Abstract
Many nutrients are known for a wide range of activities in prevention and alleviation of various diseases. Recently, their potential role in regulating human health through effects on epigenetics has become evident, although specific mechanisms are still unclear. Thus, nutriepigenetics/nutriepigenomics has emerged as a new and promising field in current epigenetics research in the past few years. In particular, polyphenols, as part of the central dynamic interaction between the genome and the environment with specificity at physiological concentrations, are well known to affect mechanisms underlying human health. This review summarizes the effects of dietary compounds on epigenetic mechanisms in the regulation of gene expression including expression of enzymes and other molecules responsible for drug absorption, distribution, metabolism and excretion in cancer, metabolic syndrome, neurodegenerative disorders and hormonal dysfunction.
Linked Articles
This article is part of a themed section on Epigenetics and Therapy. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-11
Tables of Links
| TARGETS |
|---|
| DNMT, DNA methyltransferases |
| ERα, oestrogen receptor α |
| ERβ, oestrogen receptor β |
| FASN, fatty acid synthase |
| HAT, histone acetyl-transferases |
| HDAC, histone deacetylase |
| HDAC 1 |
| HDAC 3 |
| HDAC 8 |
| Insulin receptor |
| KDM1, lysine demethylase 1 |
| SIRT1, sirtuin-1 |
| LIGANDS |
|---|
| AZA, 5-aza-2-deoxycytidine (azacitidine) |
| Curcumin |
| Daidzein |
| E2, 17β-estradiol |
| EGCG, epigallocatechin gallate |
| Folate |
| Genistein |
| Leptin |
| SAH, S-adenosylhomocysteine |
| SAM, S-adenosyl-L-methionine |
| Tamoxifen |
| TSA, trichostatin A |
These Tables lists key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,b,c).
Introduction
Stimuli and processes influencing epigenetic gene regulation and gene expression can be divided into internal factors such as inherited mutations, metabolic pathways, neuroendocrine balance, hormonal activities and external factors such as nutrients, bioactive food components, medication, tobacco, radiation, infectious organisms, stress (Choi and Friso, 2010). The resulting epigenetic patterns build an important bridge between life experiences and phenotypes of behaviour, stress response and disease susceptibility (Louis and Flint, 2007; Tammen et al., 2013). Epigenetic patterns encompass DNA methylation, histone modifications, non-coding RNAs, and chromatin remodelling (Choi and Friso, 2010), and orchestrate an enormous variety of molecular and cellular outcomes and processes essential for higher nervous system and endocrine functions, as well as evolutionary innovations (Figure 1) (Klose and Bird, 2006; Kondo, 2006; Mehler and Mattick, 2007; Tsankova et al., 2007). As these modifications are reversible and tissue-specific, they are potential targets for therapeutic purposes. Application to the therapy of a variety of diseases is already taking place, although most of the mechanisms behind the action are unknown and are of current research interest. However, epigenetic mechanisms involved in the regulation of gene expression including the control of enzymes and molecules involved in drug absorption, distribution, metabolism and excretion could be used as a pharmacologically focused set of tools for the treatment of specific disorders. A modulation of these processes through diet or specific nutrients may also prevent diseases and maintain health. Therefore, the field of ‘nutriepigenetics/nutriepigenomics’ concerned with the influence of nutrients through epigenetic modifications – is expanding. One well-established epigenetic effect of different feeding is the development of a honeybee into a queen or worker. At the early stage, all larvae are fed with royal jelly, produced in the mouth of nurse bees, but worker larvae are soon weaned and obtain a diet of pollen and nectar. In contrast, the queen larva is bathed and fed until adulthood with royal jelly. This different nurture results in morphological, behavioural and physiological differences. Thus, different phenotypic outcomes are generated from identical genomes, induced by diet (Chittka and Chittka, 2010).
Figure 1.
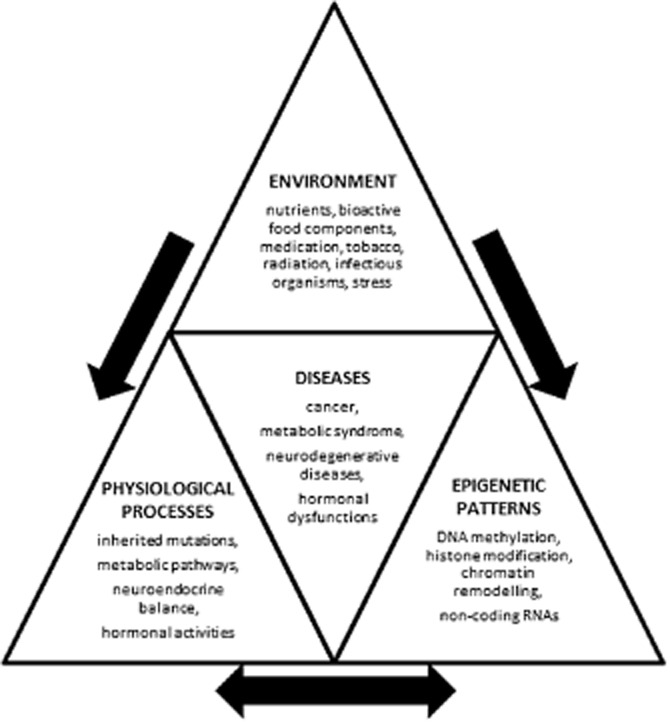
Diagram showing the influence of environment, physiological processes and epigenetic patterns on diseases, and their interaction with one another.
Secondary plant metabolites, especially polyphenols with aromatic rings and one or more hydroxyl groups, are common components of vegetables, fruits, green tea and red wine. Polyphenols are one of the largest groups of phytochemicals with important roles in plants, such as protection from photosynthetic stress and reactive oxygen species. They are known for their antioxidant properties, for example in cancer, neurodegenerative diseases, gastrointestinal disorders, but also by direct effects on enzymes, proteins, receptors and signalling pathways. Recent research indicates beneficial effects via modulation of NF-κB expression, chromatin remodelling through modulation of histone deacetylases (HDAC) and DNA methyltransferases (DNMT) activities, which, consequently, can reverse abnormal gene expression (Russell et al., 2013). For instance, curcumin, genistein, epigallocatechin gallate (EGCG), resveratrol and equol act through different mechanisms to inhibit DNMT. Theoretically, every bioactive component affecting one of the two metabolites of the 1-carbon metabolism – S-adenosylmethionine (SAM), an ubiquitous methyl donor, or S-adenosylhomocysteine (SAH), an inhibitor of methyltransferases – can potentially alter the methylation of DNA and histones (Russell et al., 2013).
Dietary components, such as butyrate, sulforaphane (SFN) and curcumin, affect histone acetyl-transferase (HAT) and HDAC activities. Several well-characterized epigenetic effect, including histone methylation, acetylation and ADP-ribosylation, as well as DNA methylation, have direct links to central metabolism through critical redox intermediates such as NAD+, SAM and 2-oxoglutarate (Cyr et al., 2013). Water-soluble B vitamins, biotin, niacin and panthothenic acid influence histone modifications. For example, biotin is a substrate for histone biotinylation and niacin for histone ADP-ribosylation. Resveratrol, butyrate, SFN and diallyl sulfide inhibit HDAC, whereas curcumin inhibits HAT (Choi and Friso, 2010).
Delineating the precise mechanisms of nutrients can be challenging, as the effects, inhibition or activation of HDACs and DNMTs can be determined not only by nutrient structure, physiological and pathological processes in the body, and nutrient interaction, but also by interaction with other lifestyle factors (Choi and Friso, 2010). For instance, the combination of a hydroxyl group at position 7 in ring B of fisetin (3,7,3′,4′-tetrahydroxyflavone), silibinin and daidzein, is required for sirtuin-1 (SIRT1) and DNMT activation, whereas a hydroxyl group at position 5 in luteolin and EGCG structure is responsible for the inhibition of SIRT1. Polyphenols such as genistein, myricetin and quercetin, with a hydroxyl group at positions 5 and 7, caused both inhibition and activation of HDACs and DNMTs depending on experimental conditions (Ayissi et al., 2014).
Methylation in a promoter and other regulatory regions of a gene is usually associated with repressed gene transcription (Tammen et al., 2013) by blocking transcription factor binding (Franks and Ling, 2010). DNA demethylation (hypomethylation), leading to gene activation, also plays an important regulatory role in gene transcription. Another DNA modification, 5-hydroxymethylcytosine (5-hmC), serves as an intermediate in the removal of methyl groups from 5-methylcytosine. The exact role of 5-hmC is not well understood and needs further investigation. Demethylation and activation recently observed in oncogenes and pro-metastatic genes in cancer (Stefanska et al., 2011) can be reversed or prevented by methyl group donors, such as folate, betaine, methyl-cobalamin and SAM, which could have potential in therapy.
