Abstract
Background and Purpose
The orphan nuclear receptor NOR1 belongs to the NR4A subfamily of the nuclear hormone receptor superfamily, and is involved in glucose and fat metabolism. However, its potential contribution to cardiovascular diseases remains to be assessed. Here, the roles of NOR1 in cardiac hypertrophy induced by isoprenaline and the underlying molecular mechanisms were investigated.
Experimental Approach
NOR1 was expressed in cardiomyocytes treated with isoprenaline. After NOR1 overexpression or knockdown in neonatal rat cardiomyocytes, cellular hypertrophy was monitored by measuring cell surface area and the mRNA of hypertrophic biomarkers. Interactions between NOR1 and PARP-1 were investigated by co-immunoprecipitation. NOR1 expression and PARP-1 activity were measured in rats with cardiac hypertrophy induced by isoprenaline.
Key Results
Treatment with isoprenaline significantly up-regulated NOR1 expression and PARP-1 activity both in vivo and in vitro. Specific gene silencing of NOR1 attenuated isoprenaline-induced cardiomyocyte hypertrophy, whereas NOR1 overexpression exacerbated cardiac hypertrophy. We identified a physical interaction between NOR1 and PARP-1, which was enhanced by NOR1 transfection and thereby led to PARP-1 activation. Overexpression of NOR1, but not C293Y, a NOR1 mutant lacking the PARP-1 binding activity, increased cellular surface area and the mRNA levels of atrial natriuretic factor and brain natriuretic polypeptide, effects blocked by the PARP-1 inhibitor 3-aminobenzamide or siRNA for PARP-1.
Conclusions and Implications
This is the first evidence that NOR1 was involved in isoprenaline-induced cardiac hypertrophy. The pro-hypertrophic effect of NOR1 can be partly attributed to its regulation of PARP-1 enzymic activity.
Tables of Links
| TARGETS | |
|---|---|
| Nuclear hormone receptorsa | Enzymesb |
| NOR1 (NR4A3), neuron-derived orphan receptor 1 | PARP-1 |
| Nurr1 (NR4A2) | SIRT1, sirtuin 1 (histone deacetylase) |
| Nur77 (NR4A1) |
| LIGANDS |
|---|
| ANF, atrial natriuretic factor |
| BNP, brain natriuretic peptide |
| Isoprenaline |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guideto PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
Although cardiac hypertrophy initially is a compensatory mechanism for increased neurohormonal stress and haemodynamic load, prolongation of hypertrophic response leads to congestive heart failure. Cardiac hypertrophy is characterized by an increase of protein synthesis, cell size and reactivation of the fetal gene programme at the cellular level (Ruwhof and van der Laarse, 2000; Frey et al., 2004). However, exactly how extracellular hypertrophic stimulation is converted into intracellular signals and how these signals change the transcriptional programme that ultimately leads to cardiac hypertrophy, are still being investigated.
The NR4A orphan nuclear receptor subfamily consists of the highly homologous receptors Nur77 (NR4A1), Nurr1 (NR4A2) and NOR1 (NR4A3). These evolutionarily highly conserved receptors serve as ligand-independent transcription factors that regulate the expression of a wide range of target genes involved in cancer, metabolism, energy balance, atherosclerosis and vascular remodelling (Maxwell and Muscat, 2006; Pols et al., 2007). The orphan nuclear receptor NOR1 was first cloned from neuronal cells, and knockout of NOR1 in mice resulted in hippocampal dysgenesis and inner ear defects (Ponnio et al., 2002; Ponnio and Conneely, 2004). To date, NOR1 has been shown to regulate a number of essential biological processes including inflammation, proliferation and cell survival (Martinez-Gonzalez et al., 2003a; Pei et al., 2005). For example, NOR1 siRNA inhibited human coronary artery smooth muscle cell proliferation (reduced de novo DNA synthesis, cell cycle progression, and vascular smooth muscle cell wound repair), suggesting that NOR1 may be involved in both spontaneous and accelerated atherosclerosis (Martinez-Gonzalez et al., 2003b). Additionally, NOR1 could directly induce expression of the adhesion molecules, ICAM-1 and VCAM-1, which subsequently aggravated atherosclerosis (Zhao et al., 2010). In spite of this progress, the potential roles of NOR1 in the cardiovascular system remain to be clarified.
There is increasing evidence that PARP-1, the key member of the PARP family, plays a pivotal role in the pathogenesis of myocardial ischaemia/reperfusion injury, diabetic cardiovascular complications, circulatory shock and atherosclerosis (Oh et al., 2009). As a DNA damage sensor in the nucleus, PARP-1 becomes activated in response to DNA single-strand breaks and then catalyses the conversion of NAD+ into long poly(ADP-ribose) chains, which can attach to acceptor proteins and thereby alter their function (Schreiber et al., 2006; Luo and Kraus, 2012; Szanto et al., 2012). Mild PARP-1 activation regulates DNA repair, gene transcription, cell survival and chromatin remodelling (Petrucco, 2003; Ame et al., 2004; Kraus, 2008). However, overactivation of PARP-1 depletes NAD+ and subsequently slows ATP formation, eventually leading to functional impairment or cell death (Virag and Szabo, 2002; Szabo et al., 2004). Previously, it has been shown that PARP-1 excessive activation participates in the development and progression of cardiac hypertrophy and heart failure induced by pressure overload (Pillai et al., 2005; Pacher and Szabo, 2007). In addition, angiotensinII (AngII) and isoprenaline can promote production of reactive nitrogen and oxygen species, which subsequently leads to DNA damage and PARP-1 activation with myocardial injury (Pillai et al., 2006; Bartha et al., 2008a). These findings suggest that PARP-1 may be a promising target, and inhibitors of PARP-1 may have potential clinical application for the prevention and treatment of cardiovascular diseases.
A recent study showed that NOR1 could directly interact with PARP-1 and that this interaction suppressed NOR1 transcriptional activity (Ohkura et al., 2008). Based on this finding, we hypothesized that the association between NOR1 and PARP-1 might also affect PARP-1 activity and thereby regulate the development and progression of cardiac hypertrophy. Therefore, in the present study, we investigated the role of NOR1 in isoprenaline-induced cardiomyocyte hypertrophy, and further tested whether the pro-hypertrophic effect of NOR1 could be attributed to its regulation of PARP-1.
Methods
Animals
All animal care and experimental procedures complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were approved by the Research Ethics Committee of Sun Yat-Sen University. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 20 animals were used in the experiments described here. Sprague-Dawley (SD) rats (male, weighing 180–220 g, SPF grade, certification No.44008500000735) were supplied by the Experimental Animal Center of Sun Yat-Sen University (Guangzhou, China).
