Abstract
Background and Purpose
The present study was undertaken to investigate an effect of dofetilide, a potent arrhythmic blocker of the voltage-gated K+ channel, hERG, on cardiac autonomic control. Combined with effects on ardiomyocytes, these properties could influence its arrhythmic potency.
Experimental Approach
The short-term variability of beat-to-beat QT interval (STVQT), induced by dofetilide is a strong surrogate of Torsades de pointes liability. Involvement of autonomic modulation in STVQT was investigated in healthy cynomolgus monkeys and beagle dogs by power spectral analysis under conditions of autonomic blockade with hexamethonium.
Key Results
Increase in STVQT induced by dofetilide in monkeys and dogs was closely associated with an enhancement of endogenous heart rate and QT interval high-frequency (HF) oscillations. These effects were fully suppressed under conditions of autonomic blockade with hexamethonium. Ventricular arrhythmias, including Torsades de pointes in monkeys, were prevented in both species when HF oscillations were suppressed by autonomic blockade. Similar enhancements of heart rate HF oscillations were found in dogs with other hERG blockers described as causing Torsades de pointes in humans.
Conclusions and Implications
These results demonstrate for the first time that beat-to-beat ventricular repolarization variability and ventricular arrhythmias induced by dofetilide are dependent on endogenous HF autonomic oscillations in heart rate. When combined with evidence of hERG-blocking properties, enhancement of endogenous HF oscillations in heart rate could constitute an earlier and more sensitive biomarker than STVQT for Torsades de pointes liability, applicable to preclinical regulatory studies conducted in healthy animals.
Tables of Links
| TARGETS |
|---|
| Ion channels |
| hERG, (Kv11.1) channels |
| LIGANDS | |
|---|---|
| Cisapride | Risperidone |
| Dofetilide | (DL) Sotalol |
| E-4031 | Terfenadine |
| Haloperidol | Thioridazine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Compounds exhibiting blocking properties at the voltage-gated K+ channel, hERG (Kv11.1), are associated with a higher risk for the life-threatening cardiac arrhythmia called Torsades de pointes (TdP). Their arrhythmic properties are attributed to their ability to cause a lengthening of repolarization of ventricular cells resulting from an inhibition of the IKr delayed rectifier repolarizing current. However, a large majority of drugs showing both this electrophysiological property and QT prolongation ability have never caused TdP in human (De Ponti et al., 2002; Redfern et al., 2003). In spite of a great deal of research over the last decade, it remains difficult to discriminate with assurance whether compunds genuinely have a ventricular arrhythmic profile from in vitro models (Abi-Gerges et al., 2011; Nalos et al., 2012; Di Veroli et al., 2014; Yang et al., 2014). Alternatively, use of in vivo models demonstrated that the short-term variability (STV) of ventricular repolarization biomarkers (left ventricular monophasic action potential, QT interval) could constitute a stronger surrogate of TdP liability than QT prolongation. However, this surrogate seems to require conditions of decreased repolarization reserve, such as chronic atrio-ventricular block (Thomsen et al., 2004) or blockade of IKs channels (Lengyel et al., 2007) that are not reached in regulatory studies (ICH S7B) conducted in healthy animals. Consequently, few arrhythmic hERG blockers have been shown to increase beat-to-beat repolarization STV in healthy animals.
Despite this, we have used this parameter in the present study as a reference biomarker to explore whether the Coumel's triangle concept (Coumel, 1993) was applicable to arrhythmic hERG blockers. According to this concept, the generation of arrhythmias requires three conditions or factors. The first one is a substrate. In the case of arrhythmic hERG blockers, it is widely accepted that ventricular repolarization constitutes a substrate favourable to TdP (Antzelevitch, 2007). The second one is a trigger factor and the third one is a modulator factor. The autonomic nervous system (ANS) has been demonstrated as representing this modulator factor in various kinds of arrhythmias (Podrid et al., 1990; Coumel, 1996; Leenhardt et al., 2012). In the case of hERG blockers, we demonstrated that thioridazine-induced QT prolongation was modulated by the sympathetic nervous system (Champeroux et al., 2010) as sympatho-activation is able to fully reverse its QT prolongation properties. Interestingly, expression of gens of the ether-a-go-go (EAG) family has been reported not only in the heart, but also in brain neurons (Vandenberg et al., 2012). In humans, expression of KCNH2-1a, a gene coding for a hERG channel subunit present in cardiac tissues, has been reported also in the hippocampus and cerebral cortex. Its level of expression was similar to that of a brain-specific isoform named KCNH2-3.1 (Huffaker et al., 2009). Both isoforms generate inward rectifying K+ currents blocked by E-4031, a selective hERG blocker. Both isoforms play a role in neuronal excitability and firing rate through hyperpolarizing properties. Conversely, E-4031 was reported to cause a depolarizing effect and increase neuronal firing in rat cortical neurons transfected with the KCNH2-3.1 isoform suggesting that hERG blockers might increase neurotransmitter release. Interestingly, mutations characterized by a loss of function of hERG channels have been linked to both the long QT syndrome type 2 (LQTS2) and a higher incidence of seizures in patients (Omichi et al., 2010; Zamorano-León et al., 2012). Taken together, these data suggest that hERG-blocking drugs could induce both cardiac (delayed repolarization) and potentially neuronal effects through depolarizing properties.