All the components of the epigenome are interdependent, and the interaction between DNA methylation, microRNA (miRNAs) and histone modifications to silence or activate gene transcription must be taken into account. It is suggested that DNA methylation and histone modifications are involved in the regulation of miRNAs. On the other hand, miRNAs alter the expression of DNMT3 and DNMT3B. For example, in cancer cells, impaired DNA methylation causes an aberrant miRNA expression (Tammen et al., 2013). Histone deacetylation and histone H3-Lys9 (H3K9) methylation are suggested to clear the way for CpG methylation. The interaction of these two mechanisms is also believed to result in long-term transcriptional silencing (Fuks, 2005). Histone acetylation at lysine and phosphorylation at serine are implicated to activate gene expression, whereas histone deacetylation, sumoylation and biotinylation are responsible for gene silencing (Brosch et al., 2008; Oliver and Denu, 2011). Methylation and ubiquitination of histones are more complex and can act as silencers or activators depending on the histone residues affected (Sawicka and Seiser, 2012). In addition, different combinations of histone modifications act together and modify the overall structure of the chromatin (Brandl et al., 2009; Haberland et al., 2009; Jimenez-Chillaron et al., 2012).
Some of the natural products reported to exert positive effects on specific human diseases are also being studied in clinical trials. Genistein has been shown to improve surrogate end points associated with metabolic syndrome through its effects on the risk of developing diabetes and cardiovascular disease (Squadrito et al., 2013). In the group receiving genistein, significantly lower fasting glucose, fasting insulin, total cholesterol, low-density lipoprotein-cholesterol (LDL-C) and triglyceride values, as well as increased high-density lipoprotein-cholesterol values were observed. On the other hand, in a randomized phase II trial of soy isoflavone supplementation for reduction of risk for breast cancer in middle-aged Western women, the authors concluded that there was no positive effect of the supplementation (Khan et al., 2012). Their discussion points out that soy exposure early in life is probably necessary for beneficial effect, and that natural sources, instead of processed supplements, are crucial for positive effects.
Curcumin is another dietrary component with potential positive effect in different types of cancer and metabolic syndrome as well. Supplementation with curcumin in pre-diabetic individuals significantly lowered the number of those individuals who eventually developed type II diabetes (Chuengsamarn et al., 2012) and improved overall function of beta-cells. Also, a phase II trial of curcumin in patients with advanced pancreatic cancer showed some beneficial effects (Dhillon et al., 2008).
In this review, we focus on the most recent knowledge of nutritional epigenetics with regard to their pharmacological potential in therapies of four of the most common disorders, worldwide: cancer, metabolic syndrome, neurodegenerative diseases and hormonal dysfunctions (Table 1).
Table 1.
Potential epigenetically active nutrients and their beneficial effect
| Disease | Nutrient | Metabolic effect/mechanism of action | Model | References |
|---|---|---|---|---|
| Cancer | ||||
| Breast cancer | Genistein | Telomerase inhibition | Breast benign cells and MCF-7 cancer cells | (Qin et al., 2009) |
| Oesophageal carcinoma | Genistein | Demethylating potential | Oesophageal squamous cell carcinoma cells | (Li et al., 2009) |
| Breast cancer | Coffee polyphenols | Inhibition of DNA methylation catalyzed by DNMT1 | Breast cancer cells | (Qin et al., 2009) |
| Colon cancer | SFN | Down-regulation of DNMT1 | Cancerous colon tissue | (Lee and Zhu, 2006) |
| Leukaemia | Curcumin | Decreased DNA methylation | Leukaemia cell line | (Traka et al., 2005) |
| Prostate cancer | Curcumin | Inhibition of acetyltransferase activity Blocking histone hyperacetylation | Prostate cancer cells and peripheral blood lymphocytes | (Liu et al., 2009) |
| Pancreatic cancer | Curcumin | miRNA expression regulation | Pancreatic cancer cells | (Sung et al., 2008) |
| Adenocarcinoma | Selenium | Inhibition of DNMT1 expression | Adenocarcinoma cell lines | (Li et al., 2009,2009) |
| Metabolic syndrome | ||||
| (+) Genistein | Improvement of insulin sensitivity in liver and muscle | Cynomolgus monkeys fed a high-fat diet | (Howard et al., 2011) | |
| (+) Fisetin | Down-regulation of TNF-α and IL-6 | Human monocytes cultured under hyperglycaemic conditions | (Kim et al., 2012) | |
| (−) Methyl donors | Impairment of fatty acid oxidation | Gestation and lactation diet in rats | (Pooya et al., 2012) | |
| (−) Vitamin B12, folate and methionine | Insulin resistance and hypertension | Maternal periconceptional diet in sheep | (Sinclair et al., 2007) | |
| (−) Vitamin B12, (+) folate | Insulin resistance | Maternal and offspring | (Yajnik et al., 2002) | |
| (+) Methyl donors | Prevention of fatty liver disease | High-fat sucrose fed rats | (Cordero et al., 2013a) | |
| (+) Folate | Decrease of homocysteine and improvement of insulin resistance | Diabetic men | (Gargari et al., 2011) | |
| (+) Betaine | Hepatoprotective effect in non-alcoholic fatty liver disease | Mice fed a high-fat diet | (Wang et al., 2010) | |
| Neurodegenerative disorders | ||||
| Polyphenols | Modulation of miRNA expression (beneficial effects on brain function) | Apolipoprotein E-deficient mice | (Milenkovic et al., 2012) | |
| Methyl-group donors inducing methylation of SAM | Reduction of amyloid-β production | Cell culture | (Scarpa et al., 2006) | |
| Folic acid | Protective effects against hyperhomocysteinaemia-induced neurotoxicity | Knockout mice | (Kalani et al., 2014) | |
| Tea catechins | Reduces glutamate-induced oxidative cytotoxicity | HT22 mouse hippocampal neurons in vitro | (Kang et al., 2013) | |
| Inhibition of L-DOPA methylation in peripheral compartment and striatum | Rats | |||
| Hormonal dysfunctions in women | ||||
| Breast cancer | AZA and TSA. | Re-expression of ERα | ERα− breast cancer cells | (Fan et al., 2008) |
| Botanical oestrogens (genistein, daidzein, equol and liquiritigenin) | Induction of ERβ; higher concentrations also ERα | MCF-7 breast cancer cells | (Jiang et al., 2013) | |
| Hormone-induced memory | 17β-estradiol | Increased histone acetylation, increase of DNMT3A, DNMT3B | Dorsal hippocampus | (Frick et al., 2011) |
Epigenetics in therapy of cancer
Cancer, one of the leading causes of death worldwide, is characterized by rapid formation of abnormal cells and out-of-control cell growth, leading to invasion of other parts of the body. In addition to the standard treatment options such as surgery, chemotherapy and radiotherapy, several epigenetically active food components have been identified as having potential for cancer prevention (Link et al., 2010). Polyphenols execute their chemopreventive action by acting on various intracellular signalling networks through all major epigenetic mechanisms, which results in their beneficial role in cancer initiation and promotion, and in reversing carcinogenesis (Manson, 2003).
Genistein, a phyto-oestrogen from soybeans, has been shown to act in breast cancer through telomerase inhibition (Li et al., 2009) and to have demethylating potential. It reversed DNA hypermethylation through inhibition of DNMT activity in a concentration-dependent manner in different cancer cell lines (Fang et al., 2005). Its effect in humans has also been demonstrated by Qin et al. (2009) in a double-blind study in premenopausal women given different doses of isoflavones daily, which induced reactivation of methylation-silenced genes, RARβ2 and CCND2. Coffee and tea polyphenols also are demethylating agents (Lee and Zhu, 2006), in human breast cancer cell lines where caffeic acid or chlorogenic acid inhibited DNA methylation catalysed by DNMT1, in a concentration-dependent manner because of the increased formation of SAH. A constituent of broccoli, sulforaphane (SFN) induced cell cycle arrest and apoptosis with down-regulation of DNMT1 in human colon cancer cells (Traka et al., 2005). Curcumin also has anticancer properties, in addition to its other beneficial actions on human health. It induced a decrease in DNA methylation in a leukaemia cell line (Liu et al., 2009), as it covalently blocked the catalytic thiolate of DNMT1 and inhibited its activity (Link et al., 2010). In addition, curcumin affects histone modifications (Marcu et al., 2006), as it inhibits HAT, binding covalently to the enzyme. HATs, in particular p300/cAMP-responsive element-binding protein (CBP), have been implicated in cancer cell growth and survival. In a study on prostate cancer cells and peripheral blood lymphocytes, curcumin promoted proteasome-dependent degradation of p300 and CBP protein, inhibited the acetyltransferase activity of purified p300 and effectively blocked histone hyperacetylation. Curcumin has also been shown to inhibit the expression of HDAC1, HDAC3 and HDAC8 (Liu et al., 2005). Another interesting naturally occurring chemical is anacardic acid, present in cashew nuts, which is an inhibitor of HAT-dependent gene transcription. Studying this compound led to synthesis and analysis of mechanisms of many analogues that could become anticancer agents (Eliseeva et al., 2007). Subsequent research elucidated the molecular mechanisms underlying the inhibition of HAT by anacardic acid, involving both inducible and constitutive NF-κB activation and downstream effects (Sung et al., 2008).