Primary culture of rat cardiomyocytes
Primary cultures of neonatal rat cardiomyocytes (NRCMs) were prepared as previously described (Yu et al., 2013). Cells from the hearts of 1- to 3-day-old SD rats were seeded at a density of 1 × 106 cells per well onto six-well plates in DMEM supplemented with 10% FBS and 5-bromodeoxyuridine (0.1 mM). After 48 h, the culture medium was replaced with 2 mL DMEM containing 1% FBS. Isoprenaline was dissolved in DMEM to prepare a 1 mM stock solution. After 24 h of serum starvation, 2 μL of the stock solution was added to medium (final concentration of 1 μM) and the cells were incubated further, as indicated. In the control group, the same volume of DMEM without isoprenaline was added to the cultures.
Experimental model, echocardiography and morphometric measures
Cardiac hypertrophy was induced by s.c. injection of isoprenaline (1.5 mg·kg−1·d−1) for 7 consecutive days. Rats given normal saline (NS) were regarded as vehicle control group. The total number of rats was 10 per group. After 7 days, two-dimensionally guided M-mode echocardiography was performed using a Technos MPX ultrasound system (ESAOTE, Genova, Italy) equipped with an 8.5 MHz imaging transducer. After assessment of echocardiography, rats were killed by exposing to a rising concentration of CO2. The hearts were carefully excised, and heart weight (HW) was determined. The left ventricle (LV) was then carefully trimmed and weighed. The weight of heart or LV was expressed as a ratio relative to body weight (BW). For morphometric measures, transverse sections of the hearts were fixed with neutral buffered formalin (10%), embedded in paraffin, cut into 5 μm cross-sections and stained with haematoxylin and eosin (H&E).
RNA isolation and quantitative RT-PCR (qRT-PCR)
Total RNA from heart tissues or cultured cells was extracted using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. One microgram of total RNA was reversely transcribed to first strand cDNA using One-step RT kit (Takara Biotechnology, Dalian, China). The mRNA levels of targeted genes were determined using SYBR-Green Quantitative PCR kit (Takara Biotechnology) by iCycler iQ system (iCycler, Bio-Rad, Hercules, CA, USA). Rat-specific primers for atrial natriuretic factor (ANF), brain natriuretic peptide (BNP), NOR1, GAPDH (listed in Supporting Information Table S1) were synthesized by Invitrogen. GAPDH served as the endogenous control.
Western blot and co-immunoprecipitation (co-IP)
For immunoprecipitation, rabbit anti-NOR1 polyclonal antibody (diluted 1:10) and mouse anti-GFP monoclonal antibody (diluted 1:10) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). For Western blotting, the other rabbit anti-NOR1 polyclonal antibody (diluted 1:300) was purchased from OriGene Technologies (Rockville, MD, USA). Mouse anti-α-tubulin monoclonal antibody (diluted 1:1000) was purchased from Sigma (St. Louis, MO, USA). Acetylated-lysine (Ac-K2-100) rabbit mAb (diluted 1:1000) and mouse anti- sirtuin 1 (SIRT1) polyclonal antibody (diluted 1:1000) were purchased from Cell Signaling Technology (Beverly, MA, USA). Rabbit anti-PARP-1 polyclonal antibody (Western blotting, diluted 1:500, immunoprecipitation: 1:100) was purchased from Proteintech (Proteintech Group, Chicago, IL, USA). Nuclear proteins were extracted with a commercially available Nuclear and Cytoplasm Extraction kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer's recommendations. Western blot analyses were performed as previously described (Zhou et al., 2006), and α-tubulin was used as a loading control. For co-IP, nuclear proteins (800 μg) were incubated with 1 μg anti-NOR1 or anti-PARP-1 antibodies for overnight (rabbit normal IgG was used as a control), followed by 4 h incubation with protein G-agarose beads (Pierce, Rockford, IL, USA) at 4°C. The immunoprecipitated proteins were detected by Western blot.
PARP-1 activity assay
PARP-1 activity was determined by a chemical quantitation method as described by Putt and Hergenrother (2004), which converted NAD into a highly fluorescent agent using recombinant human PARP-1 (Sigma). The fluorescence intensity was determined by Multimode Microplate Reader (Infinite M1000, Tecan, Switzerland) at 360 nm for excitation and 445 nm for emission. To measure the cellular PARP-1 activity, the recombinant PARP-1 was replaced by nuclear extracts from NRCMs and heart tissues.
Measurement of cell surface area
Cardiomyocytes, grown in 48-well plates, were fixed by 4% paraformaldehyde for 15 min at room temperature, followed by 0.5% Triton-100 treatment for 5 min. After incubation with 0.1% rhodamine-phalloidin for 30 min, the cells were washed with PBS and further incubated with DAPI. The images of cardiomyocytes were detected by High Content Screening system (ArrayScanVTI, Thermo Fisher Scientific, Rockford, IL, USA), and the cell surface area from randomly selected fields (50 for each group) was determined by the built-in image analysis software.
RNA interference
Small interference RNA (siRNA) for NOR1 (siNOR1), PARP-1 (siPARP-1) and negative control siRNA were purchased from Genepharma (Shanghai, China). The sequences of siRNAs are shown in Supporting Information Tables S2 and S3. Cardiomyocytes seeded in 35 mm dishes were transfected with 100 pmol of the targeted siRNA or negative control siRNA, respectively, using 5 μL lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. The qRT-PCR and Western blot assays were performed to compare silencing efficiency of different siRNAs.
Plasmid transfection
NOR1 plasmid were constructed with pEGFP-N3 or pcDNA3.1, and used NheI together with HindIII as restriction enzymes. Primers are shown in Supporting Information Table S4. Cardiomyocytes were transiently transfected with NOR1 or C293Y, a mutant of NOR1 without PARP-1 binding activity, using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Data analysis
Data are presented as mean ± SE. Statistical analyses between two groups were performed by unpaired Student's t-test. Differences among multiple groups were tested by one-way anova with Tukey's post hoc test. In all cases, differences were considered statistically significant with P < 0.05.
Materials
Isoprenaline was purchased from EMD Chemicals (San Diego, CA, USA). FBS and DMEM were purchased from Gibico (Grand Island, NY, USA). NheI, HindIII and T4 DNA Ligase were purchased from New England Biolabs, Inc. (Beverly, MA, USA).
Results
NOR1 expression was induced by isoprenaline in cultured NRCMs and rat hearts
Isoprenaline has been widely used as a stimulus of cardiac hypertrophy in vivo and in vitro (Mitsuyama et al., 2013; Ozakca et al., 2013; Yoshida et al., 2014). In this study, cultured NRCMs were incubated with 1 μM isoprenaline for the times indicated and then the expression of NOR1 was determined by quantitative RT-PCR and Western blot. As shown in Figure 1A and B, the mRNA and protein levels of NOR1 were significantly increased by isoprenaline, compared with the control. For the in vivo studies, SD rats were given s.c. injections of isoprenaline (1.5 mg·kg−1·d−1) for 7 days. The hearts of isoprenaline-treated rats were clearly larger than those of control animals receiving normal saline and presented with typical hypertrophic changes, as demonstrated by echocardiography and H&E staining. The HW/BW ratio and LV weight to BW (LVW/BW) ratio were both increased by isoprenaline (Supporting Information Figure S1). Additionally, the isoprenaline treatment clearly increased NOR1 mRNA and protein expression in rat heart tissues (Figure 1C and D).