If such interactions with neuronal activities exist and contribute to the cardiac adverse effect of arrhythmic hERG blockers, they would be expected to be mediated by the ANS. However, little is known about the presence of neuronal ERG channels in the two limbs of the ANS or in brain areas involved in the autonomic control except that ERG channels are expressed in rat peripheral sympathetic ganglia (Shi et al., 1997). The present study was designed to search for functional evidence supporting the hypothesis of interactions of hERG blockers with cardiac autonomic control. Experiments were conducted in healthy beagle dogs and cynomolgus monkeys, the two main species used in preclinical regulatory QT studies required for phase I clinical trials. In the present work, we report a detailed analysis of dofetilide effects in cynomolgus monkeys, results in dogs being reported as Supporting Information. Indeed, this hERG blocker offers in this species good conditions to study any link between possible interactions with the ANS and ventricular arrhythmias as this agent is able to induce TdP with a high incidence in cynomolgus monkeys.
Methods
All animal care and experimental procedures complied with the 2010/63/UE animal welfare European directive and were approved by ethical review (ethical committee n° CEEA-111). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 24 beagle dogs and 12 cynomolgus monkeys were used in the experiments described here.
ECG recordings in conscious animals
Adult beagle dogs (10–15 kg; CEDS, Mezilles, France) and cynomolgus monkeys (3–7 kg; originated from Mauritius and purchased from Bioprim, Baziège, France) were instrumented with radio telemetry transmitters (Data Sciences International, Saint Paul, MN, USA) as described elsewhere (Champeroux et al., 2013). After left thoracotomy, one electrode was sutured directly to the left ventricular epicardium near the apex while the second electrode was sutured to the pericardium above the right atrium to approximate a limb Lead II ECG. Analgesic treatment with buprenorphine/meloxicam was given before surgery and continued for a minimum of 2 days to alleviate any post-operative pain. A minimum period of 3 weeks was allowed for recovery from the surgery. Animals were housed in individual stainless steel cages for telemetry recordings. Out of recording periods, animals were housed in pens by groups of six animals at maximum. Environmental parameters were recorded continuously and maintained within a fixed range, room temperature at 15–21°C and 45–65% relative humidity. The artificial day/night cycle was 12 h light and 12 h darkness with light on at 07:30 h. Drinking water was provided ad libitum. Solid diet (300 g) was given daily in the morning. All dosing with drugs was performed between 15:00 and 15:30 h. ECGs were recorded continuously for a minimum of 2 h before dosing up to 24 h post dose. ECG waveforms were continuously recorded at a sampling rate of 500 Hz using the ART™ acquisition software release 4.2 (Data Sciences International). Cardiac conduction times including QT interval were calculated from a beat-to-beat analysis using internal software developed in RPL (RS/1 programming language, RS/1 release 6.3, Applied Materials, Cambridge MA, USA), Good Laboratory Practices (GLP) validated. Validation of correct location of cardiac wave markers was performed according to a standardized procedure, which covered the whole 24 h period. In most cases, the percentage of errors in location of the end of the T wave was less than 3%. Experiments were conducted following a randomized cross-over study design (vehicle vs. test items) in groups of six animals (three males and three females).