Polyphenols exert their effects also through miRNA expression regulation. Curcumin-treated pancreatic cell lines exhibited significantly altered profiles of miRNA expression (Sun et al., 2008). Moreover, when curcumin was added to treatment with gemcitabine for pancreatic cancer, the pro-apoptotic effects of gemcitabine were potentiated (Ali et al., 2010). Similarly, EGCG modulated miRNAs in human hepatocellular carcinoma where the expression of as many as 61 miRNAs was changed. One of the up-regulated miRNAs, miR-16, specifically targets anti-apoptotic protein Bcl-2. Another study compared gemcitabine-sensitive and gemcitabine-resistant pancreatic cancer cells and the effects of isoflavones from soybeans on miRNA expression (Li et al., 2009,2009). Aggressiveness in pancreatic cancer is partly attributable to its characteristic epithelial-to-mesenchymal transition, and this study suggested that isoflavones reversed the epithelial-to-mesenchymal phenotype through regulation of miRNA.
In addition to polyphenols, many other bioactive food components have demonstrated anticancer potential through influencing epigenetic processes, for example folate, selenium, retinoids, fatty acids and isothiocyanates. Selenium anticancer activity involved direct interference with DNA methylation or through its metabolites acting as HDAC inhibitors. Selenium inhibited expression of DNMT1 in an adenocarcinoma cell line and dietary deficiency induced DNA hypomethylation in animal tissues and human colon cancer cells (Davis et al., 2000). Natural organoselenium compounds and their metabolites are important anticancer mediators. They act through redox-sensitive signalling proteins and transcription factors and reduce the risk of cancer development and progression (Pinto et al., 2011). Biologically active isothiocyanates, generated from glucosinolates in cruciferous vegetables, are precursors of substances such as SFN, widely reported as an anticancer agent (Valgimigli and Iori, 2009; Cheung and Kong, 2010). The mechanism behind its anticancer action is the so called ‘one–two’ chemoprotection paradigm, where an electrophilic SFN compound targets Kelch-like ECH-associated protein 1 (Keap1), which in turn releases the transcription factor Nrf2 into the nucleus, and the resulting metabolites act as HDAC inhibitors. Following this step, tumour suppressor genes are activated and lead to cell cycle arrest and apoptosis (Dashwood and Ho, 2007).
Epigenetics in therapy of metabolic syndrome
Metabolic syndrome is defined as a cluster of dysmetabolic features, including central obesity, hypertension, dyslipidaemia, and impaired glucose utilization which together increase the risk for cardiovascular diseases and type 2 diabetes (Eckel et al., 2010). Factors such as lifestyle (Alberti et al., 2005) and diet directly influence the epigenetic regulation of key energy metabolism genes (Milagro et al., 2013). For instance, leptin involved in appetite regulation (Cordero et al., 2011), insulin receptor in glucose homeostasis (Plagemann et al., 2010), TNF-α in inflammation and insulin resistance (Cordero et al., 2011), and fatty acid synthase (FASN) in lipid storage are controlled by epigenetic regulation.
Polyphenols and other plant compounds are considered as potential therapeutic agents to treat obesity-mediated inflammation and oxidative stress, as well as other metabolic syndrome-related diseases including type 2 diabetes, atherosclerosis and hypertension (Fraga et al., 2010). EGCG, genistein, curcumin and resveratrol are some of the phytochemicals that act through epigenetic mechanisms and have been demonstrated to trigger the anti-inflammatory machinery and ameliorate some of the symptoms accompanying metabolic syndrome (Milagro et al., 2013). However, so far only genistein has been directly related to epigenetic changes. Dolinoy et al. (2006) described maternal genistein supplementation (250 mg·kg−1) as protective against obesity in Agouti mouse offspring (Dolinoy et al., 2006). These changes were mediated by modifying the foetal epigenome, with an increase in DNA methylation in a retrotransposon upstream of the transcription start site of the Agouti gene (Dolinoy et al., 2006). Similarly, DNA methylation levels were increased in liver and muscle tissues in monkeys fed with a diet supplemented with soy components, such as genistein, and this was associated with increased insulin sensitivity (homeostasis model assessment index) (Dolinoy et al., 2006; Howard et al., 2011). Other recent studies have demonstrated that genistein has anti-diabetic effects and epigenetically regulates the cAMP/PKA signalling pathway (Gilbert and Liu, 2013). The flavonoid fisetin inhibited TNF-α and IL-6 expression levels and suppressed NF-kB transcription activity in cultures of human monocytes (Kim et al., 2012). These anti-inflammatory responses may be mediated by up-regulation of HDAC activity and down-regulation of HAT, which thereby prevent NF-kB-mediated chromatin acetylation and subsequent transcription of cytokines in hyperglycaemic conditions. A dietary supplementation with apple extracts (700 mg·kg−1 body weight) – rich in the polyphenols chlorogenic acid, phloridzin, quercetin, catechin, epicatechin, procyanidin and rutin – prevented body weight gain and ameliorated hyperglycaemia, hyperleptinaemia, and insulin resistance in rats fed a high-fat sucrose diet for 8 weeks. These results were accompanied by decreased methylation of two CpG sites in the leptin promoter of rat epididymal adipocytes (Boque et al., 2013). Increased leptin production caused by an overexpression of FFAR3 can be also induced by short-chain fatty acids (SCFA) (Ichimura et al., 2009), indicating that supplementation with SCFAs may help to improve the sensitivity to, or the production of, leptin (Remely et al., 2013). Leptin is exclusively produced in adipose tissue and therefore influences the hunger–satiety cycle by suppression of appetite and regulation of fat metabolism (Schloegl et al., 2010).
Compounds implicated in one-carbon metabolism, such as methionine, betaine, folate and vitamin B12, induce methylation and influence expression of target genes (Forges et al., 2007). Pogribny et al. (2009) reported that a methyl-deficient diet in adult mice induced accumulative morphological changes in the liver similar to human non-alcoholic steatohepatitis, and was accompanied by epigenetic abnormalities, including histone modifications and DNA methylation, especially at major and minor satellites (Pogribny et al., 2009). Most of the evidence has arisen from dietary manipulation in maternal models. A methyl donor deficiency during gestation and lactation impaired PPAR-γ co-activator-mediated fatty acid oxidation in rat pups (Pooya et al., 2012). Previously, Sinclair et al. (2007) reported that a restriction of vitamin B12, folate and methionine in sheep caused DNA methylation alterations in the offspring, and was associated with insulin resistance and elevated BP later in life (Sinclair et al., 2007). In rats, perinatal folate deficiency induced modest changes in the insulin axis of the foetus (Maloney et al., 2009) and programmed glucose homeostasis in adult male offspring (Maloney et al., 2011). In humans, an association between maternal vitamin B12 deficiency, obesity and gestational diabetes has been reported (Yajnik and Deshmukh, 2008). Maternal folate supplementation was shown to reduce the risk of metabolic syndrome in Nepalese children (Stewart et al., 2009). On the other hand, maternal vitamin B12 deficiency and folate supplementation were associated with offspring insulin resistance at 6 years of age (Yajnik et al., 2002). Folate supplementation during gestation may contribute to the obese phenotype of the offspring during adulthood because of epigenetic effects on the hypothalamic mechanisms regulating food intake, which can be reversed by increasing the folate content in the pup diet (Cho et al., 2013). There is growing evidence that maternal dietary imbalances may be a cause of susceptibility of the offspring to chronic diseases later in life. However, it can be partially reversed by methyl donor supplementation in adult life. Cordero et al. (2013a,b,) reported that dietary methyl donor supplementation prevented high-fat diet-induced non-alcoholic fatty liver in rats (Cordero et al., 2013a), and that alterations in the methylation profile of hepatic genes, such as FASN (Cordero et al., 2013b) or sterol regulatory element-binding transcription factor 29 (Cordero et al., 2013a), could be implicated. Additional studies have described the beneficial effects of folate supplementation in obese individuals with type 2 diabetes (Gargari et al., 2011) and betaine supplementation in adipose tissue dysfunction and insulin resistance in mice (Wang et al., 2010).