Figure 1.
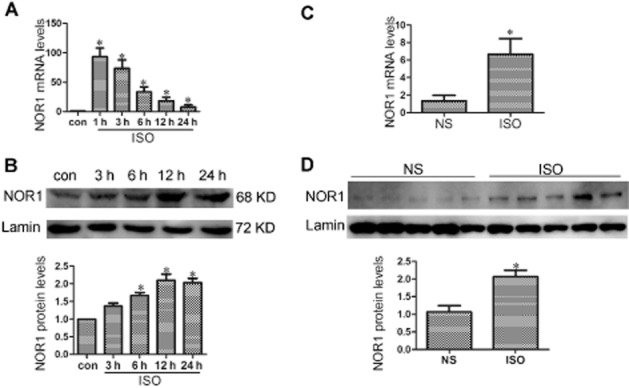
NOR1 expression was significantly increased in NRCMs and heart tissues following isoprenaline treatment. (A and B) Primary NRCMs were treated with 1 μM isoprenaline (ISO) for indicated time points. The mRNA and protein levels of NOR1 were detected by qRT-PCR and Western blot respectively. (C and D) SD rats were subjected to s.c. injection of 1.5 mg·kg−1·d−1 isoprenaline or normal saline (NS) for 7 d, and then the NOR1 expression was measured. The results were normalized by GAPDH for mRNA or Lamin for protein expression. Data were presented as means ± SE. *P < 0.05 versus control or NS group, n = 5 independent experiments.
NOR1 knockdown attenuated cardiomyocyte hypertrophy induced by isoprenaline
To explore the involvement of NOR1 in isoprenaline-induced cardiomyocyte hypertrophy, endogenous NOR1 was knocked down by using the appropriate siRNA in NRCMs. Three different siRNAs for NOR1 (S1, S3, S3) were synthesized, and S1 showed the best silencing efficacy as indicated by the results of qRT-PCR and Western blot (Figure 2A and B). As shown in Figure 2C, 1 μM isoprenaline treatment for 24 h led to significant up-regulation of NOR1 protein expression, which was inhibited by transfection with NOR1 siRNA. Moreover, knockdown of NOR1 attenuated the hypertrophic responses of cardiomyocytes stimulated by isoprenaline, as shown by decreased mRNA for ANF and BNP (Figure 2D) and cell surface area (Figure 2E).
Figure 2.
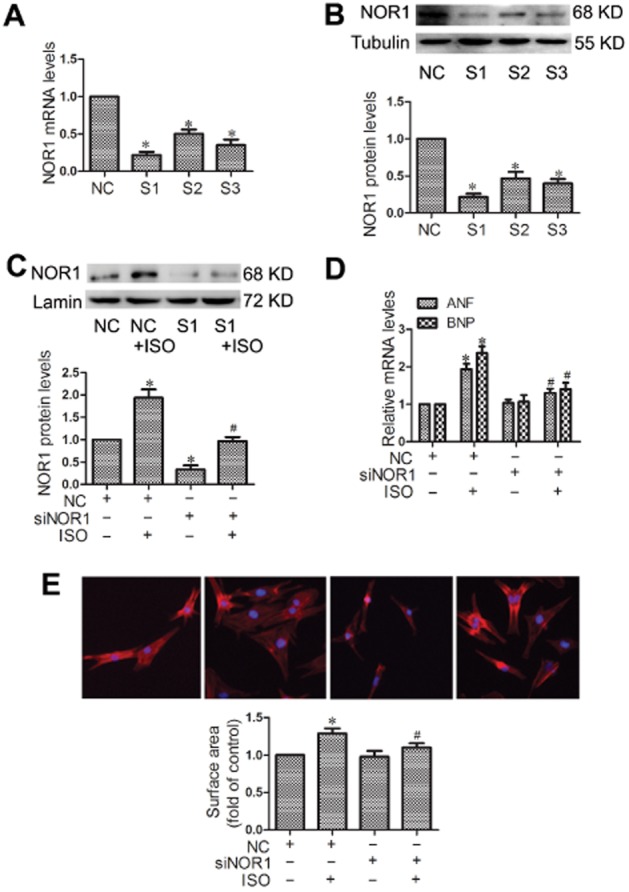
Knockdown of NOR1 blocked the hypertrophic responses induced by isoprenaline (ISO). (A and B) Cultured NRCMs were transfected with three different siRNA for NOR1 (S1, S3, S3) or negative control (NC) for 24 h, and the silencing efficacy was measured by qRT-PCR and Western blot. Cells were transfected with NOR1 siRNA (siNOR1) before incubation with 1 μM isoprenaline for 24 h. (C) Expression of NOR1 protein was detected by Western blot. (D) ANF and BNP mRNA levels were determined by qRT-PCR. (E) Cell surface area was measured by rhodamine-phalloidin staining. Data were presented as means ± SE. *P < 0.05, versus NC group, #P < 0.05 versus NC treated with isoprenaline, n = 5 independent experiments.
NOR1 overexpression induced cardiomyocyte hypertrophy
NRCMs were transfected with NOR1 or its mutant counterpart C293Y (Figure 3 A). Responses were assessed by the expression of hypertrophic markers and cell surface area. As shown in Figure 3B and C, the mRNA levels of ANF and BNP, and the cell surface area were all significantly increased by NOR1 transfection. In contrast, no similar hypertrophic responses were observed in cells transfected with C293Y, a mutant of NOR1 that lacks PARP-1 binding activity.
Figure 3.
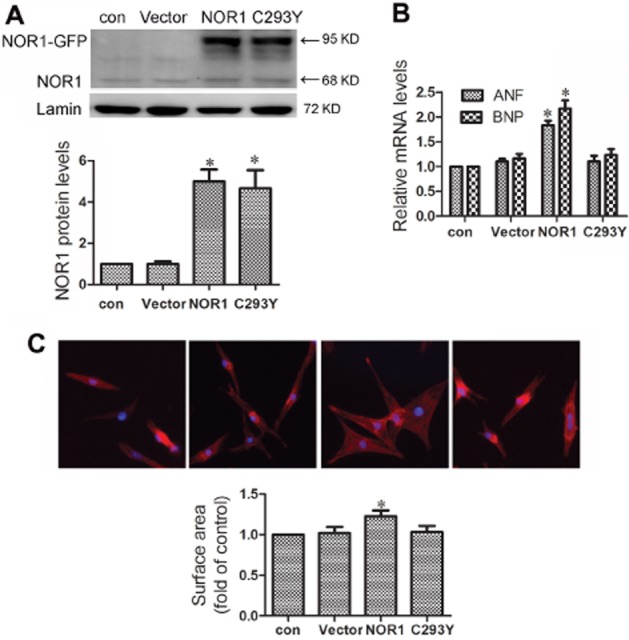
NOR1 overexpression led to hypertrophic responses in primary NRCMs. Cells were transfected with plasmids for wild-type NOR1 (NOR1), a mutant of NOR1 (C293Y) and vector. (A) Expression of NOR1 protein was measured by Western blot. (B) The mRNA levels of ANF and BNP were detected by qRT-PCR. (C) Cell surface area was measured by rhodamine-phalloidin staining. Data were presented as means ± SE. *P < 0.05 versus vector group, n = 5 independent experiments.