STV
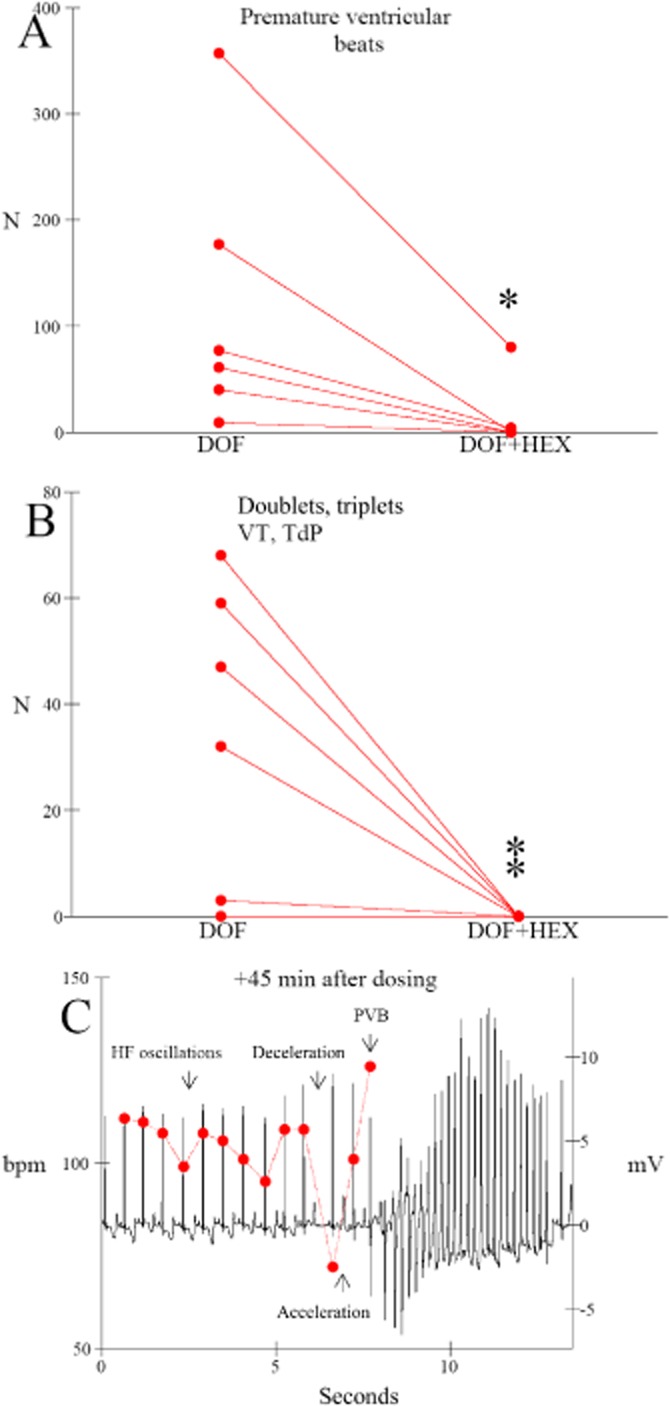
The beat-to-beat STV of QT interval, STVQT, was calculated from 30 successive beats as described elsewhere (Thomsen et al., 2004) using the following formula: 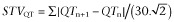 . Beat-to-beat STV of heart rate (HR), STVHR, was calculated according to the same principle.
. Beat-to-beat STV of heart rate (HR), STVHR, was calculated according to the same principle.
HR and QT interval high-frequency (HF) rhythms analysis
HR and QT interval values were re-sampled at a 1 Hz frequency. Analysis of rhythms was derived from a spectral analysis of the HR signal. This consisted of applying a fast Fourier transform on successive and continuous 256 s sequences of the 1 Hz re-sampled HR signal. HF rhythm power in bpm2 or ms2 was derived from integration of power spectrum within the 0.1–0.5 Hz frequency band (Pagani et al., 1986). HF rhythm power was calculated as 1 h reduced mean values (Champeroux et al., 2013).
HF oscillation amplitude analysis
Maximum and minimum beat-to-beat HR and QT interval values were derived from analysis of successive 10 s ECG sequences, that is the maximum period of HF rhythms (0.1 Hz). Amplitude of HF oscillations was calculated from the difference between maximum and minimum HR or QT interval values noted within each 10 s sequence (see Supporting Information Fig. S1, for a typical example of the determination of HF oscillation amplitude).
Arrhythmia analysis
Presence of arrhythmias was investigated throughout the whole post-dosing 24 h period using an internal software development in RPL (RS/1 programming language, RS/1 release 6.3, Applied Materials), GLP validated. Qualification of arrhythmias was performed manually following the Lambeth conventions (Curtis et al., 2013).