Minerals have also been shown to increase risk or protect from obesity, atherosclerosis or insulin resistance. So far, only magnesium has been linked to epigenetics (Takaya et al., 2011). Thus, low-magnesium status has been associated with diverse pathological conditions characterized by chronic inflammatory stress, such as atherosclerosis, hypertension, osteoporosis, diabetes and obesity (Nielsen, 2010). In addition, magnesium deficiency in pregnant rats induced metabolic complications in the offspring by altering cytosine methylation in the hepatic hydroxysteroid dehydrogenase-2 promoter (Takaya et al., 2011).
Epigenetics in therapy of neurodegenerative disorders
The majority of neurodegenerative diseases are defined as complex multifactorial disorders as both familial and sporadic forms are known, with genetic and environmental factors contributing to their onset. Alzheimer's diseases (AD), Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS) represent the three major neurodegenerative diseases, affecting several million people worldwide. Studies on families of patients with these diseases led to the identification of several genes and pathways responsible for the inherited forms. In parallel, hundreds of genes have been investigated in genetic association studies, and more recently, in genome-wide association studies revealing novel polymorphisms of susceptibility genes likely to contribute to the sporadic forms (Migliore and Coppede, 2009; Coppede, 2012). Among identified pathways, those involving oxidative stress have been widely explored in the last years (Markesbery, 1997). In this context, dietary and environmental enrichments have been suggested, as possible therapeutically or preventive approaches linked to the antioxidant pathway in neurodegeneration.
A variety of antioxidant compounds derived from natural products have demonstrated neuroprotective activity in either in vitro or in vivo models of neuronal cell death or neurodegeneration (Choi et al., 2012; Milenkovic et al., 2012). These natural antioxidants belong to several distinct groups based on their chemical structures: flavonoid polyphenols EGCG and quercetin (Kang et al., 2013); non-flavonoid polyphenols such as curcumin and resveratrol; phenolic acids or phenolic diterpenes such as rosmarinic acid or carnosic acid; and organosulfur compounds including the isothiocyanate, L-SFN and the thiosulfonate allicin (from garlic). One of the major advantages in using natural compounds is their ability to cross the blood–brain barrier. These compounds can act directly by scavenging free radicals or indirectly by increasing endogenous cellular antioxidant defences, for example, via activation of the Nrf2 transcription factor pathway (Kelsey et al., 2010).
Overall, there is a rapidly growing body of evidence linking epigenetic alterations to the development of neurodegenerative disorders (Migliore and Coppede, 2009; Urdinguio et al., 2009). Many of the processes with a key role in neurodegeneration, such as Aβ plaque deposition and the cleavage of amyloid precursor protein (APP) by neurosecretases in AD, the formation of Lewy bodies containing aggregates of α-synuclein and the dopaminergic neuron degeneration in PD, can be now analysed in light of the new epigenetic knowledge (Coppede, 2012; Jakovcevski and Akbarian, 2013). For instance, DNA methylation plays a critical role in learning and memory and several genes have been found to be hypomethylated in AD (Coppede, 2012). Hence, epigenetic drugs, such as the nutrients that donate methyl groups to the methylation of DNA (SAM and L-methylfolate) can help in the prevention and treatment of AD (Scarpa et al., 2006; Kalani et al., 2014). In addition, histone acetylation is thought to play a critical role in cognitive functions such as learning and memory, and many learning and memory disorders are associated with impaired histone acetylation (Day and Sweatt, 2012). Preclinical studies have suggested compounds that increase histone acetylation by inhibiting HDAC could be useful in the treatment of AD. In general, pharmacological modulation of aberrant epigenetic patterns is considered a very promising treatment in neurodegenerative diseases (Gray, 2011; Adwan and Zawia, 2013; Harrison and Dexter, 2013; Simoes-Pires et al., 2013; Varela et al., 2013).
The hippocampus undergoes selective neurodegeneration, but it is also one of the two structures in the adult brain where the formation of new neurons (neurogenesis) persists. The level of neurogenesis in the adult hippocampus has been linked directly to cognition and mood and undergoes epigenetic regulation. For example, the histone deacetylase inhibitor, valproic acid, induces neuronal differentiation of adult hippocampal progenitors most likely through the induction of neurogenic transcription factors. Several studies have reported decreased adult hippocampal neurogenesis in mouse models of AD, and mouse models of PD show a decrease in the survival rate of new hippocampal neurons. Therefore, modulation of adult hippocampal neurogenesis by diet emerges as a possible mechanism by which nutrition can influence learning and memory abilities, as well as mood. Studies in rodents have demonstrated that caloric restriction, ω-3 fatty acids, flavonoids, blueberry and low concentrations of curcumin increased adult hippocampal neurogenesis. On the contrary, folate deficiency, increased homocysteine levels, zinc deficiency, vitamin A deficiency and high-fat diets decreased or inhibited adult hippocampal neurogenesis (Stangl and Thuret, 2009).
However, there are no drugs targeting epigenetic pathways that have reached advanced clinical development so far (Phase II/III trials) (Stangl and Thuret, 2009; Ebrahimi and Schluesener, 2012; Essa et al., 2012). For many nutraceutical compounds considered to act, at least in part, as antioxidants, alternative mechanisms of action have been suggested for the neuroprotective effects such as modulation of signal transduction cascades or effects on gene expression (Kelsey et al., 2010; Reuter et al., 2011; Pham and Lee, 2012). In particular, neurodegenerative diseases present with many altered targets and pathways, so it is unlikely that we can identify individual pathways and single therapeutic targets to treat these diseases. Indeed, natural compounds display multiple components, functions, targets and pathways (Zhao, 2009). Therefore phytochemicals with already proven therapeutic potential towards a series of complex diseases could be able to effectively restore a physiological pattern of gene expression in neurodegenerative processes, but this needs to be established or confirmed in preclinical and clinical studies.
Epigenetics in therapy of hormonal dysfunction in women
Sex steroid hormones, oestrogen and progesterone, are mainly derived from the ovaries, but also from adipose tissue or oestrogenic compounds in the diet (Helferich et al., 2008). Dysfunctions related to these hormones are very common in women and impair their life by aberrant menstruation cycle, haemorrhage or complications in menopause. However, they are also important in the development of primary and secondary sexual organs, and during pregnancy. High concentrations are known to support bone density (Setchell et al., 2002; Branca, 2003; Cotter and Cashman, 2003), relieve postmenopausal symptoms (Anderson et al., 1999; Vincent and Fitzpatrick, 2000; Jenks et al., 2012; Taku et al., 2012; Ye et al., 2012) and prevent cardiovascular diseases (Anderson et al., 1999), but increased incidence of breast or ovarian cancer via over-activation of oestrogen receptors (ER) has also been reported (Hilakivi-Clarke et al., 2010). Therefore, a permanent or chronic dietary exposure to botanical oestrogens, for example genistein, daidzein, equol and liquiritigenin, or even oral contraceptives may be a risk (Hilakivi-Clarke et al., 2010).
The soy isoflavone, daidzein, is converted by intestinal bacteria into S-equol, a non-steroidal oestrogen (Kim et al., 2010). This metabolism varied not only with the ethnicity and sex of the subjects (Fujimoto et al., 2008; Hong et al., 2012; Usui et al., 2012), but also with the changing equol production potential over the lifetime (Lu et al., 1996; Frankenfeld et al., 2005; Ko et al., 2010; Franke et al., 2011; 2012,), and on derived nutrients. Lampe et al. (1998) reported a diet rich in fibre and carbohydrates as supportive in the degradation of daidzein (Lampe et al., 1998) whereas Gardana et al. (2009) stated the opposite: less dietary fibre and more lipids from animal origin (Gardana et al., 2009). Scott and Miller (2008) indicated premenopausal use of oral contraceptives as predictive of endogenous oestrogen production in mammary cells via CYP enzymes. Isoflavonoids in high blood concentrations might also act in concert to diminish CYP activity in vivo. Changes in epigenetic patterns are potential mechanisms for long-term down-regulation of CYP enzymes, as the transcription of both CYP1A1 and CYP3A4 is influenced by HDAC and DNMT activities. However, potential interactive effects of isoflavonoid combinations must be taken into consideration (Scott and Miller, 2008).