NOR1 interacts with PARP-1
NRCMs were incubated with or without 1 μM isoprenaline for 24 h, and the nuclear fraction was extracted for co-IP. In these extracts, PARP-1 was precipitated by anti-NOR1 (Figure 4A), suggesting an interaction between NOR1 and PARP-1. Additionally, this interaction was significantly enhanced by exposure to isoprenaline (Figure 4A). Moreover, the interaction between NOR1 and PARP-1 was also detected in rat heart tissues and this was increased following isoprenaline treatment, in vivo, for 7 days (Figure 4B). However, in NRCMs transfected with the NOR1 mutant C293Y, no similar interaction with PARP-1 was observed (Figure 4C).
Figure 4.
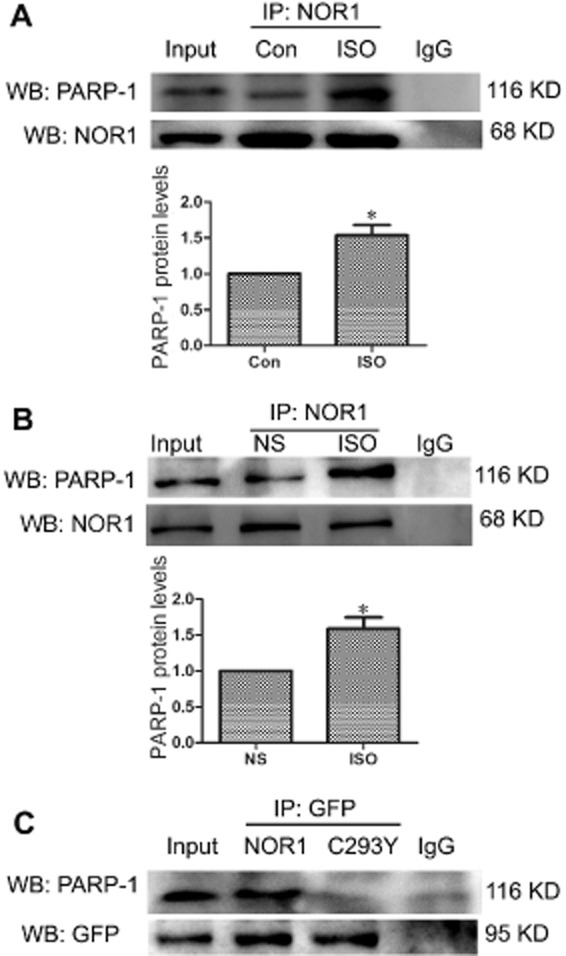
NOR1 physically interacted with PARP-1 and the interaction was significantly increased by isoprenaline (ISO) treatment. (A) Primary NRCMs were incubated with 1 μM isoprenaline for 24 h. Co-IP assay was performed to show the interaction between NOR1 and PARP-1 after immunoprecipitation of cardiomyocyte nuclear extracts with anti-NOR1 antibody. (B) SD rats were treated with 1.5 mg·kg−1·d−1 isoprenaline or NS for 7 days and the interactions between NOR1 and PARP-1 in heart tissues was detected by Co-IP. (C) After NOR1 or its mutant C293Y were transfected into cardiomyocytes, Co-IP assay was performed using anti-GFP antibody to immunoprecipitate nuclear extracts. Images representative of five independent experiments are shown.
NOR1 knockdown inhibited excessive activation of PARP-1 by isoprenaline
In NRCMs or rat heart tissues, isoprenaline treatment significantly increased the activity of PARP-1 without affecting its protein level (Figure 5A–D). NOR1 knockdown by siRNA in cultured cardiomyocytes did not alter the activity and expression of PARP-1, but inhibited isoprenaline-induced PARP-1 activation (Figure 5E and F). In addition, transfection with NOR1, but not its C293Y mutant clearly up-regulated PARP-1 activity, without affecting PARP-1 expression (Figure 5G and H), indicating that the activation of PARP-1 by NOR1 was dependent on the direct binding of PARP-1 with NOR1.
Figure 5.
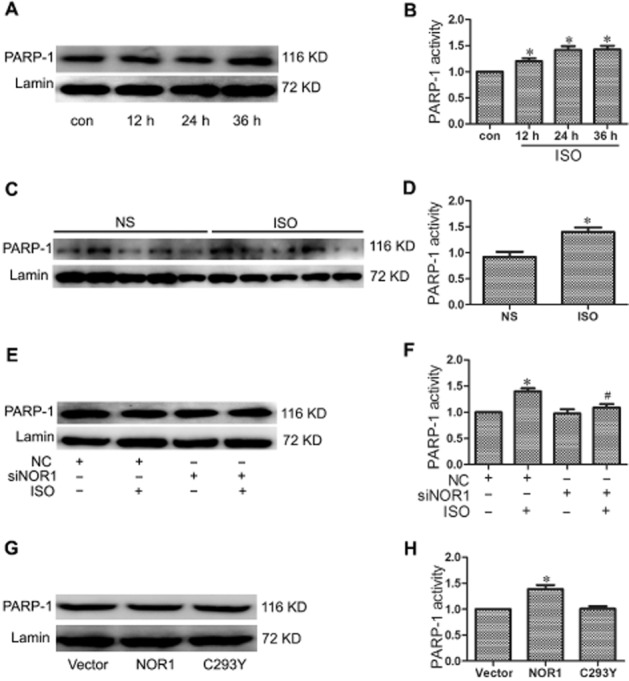
The activity of PARP-1 was regulated by NOR1 in cardiomyocyte hypertrophy. (A and B) PARP-1 expression and activity were measured in primary NRCMs treated with 1 μM isoprenaline (ISO) for the indicated time. (C and D) SD rats were treated with 1.5 mg·kg−1·d−1 isoprenaline or NS for 7 d. PARP-1 expression and activity were measured. (E and F) Cardiomyocytes were transfected with NOR1 siRNA (siNOR1) or negative control (NC), and further treated with 1 μM isoprenaline for 24 h. PARP-1 expression and activity were measured. (G and H) PARP-1 expression and activity were measured in cardiomyocytes overexpressing NOR1 or its mutant C293Y. Data are presented as means ± SE. *P < 0.05, versus control, normal saline (NS), NC, or vector, #P < 0.05 versus NC treated with isoprenaline, n = 5 independent experiments.