Autonomic blockade
Complete suppression of autonomic control was achieved with hexamethonium (20 mg·kg−1, i.v.).
Data analysis
Data were processed and analysed using GLP-validated RS/1 computer procedures (release 6.3, Applied Materials). Individual results were calculated as 1 h reduced mean values (mean calculated from 50% of discrete values centred on the median) derived from these discrete values for all parameters (QT interval, HR, STV, HF rhythms). Mean results derived from these individual values were reported as mean ± SEM. 256 s samples with presence of arrhythmias were excluded from mean calculations and statistical comparisons for all parameters. Drug-induced effects on QT interval, HR, STVQT, STVHR and HF rhythms were compared with their respective vehicle using an anova for repeated measurements. In case of a significant ‘treatment effect’ (P ≤ 0.05) or significant ‘treatment × time’ interaction (P ≤ 0.05), the analysis was continued by Fisher's least significant difference test at each time point. Effects on arrhythmia incidences were compared between groups using a non-parametric Wilcoxon test for paired data.
Materials
Dofetilide was purchased from Sequoia Research Product, Ltd (Pangbourne, UK). Hexamethonium was purchased from Sigma-Aldrich (Saint Quentin, France). These compounds were dissolved in saline (vehicle).
Results
We performed most of the analyses over very short sample periods of 10 s. This duration corresponds to a period of 0.1 Hz for HF autonomic rhythms of HR. In cynomolgus monkeys, individual inspection of ECG traces show that beat-to-beat HR and QT interval variability is very moderate when analysed over very short 10 s periods (Figure 1A). QT interval variability was markedly increased after dosing with dofetilide (Figure 1B). The Poincaré plot representation (Figure 1C) drawn from these two 10 s sequences confirms the dofetilide-induced increase in QT interval STV described earlier with this agent in other species and models (Thomsen et al., 2004). Moreover, an increase in beat-to-beat HR variability is also clearly visible in parallel to QT interval variability. In this typical example (Figure 1B), QT interval variability seems dependent on fast rhythmic oscillations of the HR through the well-known inverse rate dependence between beat-to-beat HR and QT interval (Holzgrefe et al., 2007). Moreover, changes in QT interval during oscillations may influence subsequent beat-to-beat HR values and elicit correcting HR adaptation in dofetilide-treated (1 mg·kg−1, p.o.) animals (Supporting Information Fig. S2).
Figure 1.
Typical beat-to-beat HR and QT interval variability over a 10 s period recorded in the same cynomolgus monkey 1 h before (A) and 40 min after dosing with dofetilide (B, 1 mg·kg−1, p.o.). (C) The corresponding Poincaré plot (QTn+1 vs. previous QTn) showing an increase in beat-to-beat QT interval variability after dosing with dofetilide.
Dofetilide-induced STVQT is closely associated with QT interval HF rhythmic oscillations
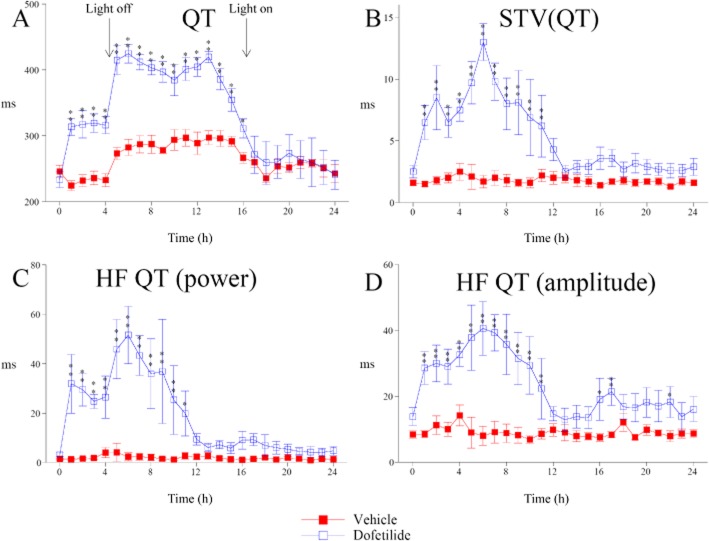
Dofetilide induced, as expected, a large and sustained increase in QT interval (Figure 2A). Note that the large increase in QT interval seen from 5 h after dosing is related to the nycthemeral cycle of HR, light being off 4.5 h after dosing. This agent caused in parallel a large increase in STVQT (Figure 2B). Interestingly, the STVQT increase appears not to be an exclusive consequence of QT prolongation as STVQT returned to baseline 12 h after dosing while QT interval value remained close to its highest level at the same time. Moreover, STVQT changes seen up to 12 h after dosing with dofetilide were more closely correlated to changes in HF QT interval rhythms power and HF QT interval oscillations amplitude than with QT or QTc interval changes (Supporting Information Fig. S3). The rhythmic feature of dofetilide-induced STVQT was first confirmed from power spectral analysis of beat-to-beat QT changes. Indeed, this analysis reveals the rhythmic aspect of QT interval variability (Figure 2C). These oscillations correspond to HF rhythms (>0.1 Hz). The time course of QT interval HF oscillations fits well with that of STVQT. These results provide a first argument suggesting that dofetilide-induced STVQT might have a rhythmic feature. Amplitude of QT interval HF oscillations was close to 40 ms at the peak of effect (Figure 2D) instead of 10 ms in vehicle-treated animals.