Oestrogens and oestrogen-related substances act through the ERs: The ERα is mainly found in breast, uterus, hypophysis and hypothalamus and drives breast cancer cell proliferation via gene transcription by direct recruitment to target genes. The ERβ is mainly present in bones, blood vessels and the hippocampus and this receptor type dampens the effects of ERα activation. A CpG methylation of the ER promoter results in transcriptional silencing, whereas an inhibition of HDAC and/or DNMT activity reactivates ER expression (Fan et al., 2008). Induction of HDAC and co-repressor complexes promotes histone deacetylation, a closed chromatin conformation. DNA hypermethylation in the ER promoter (Fan et al., 2008) and accordingly histone hypoacetylation, H3K9 methylation and recruitment of methyl-CpG-binding protein (MeCP2), MBD1 (methyl-CpG-binding domain), MBD2, DNMT1, DNMT3b and HDAC1 proteins silences the transcription (Fan et al., 2008; Mann et al., 2011). Non-genomic actions of ER include activation of other growth factor receptors: cellular tyrosine kinases, the MAPKs, PI3 kinase and the Akt signalling pathway. The extranuclear signalling of these kinase cascades includes direct modifications of histone tails or indirect influence of functions and recruitment of histone-modifying enzymes targeting cell survival and cell proliferation (Saxena and Sharma, 2010; Mann et al., 2011). The cell cycle-dependent phosphorylation via modulation of extranuclear kinases of histone H3 is driven by oestrogen. Among others, cellular proliferation and apoptosis is promoted via induction of the MAPK cascades, especially MAPK pathways involving ERK-1 and -2 that are important in breast cancer. ERα transcription also requires the lysine demethylase 1 (KDM1), which demethylates H3K9 and additional methyl marks such as H2K4me2. A therapy disabling the functions of KDM1 together with endocrine therapy also shows promising outcomes. The ER-α co-repressor proline-, glutamic acid- and leucine-rich protein-1 (PELP1) deregulation influences histone methylation at ERα genes and therefore contributes to hormone-driven tumour progression and therapy resistance (Mann et al., 2011).
An unmethylated active ER promoter is enriched in H3 and H4 acetylation and H3K4 methylation, with reduced binding of any methyl binding protein or DNMT. Induced histone acetylation promotes an open chromatin configuration and recruitment of the transcription machinery. The activated ER dimerises and binds to the promoter region at ER response elements (ERE) inducing the transactivation function via recruited HATs activity and co-activators (such as. SRC-1, SRC-2, AIB-1, PELP1, CBP, p300, PCAF, CARM1, PRMT1). Therefore, HDAC inhibitors, for example trichostatin A (TSA), and DNMT inhibitors, for example 5-aza-2-deoxycytidine (AZA), induce a re-expression of ER in vitro and in vivo (Figure 2; Fan et al., 2008; Saxena and Sharma, 2010). An additional treatment of the re-expressed ERα tumour cells with selective ER modulators (SERMs), for example tamoxifen, induces cell death of the tumour cells via binding to ERα (Fan et al., 2008; Mann et al., 2011). A specific assortment of SERMs is required to enable selective binding affinities to the different domains in the receptors. Combined therapy with DNMTs and HDAC inhibitors also reactivates silenced tumour suppressor genes (MLH1, TIMP3, CDKN2B, CDKN2A, gelsolin, maspin) (Saxena and Sharma, 2010), which is another mechanism of growth inhibition (Fan et al., 2008). The treated cells are arrested in the S phase without entering G2/M phase. Oestrogen induces the release of the treated cells from S phase to G2/M phase and SERMs block its effects, in addition the growth suppression correlates with ERα re-expression. However, an induced ERβ expression and tamoxifen binding cannot be excluded in these effects as TSA alone without apparent ERα also restored a response to tamoxifen (Fan et al., 2008). However, only tissues expressing ER, especially cancer cells, can be treated with endocrine therapies (Saxena and Sharma, 2010). According to their binding affinities, botanical oestrogens preferentially induce ERβ, although at high concentrations, reverse effects – stimulation of cell proliferation through ERα – are induced (Jiang et al., 2013). Therefore, depending on relative levels of the two ERs in target cells and the dose of botanical oestrogens, the gene expression and proliferative response can be differentially regulated and determine potential benefits and risks of botanical oestrogens (Jiang et al., 2013). Ferlay et al. (2004) showed that Asian women consuming soy as a part of their stable diet have a three- to fivefold lower breast cancer risk than Caucasian women with irregular soy consumption (Ferlay et al., 2004). Thus, a diet rich in soy may promote the ratio of ERβ to ERα, but an irregular consumption may have adverse effects. Taken together, approaches addressing the presence of ERs in invasive breast cancers are targets for further hormonal therapies.
Figure 2.
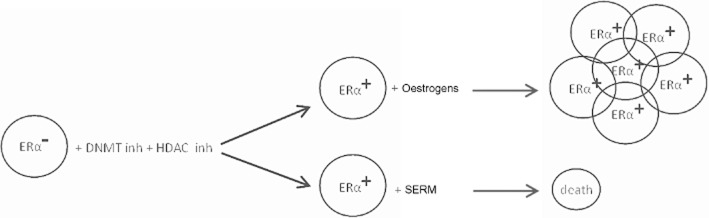
Treatment of tumour cells not expressing ERα (ERα−) with DNMT inhibitors, for example AZA, and HDAC inhibitors, for example TSA, induces re-expression of the ERα receptors in the tumour cells. Thus, the cell is sensitive to oestrogens again, which promote cell proliferation. However, treatment of the ERα+ tumour cells with SERM, for example tamoxifen, induces cell death of these cells. inh, inhibitor.
In postmenopausal women suffering from hot flushes, night sweats, mood changes, urogenital atrophy, and loss of bone density as a result of oestrogen reduction, treatment with botanical oestrogens in addition to standard hormone replacement therapy is indicated (Jiang et al., 2013). S-equol, a non-steroidal oestrogen formed from daizdein, reduced the frequency of hot flushes, menopause-related muscle pain and joint pain (Jenks et al., 2012; Taku et al., 2012; Ye et al., 2012), and improved bone density (Setchell et al., 2002). Further, oestrogen deficiency increased body weight and abdominal fat, decreased lean mass and contributed to a higher risk of metabolic disorders. In vitro as well as in vivo studies suggested soy as a potential therapeutic agent for reduction of fat mass and weight, by an increase of energy utilization (Vaughn et al., 2008), for decreased fat accumulation and a loss of visceral adipose tissue (Yamori, 2004; Nachtigal et al., 2005). Correlations with a lower body mass index, waist circumference and body fat mass were observed (Liu et al., 2013). Supplementation with S-equol in obese and overweight patients significantly improved long-term glycaemic control and cardiovascular health by lowering glycohaemoglobin, LDL-C and arterial stiffness (Usui et al., 2012). Loss of body weight and fat mass was also reported (Choquette et al., 2011). However, others reported none of these effects (Azadbakht and Nurbakhsh, 2011; Baer et al., 2011; Takahira et al., 2011; Liu et al., 2013). Differences in population characteristics, types of soy products, intervention period, methods used and microbial fluctuation in isoflavone degradation may be responsible for these discrepancies (Liu et al., 2013). Epigenetic treatments during age-related sex-steroid hormone loss in menopause should also be assessed in terms of cognitive benefits, as a loss of oestrogen is correlated with cognitive decline, dementia and AD (Frick et al., 2011; Winkler and Fox, 2013).
The potent oestrogen, 17β-estradiol (E2), enhanced the object recognition memory not only via genomic mechanisms, but also via epigenetic mechanism. Increased histone acetylation is suggested to enhance hippocampal long-term memory. A dorsal hippocampal infusion of E2 significantly increased the ERK-dependent acetylation of H3. In addition, evidence is accumulating that DNMT inhibitors (AZA, zebularine) block contextual fear memory and hippocampal long-term memory. DNMT3A and DNMT3B may play a role in memory-enhancing effects as a significant increase in both de novo methyltransferases was observed after 45 min infusion of E2 and increased DNMT3B protein 4 h after infusion. However, they also prevented an increase in histone H3 acetylation. Therefore, a synergy between both epigenetic regulations in the modulation of memory is suggested, although these interactions have to be explored more thoroughly and side effects must be taken into consideration (Frick et al., 2011).
Conclusions
There is a growing body of evidence that a diet containing epigenetically active food compounds plays an important role in numerous aspects of health. Crucial data have been gathered on the involvement of epigenetic mechanisms in different disorders. Therefore, further research should address the (i) molecular basis of epigenetic modifications together with possible interactions; (ii) epigenetic alterations in disease progression; (iii) elucidation of therapeutic mode of action; and (iv) development of new therapeutic agents targeting epigenetic modifications.
Conflict of interest
The authors declare to have no actual or potential competing interests that might be perceived as influencing the results or interpretation of a reported study.