PARP-1 inhibition protected cardiomyocytes from isoprenaline-induced hypertrophy
The PARP-1 inhibitor, 3-aminobenzamide (3AB) has been widely used to investigate the function of PARP-1 (Decker et al., 1999). In this study, 3AB or PARP-1 siRNA (siPARP-1) were used to inhibit PARP-1 in NRCMs (Figure 6A–C), with the aim of elucidating the invovlement of PARP-1 in the cardiac hypertrophy induced by isoprenaline. As shown in Figure 6D and E, siPARP-1 or 3AB significantly attenuated the hypertrophic responses of cardiomyocytes induced by isoprenaline, as shown by decreased levels of mRNA for ANF and BNP and decreased cell surface area.
Figure 6.
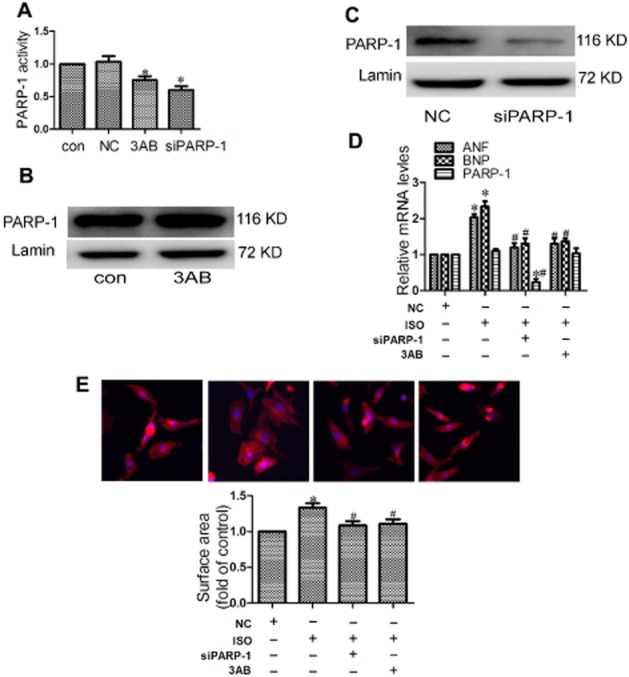
PARP-1 inhibition protected cardiomyocytes from hypertrophy induced by isoprenaline (ISO). (A–C) Primary NRCMs were transfected with PARP-1 siRNA (siPARP-1) and negative control (NC), or incubated with 500 μM 3AB. The expression and activity of PARP-1 were measured. (D–E) Cardiomyocytes were transfected with PARP-1 siRNA (siPARP-1) or were treated with 3AB (500 μM) following incubation with 1 μM isoprenaline for 24 h. Hypertrophic responses were measured by the mRNA levels of hypertrophic biomarkers and cell surface area. Data were presented as means ± SE. *P < 0.05 versus NC group, #P < 0.05 versus NC treated with isoprenaline, n = 4 independent experiment).
PARP-1 inhibition attenuated hypertrophic responses induced by NOR1 overexpression
The role of PARP-1 in NOR1-induced cardiomyocyte hypertrophy induced by NOR1 overexpression was investigated by exposure to 3AB (500 μM) or siPARP-1 transfection, As shown in Figure 7A and B, these treatments clearly reversed the increase in ANF and BNP mRNA levels and in cell surface area.
Figure 7.
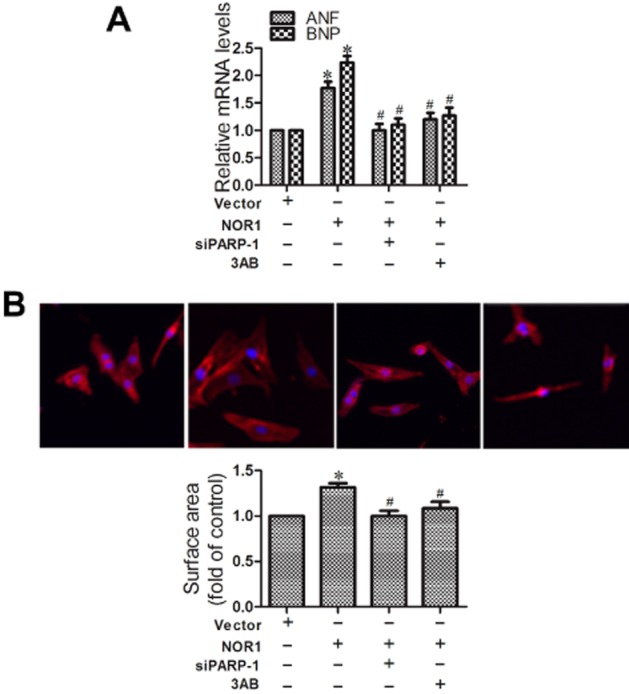
PARP-1 inhibition attenuated hypertrophic responses induced by NOR1 overexpression. (A and B) Cardiomyocytes were transfected with PARP-1 siRNA (siPARP-1) and negative control (NC), or incubated with 3AB (500 μM) before transfection with NOR1 for 36 h. Hypertrophic responses were measured by the mRNA levels of hypertrophic biomarkers and cell surface area. Data were presented as means ± SE. *P < 0.05 versus vector, #P < 0.05 versus NOR1, n = 5 independent experiments.
NOR1 improved acetylation of PARP-1
The mechanism underlying the activation of PARP-1 by NOR1 overexpression was unclear. It has been previously reported that acetylation of PARP-1 after stress of cardiomyocytes results in its activation, which could be reversed by the histone deacetylase SIRT1 (Rajamohan et al., 2009). Thus, the effect of NOR1 overexpression on PARP-1 acetylation was investigated. As demonstrated in Figure 8, NOR1 transfection in NRCMs significantly enhanced NOR1/PARP-1 interaction and elevated the acetylation level of PARP-1. Additionally, NOR1 overexpression suppressed the interaction between PARP-1 and SIRT1, which might explain the high acetylation level of PARP-1 following NOR1 transfection (Figure 8).
Figure 8.
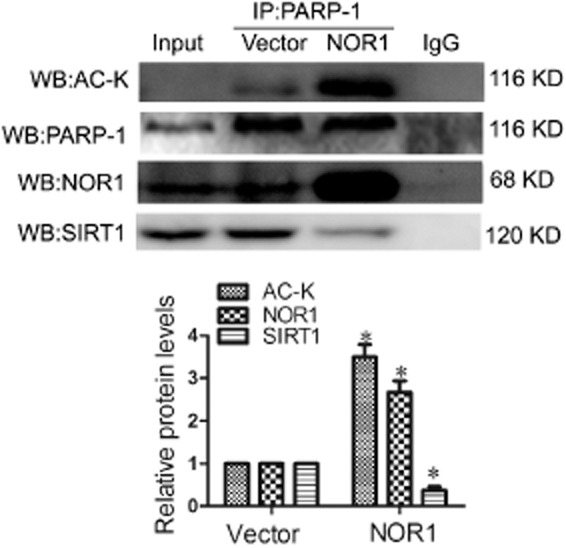
NOR1 overexpression improved acetylation of PARP-1. NOR1 was overexpressed in primary NRCMs. Co-IP assays were performed to show the effect of NOR1 overexpression on acetylation of PARP-1 as well as the interactions of NOR1/PARP-1 and SIRT1/PARP-1. *P < 0.05 versus vector, n = 5 independent experiments.