Figure 2.
Effect of dofetilide (1 mg·kg−1, p.o.) on QT interval and short-term QT interval variability in cynomolgus monkeys. (A) Dofetilide-induced QT prolongation. (B) Dofetilide also caused a marked increase in STVQT. (C) Power spectral analysis reveals QT interval HF (>0.1 Hz) rhythmic oscillations in dofetilide treated animals. (D) amplitude of QT interval HF oscillations. The time course of changes in QT interval HF rhythmic oscillations fits with those of STVQT. Data are presented as mean values ± SEM; n = 6. *P ≤ 0.05, **P ≤ 0.01, significantly different from vehicle.
Dofetilide increased the HF oscillations in HR
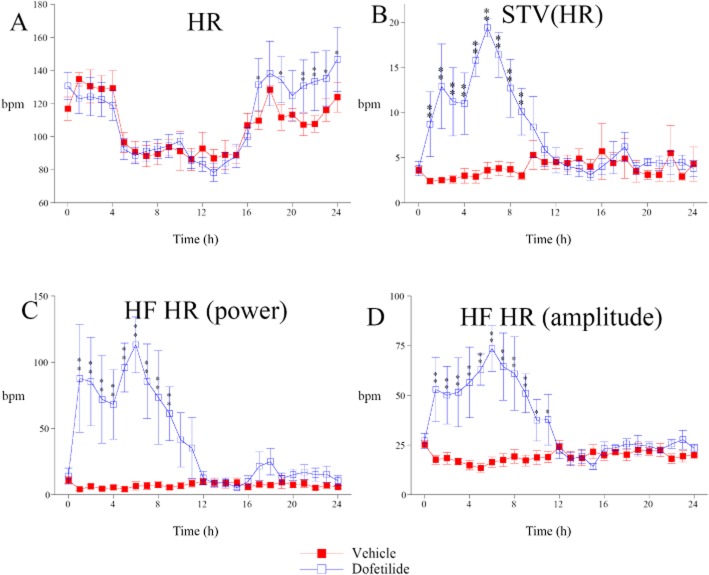
While dofetilide had almost no effect on mean HR in cynomolgus monkeys (Figure 3A), it caused a marked increase in beat-to-beat HR STV (STVHR, Figure 3B). As for STVQT, power spectral analysis of HR variability reveals that dofetilide-induced STVHR has a rhythmic feature within the same HF band (Figure 3C). Indeed, amplitude of the HF oscillations in HR was markedly increased, close to 70 beats per min at the peak of effect (Figure 3D), when compared with vehicle-treated animals. The time course of HF oscillations in HR fits with that of QT interval HF oscillations and STVQT. As HF oscillations in HR are classically attributed to rhythmic activity of the parasympathetic nervous system (Pagani et al., 1986), these results show that dofetilide enhanced endogenous parasympathetic rhythms of HR.
Figure 3.
Effects of dofetilide (1 mg·kg−1, p.o.) on HR and short-term HR variability in cynomolgus monkeys. (A) Dofetilide was quite devoid of effect on mean HR. (B) In parallel, dofetilide increased beat-to-beat STVHR. (C) Power spectral analysis of HR variability reveals HF (>0.1 Hz) rhythmic oscillations of HR in dofetilide-treated animals. (D) Amplitude of HR HF oscillations. Data are presented as mean values ± SEM; n = 6. *P ≤ 0.05, **P ≤ 0.01, significantly different from vehicle.