Glossary
- 5-hmC
5-hydroxymethylcytosine
- AD
Alzheimer's disease
- AZA
5-aza-2-deoxycytidine
- CBP
CREB-Binding Protein
- DNMT
DNA methyltransferases
- E2
17β-estradiol
- EGCG
epigallocatechin gallate
- ER
oestrogen receptor
- ERE
oestrogen receptor response elements
- HAT
histone acetyl-transferase
- HDAC
histone deacetylase
- KDM1
lysine demethylase 1
- MBD
methyl-CpG-binding domain
- miRNA
microRNA
- Nrf2
nuclear factor erythroid-derived 2-related factor 2
- PD
Parkinson's disease
- PELP
proline-, glutamic acid- and leucine-rich protein-1
- SAH
S-adenosylhomocysteine
- SAM
S-adenosyl-L-methionine
- SCFA
short-chain fatty acid
- SERM
selective oestrogen receptor modulators
- SFN
sulforaphane
- SIRT1
sirtuin-1
- TSA
trichostatin A
References
- Adwan L, Zawia NH. Epigenetics: a novel therapeutic approach for the treatment of Alzheimer's disease. Pharmacol Ther. 2013;1:41–50. doi: 10.1016/j.pharmthera.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet. 2005;9491:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. British Journal of Pharmacology. 2013a;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol. 2013b;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic Receptors. British Journal of Pharmacology. 2013c;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;9:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Anderson JJ, Anthony MS, Cline JM, Washburn SA, Garner SC. Health potential of soy isoflavones for menopausal women. Public Health Nutr. 1999;4:489–504. doi: 10.1017/s1368980099000671. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Smith BM, Washnock CS. Cardiovascular and renal benefits of dry bean and soybean intake. Am J Clin Nutr. 1999;3(Suppl):464S–474S. doi: 10.1093/ajcn/70.3.464s. [DOI] [PubMed] [Google Scholar]
- Ayissi VB, Ebrahimi A, Schluesenner H. Epigenetic effects of natural polyphenols: a focus on SIRT1-mediated mechanisms. Mol Nutr Food Res. 2014;1:22–32. doi: 10.1002/mnfr.201300195. [DOI] [PubMed] [Google Scholar]
- Azadbakht L, Nurbakhsh S. Effect of soy drink replacement in a weight reducing diet on anthropometric values and blood pressure among overweight and obese female youths. Asia Pac J Clin Nutr. 2011;3:383–389. [PubMed] [Google Scholar]
- Baer DJ, Stote KS, Paul DR, Harris GK, Rumpler WV, Clevidence BA. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J Nutr. 2011;8:1489–1494. doi: 10.3945/jn.111.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boque N, de la Iglesia R, de la Garza AL, Milagro FI, Olivares M, Banuelos O, et al. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol Nutr Food Res. 2013;8:1473–1478. doi: 10.1002/mnfr.201200686. [DOI] [PubMed] [Google Scholar]
- Branca F. Dietary phyto-oestrogens and bone health. Proc Nutr Soc. 2003;4:877–887. doi: 10.1079/PNS2003309. [DOI] [PubMed] [Google Scholar]
- Brandl A, Heinzel T, Kramer OH. Histone deacetylases: salesmen and customers in the post-translational modification market. Biol Cell. 2009;4:193–205. doi: 10.1042/BC20080158. [DOI] [PubMed] [Google Scholar]
- Brosch G, Loidl P, Graessle S. Histone modifications and chromatin dynamics: a focus on filamentous fungi. FEMS Microbiol Rev. 2008;3:409–439. doi: 10.1111/j.1574-6976.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;1:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka A, Chittka L. Epigenetics of royalty. PLoS Biol. 2010;11:e1000532. doi: 10.1371/journal.pbio.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CE, Sanchez-Hernandez D, Reza-Lopez SA, Huot PS, Kim YI, Anderson GH. High folate gestational and post-weaning diets alter hypothalamic feeding pathways by DNA methylation in Wistar rat offspring. Epigenetics. 2013;7:710–719. doi: 10.4161/epi.24948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DY, Lee YJ, Hong JT, Lee HJ. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease. Brain Res Bull. 2012;2–3:144–153. doi: 10.1016/j.brainresbull.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Choi SW, Friso S. Epigenetics: a new bridge between nutrition and health. Adv Nutr. 2010;1:8–16. doi: 10.3945/an.110.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquette S, Riesco E, Cormier E, Dion T, Aubertin-Leheudre M, Dionne IJ. Effects of soya isoflavones and exercise on body composition and clinical risk factors of cardiovascular diseases in overweight postmenopausal women: a 6-month double-blind controlled trial. Br J Nutr. 2011;8:1199–1209. doi: 10.1017/S0007114510004897. [DOI] [PubMed] [Google Scholar]
- Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;11:2121–2127. doi: 10.2337/dc12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppede F. Genetics and epigenetics of Parkinson's disease. ScientificWorldJournal. 2012;2012:489830. doi: 10.1100/2012/489830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero P, Campion J, Milagro FI, Goyenechea E, Steemburgo T, Javierre BM, et al. Leptin and TNF-alpha promoter methylation levels measured by MSP could predict the response to a low-calorie diet. J Physiol Biochem. 2011;3:463–470. doi: 10.1007/s13105-011-0084-4. [DOI] [PubMed] [Google Scholar]
- Cordero P, Campion J, Milagro FI, Martinez JA. Transcriptomic and epigenetic changes in early liver steatosis associated to obesity: effect of dietary methyl donor supplementation. Mol Genet Metab. 2013a;3:388–395. doi: 10.1016/j.ymgme.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Cordero P, Gomez-Uriz AM, Campion J, Milagro FI, Martinez JA. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2013b;8:105–113. doi: 10.1007/s12263-012-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter A, Cashman KD. Genistein appears to prevent early postmenopausal bone loss as effectively as hormone replacement therapy. Nutr Rev. 2003;10:346–351. doi: 10.1301/nr.2003.oct.346-351. [DOI] [PubMed] [Google Scholar]
- Cyr AR, Hitchler MJ, Domann FE. Regulation of SOD2 in cancer by histone modifications and CpG methylation: closing the loop between redox biology and epigenetics. Antioxid Redox Signal. 2013;15:1946–1955. doi: 10.1089/ars.2012.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol. 2007;5:363–369. doi: 10.1016/j.semcancer.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CD, Uthus EO, Finley JW. Dietary selenium and arsenic affect DNA methylation in vitro in Caco-2 cells and in vivo in rat liver and colon. J Nutr. 2000;12:2903–2909. doi: 10.1093/jn/130.12.2903. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology. 2012;1:247–260. doi: 10.1038/npp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;4:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi A, Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res Rev. 2012;2:329–345. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;9710:181–183. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- Eliseeva ED, Valkov V, Jung M, Jung MO. Characterization of novel inhibitors of histone acetyltransferases. Mol Cancer Ther. 2007;9:2391–2398. doi: 10.1158/1535-7163.MCT-07-0159. [DOI] [PubMed] [Google Scholar]
- Essa MM, Vijayan RK, Castellano-Gonzalez G, Memon MA, Braidy N, Guillemin GJ. Neuroprotective effect of natural products against Alzheimer's disease. Neurochem Res. 2012;9:1829–1842. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- Fan J, Yin WJ, Lu JS, Wang L, Wu J, Wu FY, et al. ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol. 2008;8:883–890. doi: 10.1007/s00432-008-0354-x. [DOI] [PubMed] [Google Scholar]
- Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;19(Pt 1):7033–7041. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2004;6:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Forges T, Monnier-Barbarino P, Alberto JM, Gueant-Rodriguez RM, Daval JL, Gueant JL. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update. 2007;3:225–238. doi: 10.1093/humupd/dml063. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med. 2010;6:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Franke AA, Lai JF, Halm BM, Pagano I, Kono N, Mack WJ, et al. Equol production changes over time in postmenopausal women. J Nutr Biochem. 2011;23:573–579. doi: 10.1016/j.jnutbio.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke AA, Lai JF, Pagano I, Morimoto Y, Maskarinec G. Equol production changes over time in pre-menopausal women. Br J Nutr. 2012;8:1201–1206. doi: 10.1017/S0007114511004223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenfeld CL, Atkinson C, Thomas WK, Gonzalez A, Jokela T, Wahala K, et al. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br J Nutr. 2005;6:873–876. doi: 10.1079/bjn20051565. [DOI] [PubMed] [Google Scholar]
- Franks PW, Ling C. Epigenetics and obesity: the devil is in the details. BMC Med. 2010;8:88. doi: 10.1186/1741-7015-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Zhao Z, Fan L. The epigenetics of estrogen: epigenetic regulation of hormone-induced memory enhancement. Epigenetics. 2011;6:675–680. doi: 10.4161/epi.6.6.16177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Tanaka M, Hirao Y, Nagata Y, Mori M, Miyanaga N, et al. Age-stratified serum levels of isoflavones and proportion of equol producers in Japanese and Korean healthy men. Prostate Cancer Prostatic Dis. 2008;3:252–257. doi: 10.1038/sj.pcan.4501030. [DOI] [PubMed] [Google Scholar]
- Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;5:490–495. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gardana C, Canzi E, Simonetti P. The role of diet in the metabolism of daidzein by human faecal microbiota sampled from Italian volunteers. J Nutr Biochem. 2009;12:940–947. doi: 10.1016/j.jnutbio.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Gargari BP, Aghamohammadi V, Aliasgharzadeh A. Effect of folic acid supplementation on biochemical indices in overweight and obese men with type 2 diabetes. Diabetes Res Clin Pract. 2011;1:33–38. doi: 10.1016/j.diabres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic beta-cell function. Food Funct. 2013;2:200–212. doi: 10.1039/c2fo30199g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SG. Epigenetic treatment of neurological disease. Epigenomics. 2011;4:431–450. doi: 10.2217/epi.11.67. [DOI] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;1:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison IF, Dexter DT. Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson's disease? Pharmacol Ther. 2013;1:34–52. doi: 10.1016/j.pharmthera.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;5:219–226. doi: 10.1007/s10787-008-8020-0. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr. 2010;12:2326S–2334S. doi: 10.3945/jn.110.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KW, Ko KP, Ahn Y, Kim CS, Park SJ, Park JK, et al. Epidemiological profiles between equol producers and nonproducers: a genomewide association study of the equol-producing phenotype. Genes Nutr. 2012;7:567–574. doi: 10.1007/s12263-012-0292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard TD, Ho SM, Zhang L, Chen J, Cui W, Slager R, et al. Epigenetic changes with dietary soy in cynomolgus monkeys. PLoS ONE. 2011;10:e26791. doi: 10.1371/journal.pone.0026791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009;3–4:82–88. doi: 10.1016/j.prostaglandins.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Jakovcevski M, Akbarian S. Epigenetic mechanisms in neurological disease. Nat Med. 2013;8:1194–1204. doi: 10.1038/nm.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks BH, Iwashita S, Nakagawa Y, Ragland K, Lee J, Carson WH, et al. A pilot study on the effects of S-equol compared to soy isoflavones on menopausal hot flash frequency and other menopausal symptoms. J Womens Health (Larchmt) 2012;21:674–682. doi: 10.1089/jwh.2011.3153. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Gong P, Madak-Erdogan Z, Martin T, Jeyakumar M, Carlson K, et al. Mechanisms enforcing the estrogen receptor beta selectivity of botanical estrogens. FASEB J. 2013;11:4406–4418. doi: 10.1096/fj.13-234617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Chillaron JC, Diaz R, Martinez D, Pentinat T, Ramon-Krauel M, Ribo S, et al. The role of nutrition on epigenetic modifications and their implications on health. Biochimie. 2012;11:2242–2263. doi: 10.1016/j.biochi.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Givvimani S, Brown K, Metreveli N, Tyagi SC, et al. Nutri-epigenetics ameliorates blood–brain barrier damage and neurodegeneration in hyperhomocysteinemia: role of folic acid. J Mol Neurosci. 2014;2:202–215. doi: 10.1007/s12031-013-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KS, Yamabe N, Wen Y, Fukui M, Zhu BT. Beneficial effects of natural phenolics on levodopa methylation and oxidative neurodegeneration. Brain Res. 2013;1497:1–14. doi: 10.1016/j.brainres.2012.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;11:7792–7814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Chatterton RT, Michel N, Bryk M, Lee O, Ivancic D, et al. Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer Prev Res (Phila) 2012;2:309–319. doi: 10.1158/1940-6207.CAPR-11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim SH, Yun JM. Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms. Evid Based Complement Alternat Med. 2012;2012:639469. doi: 10.1155/2012/639469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Marsh EN, Kim SU, Han J. Conversion of (3S,4R)-tetrahydrodaidzein to (3S)-equol by THD reductase: proposed mechanism involving a radical intermediate. Biochemistry. 2010;26:5582–5587. doi: 10.1021/bi100465y. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;2:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Ko TF, Tsai HS, Lin SM, Liu CD, Learn SP, Chiou RY. GC-MS determined distribution of urinary equol producers as affected by age, gender, and repeated ingestions of soymilk. J Food Sci. 2010;9:H306–H310. doi: 10.1111/j.1750-3841.2010.01860.x. [DOI] [PubMed] [Google Scholar]
- Kondo T. Epigenetic alchemy for cell fate conversion. Curr Opin Genet Dev. 2006;5:502–507. doi: 10.1016/j.gde.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;3:335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Zhu BT. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis. 2006;2:269–277. doi: 10.1093/carcin/bgi206. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu L, Andrews LG, Tollefsbol TO. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int J Cancer. 2009;2:286–296. doi: 10.1002/ijc.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, VandenBoom TG, 2nd, Kong D, Wang Z, Ali S, Philip PA, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;16:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol. 2010;12:1771–1792. doi: 10.1016/j.bcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HL, Chen Y, Cui GH, Zhou JF. Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation. Acta Pharmacol Sin. 2005;5:603–609. doi: 10.1111/j.1745-7254.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, et al. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett. 2009;3:706–709. doi: 10.1016/j.bmcl.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Ho SC, Chen YM, Woo J. A six-month randomized controlled trial of whole soy and isoflavones daidzein on body composition in equol-producing postmenopausal women with prehypertension. J Obes. 2013;2013:359763. doi: 10.1155/2013/359763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Flint HJ. Development of a semiquantitative degenerate real-time PCR-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. Appl Environ Microbiol. 2007;6:2009–2012. doi: 10.1128/AEM.02561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LJ, Anderson KE, Grady JJ, Nagamani M. Effects of soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev. 1996;1:63–70. [PubMed] [Google Scholar]
- Maloney CA, Hay SM, Rees WD. The effects of feeding rats diets deficient in folic acid and related methyl donors on the blood pressure and glucose tolerance of the offspring. Br J Nutr. 2009;9:1333–1340. doi: 10.1017/S0007114508066798. [DOI] [PubMed] [Google Scholar]
- Maloney CA, Hay SM, Young LE, Sinclair KD, Rees WD. A methyl-deficient diet fed to rat dams during the peri-conception period programs glucose homeostasis in adult male but not female offspring. J Nutr. 2011;1:95–100. doi: 10.3945/jn.109.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M, Cortez V, Vadlamudi RK. Epigenetics of estrogen receptor signaling: role in hormonal cancer progression and therapy. Cancers (Basel) 2011;3:1691–1707. doi: 10.3390/cancers3021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson MM. Cancer prevention – the potential for diet to modulate molecular signalling. Trends Mol Med. 2003;1:11–18. doi: 10.1016/s1471-4914(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Marcu MG, Jung YJ, Lee S, Chung EJ, Lee MJ, Trepel J, et al. Curcumin is an inhibitor of p300 histone acetylatransferase. Med Chem. 2006;2:169–174. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;1:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mattick JS. Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol Rev. 2007;3:799–823. doi: 10.1152/physrev.00036.2006. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res. 2009;1–2:82–97. doi: 10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Milagro FI, Mansego ML, De Miguel C, Martinez JA. Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol Aspects Med. 2013;4:782–812. doi: 10.1016/j.mam.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Milenkovic D, Deval C, Gouranton E, Landrier JF, Scalbert A, Morand C, et al. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: a new mechanism of the action of polyphenols. PLoS ONE. 2012;1:e29837. doi: 10.1371/journal.pone.0029837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtigal MC, Patterson RE, Stratton KL, Adams LA, Shattuck AL, White E. Dietary supplements and weight control in a middle-age population. J Altern Complement Med. 2005;5:909–915. doi: 10.1089/acm.2005.11.909. [DOI] [PubMed] [Google Scholar]