Discussion
The NR4A orphan nuclear receptors (Nur77, Nurr1 and NOR1) share a highly conserved DNA binding domain and a less conserved C-terminal putative ligand binding domain (Martinez-Gonzalez and Badimon, 2005; Pols et al., 2007). However, the amino acid sequences of the N-terminal activation function domain, which mediates the ligand-independent activation of gene expression and influences transactivation and co-factor recruitment, are very different (Wansa et al., 2002). During recent years, the roles of these orphan receptors in pathogenesis of cardiovascular diseases have been intensively investigated. We now know that Nur77 participates in AngII-induced cardiac hypertrophy by regulating the mTOR signal pathway (Wang et al., 2013). Translocation of Nur77 from the nucleus to the mitochondria in cardiomyocytes results in the loss of mitochondrial integrity and subsequent apoptosis in response to ischaemia/reperfusion injury (Cheng et al., 2011). Also, Nur77 and Nurr1 attenuated atherosclerosis in mice via inhibition of NF-κB (Hamers et al., 2013). In contrast, NOR1 was a detrimental factor in atherosclerosis, as it increased SMC proliferation and production of adhesion molecules, and exacerbated vascular lesion formation (Martinez-Gonzalez et al., 2003b; Zhao et al., 2010).
Although major advances have been made in elucidating the fundamental roles of NOR1 in atherosclerosis, little is known regarding its function and related molecular mechanisms in other cardiovascular diseases. Recently, Myers et al. have identified cross-talk between β-adrenoceptors and NR4A signalling in vivo (Myers et al., 2009). They reported that the mRNA level of NOR1 in mouse heart tissue was markedly induced 1 h after a single i.p. injection of isoprenaline and remained significant up to 4 h. However, these findings only indicated an instant and short-term change of NOR1 expression in response to isoprenaline stimulation. The consequences of NOR1 up-regulation especially those associated with cardiovascular diseases still need to be elucidated, as injection of a single dose of isoprenaline is not sufficient to produce sustained stress leading to morphological and pathological changes. In our study, a rat model of cardiac hypertrophy was established by s.c. injection of isoprenaline for 7 consecutive days. We observed that the mRNA and protein expression of NOR1 were significantly increased by this isoprenaline treatment in vivo (Figure 1). Additionally, isoprenaline and NOR1 overexpression both resulted in hypertrophic responses in cultured NRCMs (Figures 2 and 3). More importantly, gene silencing of NOR1 attenuated cardiomyocyte hypertrophy induced by isoprenaline or phenylephrine (Figure 2 and Figure S2). Thus, the present work provides new evidence that NOR1 is involved in cardiac hypertrophy and it suggests that cardiac hypertrophy could be treated by targeting NOR1. Furthermore, NOR1 directly interacted with PARP-1 and thereby increased PARP-1 activity, an effect that might be critical for the pro-hypertrophic effect of NOR1.
PARP-1 is the most extensively studied member of the PARP family and it acts primarily as a DNA damage sensor in the nucleus, being activated in response to DNA single-strand break,s induced by free radicals and cell injury (Luo and Kraus, 2012). Poly(ADP-ribosylation) by PARP-1 requires NAD+ as a substrate for generating ADP-ribose monomers. The overactivation of PARP-1 may deplete the stores of cellular NAD+ and may induce a progressive ATP depletion, thereby altering the functions and activities of NAD+-dependent enzymes, which are involved in the control of critical cellular processes such as differentiation, proliferation, apoptosis, metabolism and ageing (Virag and Szabo, 2002; Blander and Guarente, 2004; Szabo et al., 2004; Luo and Kraus, 2012). Thus, PARP-1 is involved in a variety of cardiovascular diseases including myocardial ischaemia/reperfusion injury, circulatory shock, diabetic cardiovascular complications, as well as atherosclerosis (Szabo, 2005; Pillai et al., 2006; Oh et al., 2009). Moreover, PARP-1 has recently been shown to be critical for the development of cardiac hypertrophy. Significant up-regulation of PARP-1 activity has been observed in various animal models of pathological cardiac hypertrophy (Pillai et al., 2005; Bartha et al., 2008b) and PARP-1-deficient mice were protected from AngII-mediated hypertrophy (Palfi et al., 2006). Treatment with the PARP-1 inhibitor L-2286 reduced the progression of cardiac hypertrophy following pressure overload (Bartha et al., 2008a,b,). A novel PARP-1 inhibitor AG-690/11026014 (6014) was identified by virtual screening and protected cardiomyocytes from AngII-induced hypertrophy (Liu et al., 2014). In line with these studies, we also found significant activation of PARP-1 in cardiac hypertrophy induced by isoprenaline, and knockdown of PARP-1 or the PARP-1 inhibitor 3AB inhibited the cardiac hypertrophy induced by isoprenaline (Figures 5 and 6).
Given the links of both NOR1 and PARP-1 to cardiomyocyte hypertrophy, we hypothesized that these two detrimental factors might functionally overlap. The data from the co-IP assays clearly indicated a physical interaction between NOR1 and PARP-1 in cardiomyocytes, which was enhanced by isoprenaline treatment or NOR1 overexpression (Figures 4 and 8). Although NOR1 directly interacted with PARP-1 in 293T cells and PARP-1 suppressed the transcriptional activities of NOR1 (Ohkura et al., 2008), little information is available on whether such NOR1/PARP-1 interactions would affect the function of PARP-1. Here, we further observed that isoprenaline or overexpression of wild-type NOR1 strengthened the interaction between NOR1 and PARP-1, and subsequently increased PARP-1 activity (Figures 4 and 5). In contrast, overexpression of C293Y, a NOR1 mutant containing a single Cys replacement to Tyr at codon 293 that abolishing its binding with PARP-1, failed to increase the activity of PARP-1 and did not stimulate cardiomyocyte hypertrophy (Figures 5). These results suggested that the effect of NOR1 on PARP-1 activation might depend on the direct interaction between them. Through gain- and loss-of-function approaches in cultured cardiomyocytes, we also found that NOR1 knockdown by RNA interference exhibited cardioprotective effects against hypertrophic stimuli, which could be attributed to the inhibition of NOR1 and PARP-1 interaction and subsequent PARP-1 activation (Figure 5).
