita M, Niikura K, et al. Epigenetic transcriptional activation of monocyte chemotactic protein 3 contributes to long-lasting neuropathic pain. Brain. 2013;136:828–843. doi: 10.1093/brain/aws330. [DOI] [PubMed] [Google Scholar]
- Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- Kalkman CJ, Visser K, Moen J, Bonsel GJ, Grobbee DE, Moons KG. Preoperative prediction of severe postoperative pain. Pain. 2003;105:415–423. doi: 10.1016/S0304-3959(03)00252-5. [DOI] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- Kern U, Busch V, Rockland M, Kohl M, Birklein F. Prevalence and risk factors of phantom limb pain and phantom limb sensations in Germany. A nationwide field survey. Schmerz. 2009;23:479–488. doi: 10.1007/s00482-009-0786-5. [DOI] [PubMed] [Google Scholar]
- Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg. 2004;98:1050–1055. doi: 10.1213/01.ANE.0000104582.71710.EE. [DOI] [PubMed] [Google Scholar]
- Kruidenier L, Chung CW, Cheng Z, Liddle J, Che K, Joberty G, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404–408. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulin JP, Maurette P, Corcuff JB, Rivat C, Chauvin M, Simonnet G. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg. 2002;94:1263–1269. doi: 10.1097/00000539-200205000-00040. [DOI] [PubMed] [Google Scholar]
- Liang DY, Li X, Clark JD. Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain. 2013;14:36–47. doi: 10.1016/j.jpain.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirk P, Hollmann M. Outcome after regional anesthesia: weighing risks and benefits. Minerva Anestesiol. 2013;80:610–618. [PubMed] [Google Scholar]
- Lirk P, Berger R, Hollmann MW, Fiegl H. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro. Br J Anaesth. 2012;109:200–207. doi: 10.1093/bja/aes128. [DOI] [PubMed] [Google Scholar]
- Lirk P, Hollmann M, Fleischer M, Hauck-Weber NC, Fiegl H. Lignocaine and ropivacaine, but not bupivacaine, demethylate deoxyribonucleic acid in breast cancer cells in vitro. Br J Anaesth. 2014;113:i32–i38. doi: 10.1093/bja/aeu201. (Suppl. 1): [DOI] [PubMed] [Google Scholar]
- Mann J, Mann DA. Epigenetic regulation of wound healing and fibrosis. Curr Opin Rheumatol. 2013;25:101–107. doi: 10.1097/BOR.0b013e32835b13e1. [DOI] [PubMed] [Google Scholar]
- Martin DS, Khosravi M, Grocott MP, Mythen MG. Concepts in hypoxia reborn. Crit Care. 2010;14:315. doi: 10.1186/cc9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Cherif K, Gentili ME, Enel D, Abe E, Alvarez JC, et al. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology. 2008;109:118–123. doi: 10.1097/ALN.0b013e31817b5a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs. 2010;70:1149–1163. doi: 10.2165/10898560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Naguib M, Bie B, Ting AH. Fundamental concepts of epigenetics for consideration in anesthesiology. Curr Opin Anaesthesiol. 2012;25:434–443. doi: 10.1097/ACO.0b013e3283556211. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Naso-Kaspar CK, Herndon GW, Wyman JF, Felo JA, Lavins ES, Gilson TP. ‘Lingering’ opiate deaths? Concentration of opiates in medulla and femoral blood. J Anal Toxicol. 2013;37:507–511. doi: 10.1093/jat/bkt061. [DOI] [PubMed] [Google Scholar]
- Navada SC, Steinmann J, Lubbert M, Silverman LR. Clinical development of demethylating agents in hematology. J Clin Invest. 2014;124:40–46. doi: 10.1172/JCI69739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB. Heritability of responses to painful stimuli in women: a classical twin study. Brain. 2007;130:3041–3049. doi: 10.1093/brain/awm233. [DOI] [PubMed] [Google Scholar]
- Nyman T, Mulder M, Iliadou A, Svartengren M, Wiktorin C. High heritability for concurrent low back and neck-shoulder pain: a study of twins. Spine (Phila Pa 1976) 2011;36:E1469–E1476. doi: 10.1097/BRS.0b013e3181e2c878. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Multiple opiate receptors: deja vu all over again. Neuropharmacology. 2004;47(Suppl. 1):312–323. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan YX. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev. 2013;65:1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi J, Aloisi AM, Dahan A, Filitz J, Langford R, Likar R, et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract. 2010;10:428–450. doi: 10.1111/j.1533-2500.2010.00378.x. [DOI] [PubMed] [Google Scholar]
- Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- Rafehi H, El-Osta A, Karagiannis TC. Genetic and epigenetic events in diabetic wound healing. Int Wound J. 2011;8:12–21. doi: 10.1111/j.1742-481X.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber GE, McFarland LV, Hubbard S, Maynard C, Blough DK, Gambel JM, et al. Servicemembers and veterans with major traumatic limb loss from Vietnam war and OIF/OEF conflicts: survey methods, participants, and summary findings. J Rehabil Res Dev. 2010;47:275–297. doi: 10.1682/jrrd.2010.01.0009. [DOI] [PubMed] [Google Scholar]
- Richardson C, Glenn S, Nurmikko T, Horgan M. Incidence of phantom phenomena including phantom limb pain 6 months after major lower limb amputation in patients with peripheral vascular disease. Clin J Pain. 2006;22:353–358. doi: 10.1097/01.ajp.0000177793.01415.bd. [DOI] [PubMed] [Google Scholar]
- Sampson HW, Chaput CD, Brannen J, Probe RA, Guleria RS, Pan J, et al. Alcohol induced epigenetic perturbations during the inflammatory stage of fracture healing. Exp Biol Med (Maywood) 2011;236:1389–1401. doi: 10.1258/ebm.2011.011207. [DOI] [PubMed] [Google Scholar]
- Schlagenhauff B, Ellwanger U, Breuninger H, Stroebel W, Rassner G, Garbe C. Prognostic impact of the type of anaesthesia used during the excision of primary cutaneous melanoma. Melanoma Res. 2000;10:165–169. [PubMed] [Google Scholar]
- Scott LJ, Perry CM. Tramadol: a review of its use in perioperative pain. Drugs. 2000;60:139–176. doi: 10.2165/00003495-200060010-00008. [DOI] [PubMed] [Google Scholar]
- Sharma A, Jamal MM. Opioid induced bowel disease: a twenty-first century physicians' dilemma. Considering pathophysiology and treatment strategies. Curr Gastroenterol Rep. 2013;15:334. doi: 10.1007/s11894-013-0334-4. [DOI] [PubMed] [Google Scholar]
- Shaw T, Martin P. Epigenetic reprogramming during wound healing: loss of polycomb-mediated silencing may enable upregulation of repair genes. EMBO Rep. 2009;10:881–886. doi: 10.1038/embor.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–115. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li T, Wang N, Yun Y, Gan TJ. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2012;55:1183–1194. doi: 10.1097/DCR.0b013e318259bcd8. [DOI] [PubMed] [Google Scholar]
- Tajerian M, Alvarado S, Millecamps M, Vachon P, Crosby C, Bushnell MC, et al. Peripheral nerve injury is associated with chronic, reversible changes in global DNA methylation in the mouse prefrontal cortex. PLoS ONE. 2013;8:e55259. doi: 10.1371/journal.pone.0055259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff AE, Menon U, Gentry-Maharaj A, Ramus SJ, Weisenberger DJ, Shen H, et al. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res. 2010;20:440–446. doi: 10.1101/gr.103606.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votta-Velis EG, Piegeler T, Minshall RD, Aguirre J, Beck-Schimmer B, Schwartz DE, et al. Regional anaesthesia and cancer metastases: the implication of local anaesthetics. Acta Anaesthesiol Scand. 2013;57:1211–1229. doi: 10.1111/aas.12210. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard RM, Stanisor OI, van Diest SA, Welting O, Wouters MM, Cailotto C, et al. Susceptibility to stress induced visceral hypersensitivity in maternally separated rats is transferred across generations. Neurogastroenterol Motil. 2013;25:e780–e790. doi: 10.1111/nmo.12202. [DOI] [PubMed] [Google Scholar]
- Zeybel M, Hardy T, Wong YK, Mathers JC, Fox CR, Gackowska A, et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med. 2012;18:1369–1377. doi: 10.1038/nm.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med. 2011;17:1448–1455. doi: 10.1038/nm.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Yu Z, Zhou Y, Tao L, Pang Y, Chen T, et al. Live cell imaging analysis of the epigenetic regulation of the human endothelial cell migration at single-cell resolution. Lab Chip. 2012;12:3063–3072. doi: 10.1039/c2lc40192d. [DOI] [PubMed] [Google Scholar]