- Nielsen FH. Magnesium, inflammation, and obesity in chronic disease. Nutr Rev. 2010;6:333–340. doi: 10.1111/j.1753-4887.2010.00293.x. [DOI] [PubMed] [Google Scholar]
- Oliver SS, Denu JM. Dynamic interplay between histone H3 modifications and protein interpreters: emerging evidence for a ‘histone language’. Chembiochem. 2011;2:299–307. doi: 10.1002/cbic.201000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TX, Lee J. Dietary regulation of histone acetylases and deacetylases for the prevention of metabolic diseases. Nutrients. 2012;12:1868–1886. doi: 10.3390/nu4121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JT, Lee JI, Sinha R, MacEwan ME, Cooper AJ. Chemopreventive mechanisms of alpha-keto acid metabolites of naturally occurring organoselenium compounds. Amino Acids. 2011;1:29–41. doi: 10.1007/s00726-010-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann A, Roepke K, Harder T, Brunn M, Harder A, Wittrock-Staar M, et al. Epigenetic malprogramming of the insulin receptor promoter due to developmental overfeeding. J Perinat Med. 2010;4:393–400. doi: 10.1515/jpm.2010.051. [DOI] [PubMed] [Google Scholar]
- Pogribny IP, Tryndyak VP, Bagnyukova TV, Melnyk S, Montgomery B, Ross SA, et al. Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol. 2009;1:176–186. doi: 10.1016/j.jhep.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooya S, Blaise S, Moreno Garcia M, Giudicelli J, Alberto JM, Gueant-Rodriguez RM, et al. Methyl donor deficiency impairs fatty acid oxidation through PGC-1alpha hypomethylation and decreased ER-alpha, ERR-alpha, and HNF-4alpha in the rat liver. J Hepatol. 2012;2:344–351. doi: 10.1016/j.jhep.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Zhu W, Shi H, Hewett JE, Ruhlen RL, MacDonald RS, et al. Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr Cancer. 2009;2:238–244. doi: 10.1080/01635580802404196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remely M, Aumueller E, Merold C, Dworzak S, Hippe B, Zanner J, et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2013;1:85–92. doi: 10.1016/j.gene.2013.11.081. [DOI] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;2:93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, et al. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;3:523–535. doi: 10.1002/mnfr.201200594. [DOI] [PubMed] [Google Scholar]
- Sawicka A, Seiser C. Histone H3 phosphorylation – a versatile chromatin modification for different occasions. Biochimie. 2012;11:2193–2201. doi: 10.1016/j.biochi.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NK, Sharma D. Epigenetic reactivation of estrogen receptor: promising tools for restoring response to endocrine therapy. Mol Cell Pharmacol. 2010;5:191–202. [PMC free article] [PubMed] [Google Scholar]
- Scarpa S, Cavallaro RA, D'Anselmi F, Fuso A. Gene silencing through methylation: an epigenetic intervention on Alzheimer disease. J Alzheimers Dis. 2006;4:407–414. doi: 10.3233/jad-2006-9406. [DOI] [PubMed] [Google Scholar]
- Schloegl H, Percik R, Horstmann A, Villringer A, Stumvoll M. Peptide hormones regulating appetite – focus on neuroimaging studies in humans. Diabetes Metab Res Rev. 2010;2:104–112. doi: 10.1002/dmrr.1154. [DOI] [PubMed] [Google Scholar]
- Scott RR, Miller WL. Genetic and clinical features of p450 oxidoreductase deficiency. Horm Res. 2008;5:266–275. doi: 10.1159/000114857. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol – a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;12:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- Simoes-Pires C, Zwick V, Nurisso A, Schenker E, Carrupt PA, Cuendet M. HDAC6 as a target for neurodegenerative diseases: what makes it different from the other HDACs? Mol Neurodegener. 2013;8:7. doi: 10.1186/1750-1326-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A. 2007;49:19351–19356. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squadrito F, Marini H, Bitto A, Altavilla D, Polito F, Adamo EB, et al. Genistein in the metabolic syndrome: results of a randomized clinical trial. J Clin Endocrinol Metab. 2013;8:3366–3374. doi: 10.1210/jc.2013-1180. [DOI] [PubMed] [Google Scholar]
- Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanska B, Huang J, Bhattacharyya B, Suderman M, Hallett M, Han ZG, et al. Definition of the landscape of promoter DNA hypomethylation in liver cancer. Cancer Res. 2011;17:5891–5903. doi: 10.1158/0008-5472.CAN-10-3823. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Christian P, LeClerq SC, West KP, Jr, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school-age children in rural Nepal. Am J Clin Nutr. 2009;1:132–140. doi: 10.3945/ajcn.2008.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008;3:464–473. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- Sung B, Pandey MK, Ahn KS, Yi T, Chaturvedi MM, Liu M, et al. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood. 2008;10:4880–4891. doi: 10.1182/blood-2007-10-117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahira M, Noda K, Fukushima M, Zhang B, Mitsutake R, Uehara Y, et al. Randomized, double-blind, controlled, comparative trial of formula food containing soy protein vs. milk protein in visceral fat obesity – FLAVO study. Circ J. 2011;9:2235–2243. doi: 10.1253/circj.cj-10-1013. [DOI] [PubMed] [Google Scholar]
- Takaya J, Iharada A, Okihana H, Kaneko K. Magnesium deficiency in pregnant rats alters methylation of specific cytosines in the hepatic hydroxysteroid dehydrogenase-2 promoter of the offspring. Epigenetics. 2011;5:573–578. doi: 10.4161/epi.6.5.15220. [DOI] [PubMed] [Google Scholar]
- Taku K, Melby MK, Kronenberg F, Kurzer MS, Messina M. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: systematic review and meta-analysis of randomized controlled trials. Menopause. 2012;19:776–790. doi: 10.1097/gme.0b013e3182410159. [DOI] [PubMed] [Google Scholar]
- Tammen SA, Friso S, Choi SW. Epigenetics: the link between nature and nurture. Mol Aspects Med. 2013;4:753–764. doi: 10.1016/j.mam.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M, Gasper AV, Smith JA, Hawkey CJ, Bao Y, Mithen RF. Transcriptome analysis of human colon Caco-2 cells exposed to sulforaphane. J Nutr. 2005;8:1865–1872. doi: 10.1093/jn/135.8.1865. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;5:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol. 2009;11:1056–1072. doi: 10.1016/S1474-4422(09)70262-5. [DOI] [PubMed] [Google Scholar]
- Usui T, Tochiya M, Sasaki Y, Muranaka K, Yamakage H, Himeno A, et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin Endocrinol (Oxf) 2012;78:365–372. doi: 10.1111/j.1365-2265.2012.04400.x. [DOI] [PubMed] [Google Scholar]
- Valgimigli L, Iori R. Antioxidant and pro-oxidant capacities of ITCs. Environ Mol Mutagen. 2009;3:222–237. doi: 10.1002/em.20468. [DOI] [PubMed] [Google Scholar]
- Varela MA, Roberts TC, Wood MJ. Epigenetics and ncRNAs in brain function and disease: mechanisms and prospects for therapy. Neurother. 2013;4:621–631. doi: 10.1007/s13311-013-0212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn N, Rizzo A, Doane D, Beverly JL, Gonzalez de Mejia E. Intracerebroventricular administration of soy protein hydrolysates reduces body weight without affecting food intake in rats. Plant Foods Hum Nutr. 2008;1:41–46. doi: 10.1007/s11130-007-0067-3. [DOI] [PubMed] [Google Scholar]
- Vincent A, Fitzpatrick LA. Soy isoflavones: are they useful in menopause? Mayo Clin Proc. 2000;11:1174–1184. doi: 10.4065/75.11.1174. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z. Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2010;5:G634–G642. doi: 10.1152/ajpgi.00249.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler JM, Fox HS. Transcriptome meta-analysis reveals a central role for sex steroids in the degeneration of hippocampal neurons in Alzheimer's disease. BMC Syst Biol. 2013;7:51. doi: 10.1186/1752-0509-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajnik CS, Deshmukh US. Maternal nutrition, intrauterine programming and consequential risks in the offspring. Rev Endocr Metab Disord. 2008;3:203–211. doi: 10.1007/s11154-008-9087-z. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Lubree HG, Rege SS, Naik SS, Deshpande JA, Deshpande SS, et al. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab. 2002;12:5575–5580. doi: 10.1210/jc.2002-020434. [DOI] [PubMed] [Google Scholar]
- Yamori Y. Worldwide epidemic of obesity: hope for Japanese diets. Clin Exp Pharmacol Physiol. 2004;31(Suppl):S2–S4. doi: 10.1111/j.1440-1681.2004.04120.x. [DOI] [PubMed] [Google Scholar]
- Ye YB, Wang ZL, Zhuo SY, Lu W, Liao HF, Verbruggen MA, et al. Soy germ isoflavones improve menopausal symptoms but have no effect on blood lipids in early postmenopausal Chinese women: a randomized placebo-controlled trial. Menopause. 2012;19:791–798. doi: 10.1097/gme.0b013e31823dbeda. [DOI] [PubMed] [Google Scholar]
- Zhao B. Natural antioxidants protect neurons in Alzheimer's disease and Parkinson's disease. Neurochem Res. 2009;4:630–638. doi: 10.1007/s11064-008-9900-9. [DOI] [PubMed] [Google Scholar]