It is still unclear exactly how NOR-1 could activate PARP-1. Although many studies have demonstrated that NOR1 functions as a transcription factor, in our experiments, it is unlikely that NOR1 altered the transcription of PARP-1, as the expression of PARP-1 was unchanged following NOR1 overexpression or silencing (Zhao et al., 2010; Tessem et al., 2014). It is also unlikely that NOR1 activates PARP-1 by causing DNA injury, as the interaction between NOR1 and PARP-1 was not dependent on DNA (Ohkura et al., 2008). The activation of PARP-1 is dependent on its acetylation, which could be regulated by the histone deacetylase SIRT1 (Rajamohan et al., 2009). Two sequences in PARP-1, the N terminal residues 1–214 and the residues 477–524 are required for full interaction of PARP-1 and SIRT1 (Rajamohan et al., 2009), with higher affinity binding of SIRT1 to the N-terminal sequence (Rajamohan et al., 2009). However, the closely related sequence, residues 7–232, is required for PARP-1 binding to NOR1 (Ohkura et al., 2008). These findings would suggest that there could be competition between NOR1 and SIRT1 for binding to PARP-1, with consequent inhibition of SIRT1 binding to PARP-1 by NOR1. Indeed, we found that the increased NOR1/PARP-1 interaction was accompanied by a decreased SIRT1/PARP-1 interaction, and that the acetylation level of PARP-1 was increased by NOR1 overexpression (Figure 8). These observations would support our hypothesis and suggest that NOR1 activates PARP-1 by preventing the binding of, and subsequent deacetylation by, SIRT1.
In summary, the present study showed that NOR1 expression was up-regulated in response to hypertrophic stimuli in vivo and in vitro. NOR1 knockdown protected cardiomyocytes from hypertrophy induced by isoprenaline, which might be partly attributed to the inhibition of NOR1/PARP-1 interaction and subsequent PARP-1 activation. Understanding this novel role of NOR1 may provide new therapeutic strategies against cardiac hypertrophy, by regulating NOR1.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81072641; No. 81273499; No. 81200096); the National Science and Technology Major Project of China ‘Key New Drug Creation and Manufacturing Program’ (No. 2011ZX09401-307); Team item of Natural Science Foundation of Guangdong Province (No. S2011030003190); Major Project of Department of Education of Guangdong Province (No. CXZD1006).
Glossary
- 3AB
3-aminobenzamide
- ANF
atrial natriuretic factor
- BNP
brain natriuretic peptide
- BW
body weight
- HW
heart weight
- LV
left ventricle
- LVW
left ventricle weight
- SIRT1
sirtuin 1
- NOR1
neuron-derived orphan receptor 1
Authors contributions
P.-Q. L. and J.-T. Y. conceived and designed the experiments. X.-J. F., H. G., S. G., H. L., J. L., J.-J. W. and X.-Y. H. performed the experiments. J.-T. Y., X.-J. F. and Z.-M. L. analysed the data. J. L., M. L. and J. Z. contributed reagents/materials/analysis tools. X.-J. F. and H. G. wrote the paper.
Conflicts of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 SD rats were subjected to s.c. injections of 1.5 mg·kg−1·d−1 isoprenaline for 7 d.
Figure S2 Knockdown of NOR1 blocked the hypertrophic responses induced by PE. Cultured NRCMs were transfected with siRNA for NOR1 (siNOR1) or negative control (NC) before treated by 10 μM phenylephrine (PE) for 24 h.
Table S1 Primer sequences for quantitative RT-PCR.
Table S2 Sequences of siRNAs for NOR1.
Table S3 Sequences of siRNAs for PARP-1.
Table S4 Sequences of NOR1 and PCAF plasmids.
Table S5 Sequences of siRNAs for NR4A1.
Table S6 Sequences of siRNAs for NR4A2.
Table S7 Gravimetric parameters of SD rats treated with isoprenaline.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol. 2013a;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- Bartha E, Kiss GN, Kalman E, Kulcsar G, Kalai T, Hideg K, et al. Effect of L-2286, a poly(ADP-ribose)polymerase inhibitor and enalapril on myocardial remodeling and heart failure. J Cardiovasc Pharmacol. 2008a;52:253–261. doi: 10.1097/FJC.0b013e3181855cef. [DOI] [PubMed] [Google Scholar]
- Bartha E, Kiss GN, Kalman E, Kulcsar G, Kalai T, Hideg K, et al. Effect of L-2286, a poly(ADP-ribose)polymerase inhibitor and enalapril on myocardial remodeling and heart failure. J Cardiovasc Pharmacol. 2008b;52:253–261. doi: 10.1097/FJC.0b013e3181855cef. [DOI] [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Volkers M, Din S, Avitabile D, Khan M, Gude N, et al. Mitochondrial translocation of Nur77 mediates cardiomyocyte apoptosis. Eur Heart J. 2011;32:2179–2188. doi: 10.1093/eurheartj/ehq496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker P, Miranda EA, de Murcia G, Muller S. An improved nonisotopic test to screen a large series of new inhibitor molecules of poly(ADP-ribose) polymerase activity for therapeutic applications. Clin Cancer Res. 1999;5:1169–1172. [PubMed] [Google Scholar]
- Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- Hamers AA, Hanna RN, Nowyhed H, Hedrick CC, de Vries CJ. NR4A nuclear receptors in immunity and atherosclerosis. Curr Opin Lipidol. 2013;24:381–385. doi: 10.1097/MOL.0b013e3283643eac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WL. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr Opin Cell Biol. 2008;20:294–302. doi: 10.1016/j.ceb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Li Z, Chen GW, Li ZM, Wang LP, Ye JT, et al. AG-690/11026014, a novel PARP-1 inhibitor, protects cardiomyocytes from AngII-induced hypertrophy. Mol Cell Endocrinol. 2014;392:14–22. doi: 10.1016/j.mce.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Luo X, Kraus WL. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012;26:417–432. doi: 10.1101/gad.183509.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005;65:609–618. doi: 10.1016/j.cardiores.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez J, Rius J, Castello A, Cases-Langhoff C, Badimon L. Neuron-derived orphan receptor-1 (NOR-1) modulates vascular smooth muscle cell proliferation. Circ Res. 2003a;92:96–103. doi: 10.1161/01.es.0000050921.53008.47. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez J, Rius J, Castello A, Cases-Langhoff C, Badimon L. Neuron-derived orphan receptor-1 (NOR-1) modulates vascular smooth muscle cell proliferation. Circ Res. 2003b;92:96–103. doi: 10.1161/01.es.0000050921.53008.47. [DOI] [PubMed] [Google Scholar]
- Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e2. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama S, Takeshita D, Obata K, Zhang GX, Takaki M. Left ventricular mechanical and energetic changes in long-term isoproterenol-induced hypertrophied hearts of SERCA2a transgenic rats. J Mol Cell Cardiol. 2013;59:95–106. doi: 10.1016/j.yjmcc.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Myers SA, Eriksson N, Burow R, Wang SC, Muscat GE. Beta-adrenergic signaling regulates NR4A nuclear receptor and metabolic gene expression in multiple tissues. Mol Cell Endocrinol. 2009;309:101–108. doi: 10.1016/j.mce.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Oh KS, Lee S, Yi KY, Seo HW, Koo HN, Lee BH. A novel and orally active poly(ADP-ribose) polymerase inhibitor, KR-33889 [2-[methoxycarbonyl(4-methoxyphenyl) methylsulfanyl]-1H-benzimidazole-4-carboxylic acid amide], attenuates injury in in vitro model of cell death and in vivo model of cardiac ischemia. J Pharmacol Exp Ther. 2009;328:10–18. doi: 10.1124/jpet.108.143719. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Nagamura Y, Tsukada T. Differential transactivation by orphan nuclear receptor NOR1 and its fusion gene product EWS/NOR1: possible involvement of poly(ADP-ribose) polymerase I, PARP-1. J Cell Biochem. 2008;105:785–800. doi: 10.1002/jcb.21876. [DOI] [PubMed] [Google Scholar]
- Ozakca I, Arioglu-Inan E, Esfahani H, Altan VM, Balligand JL, Kayki-Mutlu G, et al. Nebivolol prevents desensitization of beta-adrenoceptor signaling and induction of cardiac hypertrophy in response to isoprenaline beyond beta1-adrenoceptor blockage. Am J Physiol Heart Circ Physiol. 2013;304:H1267–H1276. doi: 10.1152/ajpheart.00352.2012. [DOI] [PubMed] [Google Scholar]
- Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfi A, Toth A, Hanto K, Deres P, Szabados E, Szereday Z, et al. PARP inhibition prevents postinfarction myocardial remodeling and heart failure via the protein kinase C/glycogen synthase kinase-3beta pathway. J Mol Cell Cardiol. 2006;41:149–159. doi: 10.1016/j.yjmcc.2006.03.427. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- Petrucco S. Sensing DNA damage by PARP-like fingers. Nucleic Acids Res. 2003;31:6689–6699. doi: 10.1093/nar/gkg890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JB, Russell HM, Raman J, Jeevanandam V, Gupta MP. Increased expression of poly(ADP-ribose) polymerase-1 contributes to caspase-independent myocyte cell death during heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H486–H496. doi: 10.1152/ajpheart.00437.2004. [DOI] [PubMed] [Google Scholar]
- Pillai JB, Gupta M, Rajamohan SB, Lang R, Raman J, Gupta MP. Poly(ADP-ribose) polymerase-1-deficient mice are protected from angiotensin II-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H1545–H1553. doi: 10.1152/ajpheart.01124.2005. [DOI] [PubMed] [Google Scholar]
- Pols TW, Bonta PI, de Vries CJ. NR4A nuclear orphan receptors: protective in vascular disease? Curr Opin Lipidol. 2007;18:515–520. doi: 10.1097/MOL.0b013e3282ef77d1. [DOI] [PubMed] [Google Scholar]
- Ponnio T, Conneely OM. nor-1 regulates hippocampal axon guidance, pyramidal cell survival, and seizure susceptibility. Mol Cell Biol. 2004;24:9070–9078. doi: 10.1128/MCB.24.20.9070-9078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnio T, Burton Q, Pereira FA, Wu DK, Conneely OM. The nuclear receptor Nor-1 is essential for proliferation of the semicircular canals of the mouse inner ear. Mol Cell Biol. 2002;22:935–945. doi: 10.1128/MCB.22.3.935-945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putt KS, Hergenrother PJ. An enzymatic assay for poly(ADP-ribose) polymerase-1 (PARP-1) via the chemical quantitation of NAD(+): application to the high-throughput screening of small molecules as potential inhibitors. Anal Biochem. 2004;326:78–86. doi: 10.1016/j.ab.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Rajamohan SB, Pillai VB, Gupta M, Sundaresan NR, Birukov KG, Samant S, et al. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res. 2000;47:23–37. doi: 10.1016/s0008-6363(00)00076-6. [DOI] [PubMed] [Google Scholar]
- Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- Szabo C. Cardioprotective effects of poly(ADP-ribose) polymerase inhibition. Pharmacol Res. 2005;52:34–43. doi: 10.1016/j.phrs.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Szabo G, Liaudet L, Hagl S, Szabo C. Poly(ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc Res. 2004;61:471–480. doi: 10.1016/j.cardiores.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Szanto M, Brunyanszki A, Kiss B, Nagy L, Gergely P, Virag L, et al. Poly(ADP-ribose) polymerase-2: emerging transcriptional roles of a DNA-repair protein. Cell Mol Life Sci. 2012;69:4079–4092. doi: 10.1007/s00018-012-1003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessem JS, Moss LG, Chao LC, Arlotto M, Lu D, Jensen MV, et al. Nkx6.1 regulates islet beta-cell proliferation via Nr4a1 and Nr4a3 nuclear receptors. Proc Natl Acad Sci U S A. 2014;111:5242–5247. doi: 10.1073/pnas.1320953111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- Wang RH, He JP, Su ML, Luo J, Xu M, Du XD, et al. The orphan receptor TR3 participates in angiotensin II-induced cardiac hypertrophy by controlling mTOR signalling. EMBO Mol Med. 2013;5:137–148. doi: 10.1002/emmm.201201369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansa KD, Harris JM, Muscat GE. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J Biol Chem. 2002;277:33001–33011. doi: 10.1074/jbc.M203572200. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yamashita M, Horimai C, Hayashi M. Kruppel-like factor 4 protein regulates isoproterenol-induced cardiac hypertrophy by modulating myocardin expression and activity. J Biol Chem. 2014;289:26107–26118. doi: 10.1074/jbc.M114.582809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SS, Cai Y, Ye JT, Pi RB, Chen SR, Liu PQ, et al. Sirtuin 6 protects cardiomyocytes from hypertrophy in vitro via inhibition of NF-kappaB-dependent transcriptional activity. Br J Pharmacol. 2013;168:117–128. doi: 10.1111/j.1476-5381.2012.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Howatt DA, Gizard F, Nomiyama T, Findeisen HM, Heywood EB, et al. Deficiency of the NR4A orphan nuclear receptor NOR1 decreases monocyte adhesion and atherosclerosis. Circ Res. 2010;107:501–511. doi: 10.1161/CIRCRESAHA.110.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SG, Zhou SF, Huang HQ, Chen JW, Huang M, Liu PQ. Proteomic analysis of hypertrophied myocardial protein patterns in renovascularly hypertensive and spontaneously hypertensive rats. J Proteome Res. 2006;5:2901–2908. doi: 10.1021/pr050456l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 SD rats were subjected to s.c. injections of 1.5 mg·kg−1·d−1 isoprenaline for 7 d.
Figure S2 Knockdown of NOR1 blocked the hypertrophic responses induced by PE. Cultured NRCMs were transfected with siRNA for NOR1 (siNOR1) or negative control (NC) before treated by 10 μM phenylephrine (PE) for 24 h.
Table S1 Primer sequences for quantitative RT-PCR.
Table S2 Sequences of siRNAs for NOR1.
Table S3 Sequences of siRNAs for PARP-1.
Table S4 Sequences of NOR1 and PCAF plasmids.
Table S5 Sequences of siRNAs for NR4A1.
Table S6 Sequences of siRNAs for NR4A2.
Table S7 Gravimetric parameters of SD rats treated with isoprenaline.