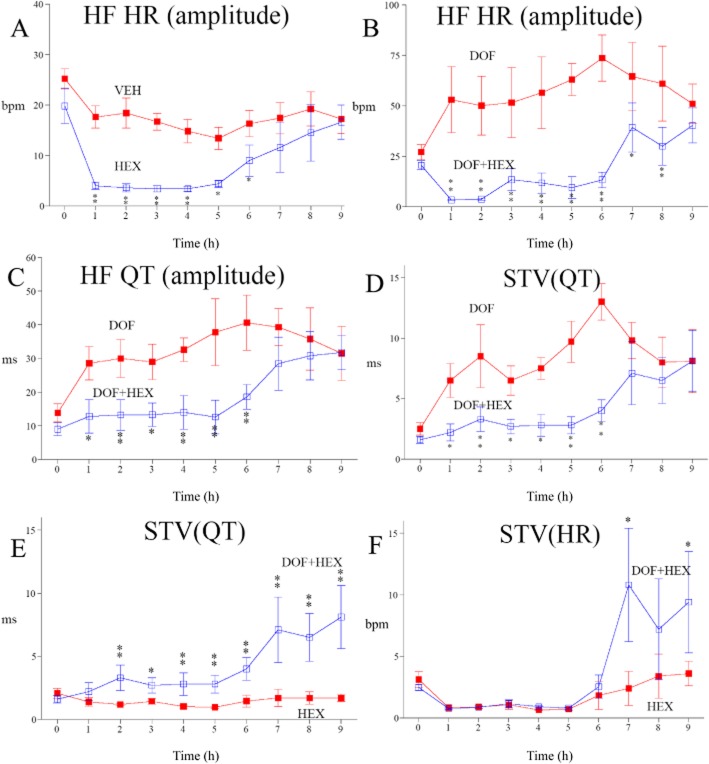
Autonomic blockade by hexamethonium prevents dofetilide-induced STVQT
We used hexamethonium, a ganglioplegic agent, to investigate the involvement of the ANS in dofetilide effects on STVQT and HF rhythms. This agent blocks both the parasympathetic and sympathetic limbs of the ANS. Hexamethonium alone fully suppressed the endogenous HF parasympathetic rhythms of HR (Figure 4A). Duration of its effect was 6 h in cynomolgus monkeys. Hexamethonium had moderate effect on mean HR levels and QT interval (Supporting Information Fig. S4). Comparisons of the effects of dofetilide in the absence and the presence of hexamethonium show that increases in endogenous parasympathetic rhythms of HR (Figure 4B), in QT interval HF oscillations (Figure 4C) and STVQT (Figure 4D) were fully suppressed for the whole duration of autonomic blockade. These results confirm that the effects of dofetilide on HR HF rhythms, QT interval HF oscillations and STVQT have an autonomic component. However, comparison of dofetilide in the presence of hexamethonium versus hexamethonium alone revealed a residual source of QT interval variability (STVQT) that was resistant to autonomic blockade (Figure 4E). Magnitude of this residual source of QT interval variability caused by dofetilide is very much lower than that seen under intact autonomic control. This QT interval variability cannot be explained by a residual source of HR variability as STVHR was dramatically reduced following autonomic blockade both in the presence or absence of dofetilide (Figure 4F). The experiments with dofetilide combined with hexamethonium in beagle dogs lead to the same findings and conclusions (Supporting Information Fig. S5).
Figure 4.
Effects of autonomic blockade by hexamethonium (20 mg·kg−1, i.v., HEX) on dofetilide-induced HR and short-term QT interval variability in cynomolgus monkeys. (A) Hexamethonium alone fully abolished endogenous HF oscillations in HR for 6 h, compared with vehicle (VEH). During this period, this ganglioplegic agent markedly reduced dofetilide (1 mg·kg−1, p.o., DOF) induced increases in HF oscillations in HR (B), QT interval HF oscillations (C) and STVQT (D). (E) A residual QT interval variability (STVQT) insensitive to autonomic blockade was still visible in the presence of dofetilide plus hexamethonium versus hexamethonium alone. (F) On the other hand, dofetilide did not cause any further beat-to-beat STVHR under autonomic blockade when compared with hexamethonium alone. Data are presented as mean values ± SEM; n = 6. P ≤ 0.05, **P ≤ 0.01, significantly different from vehicle or hexamethonium alone.
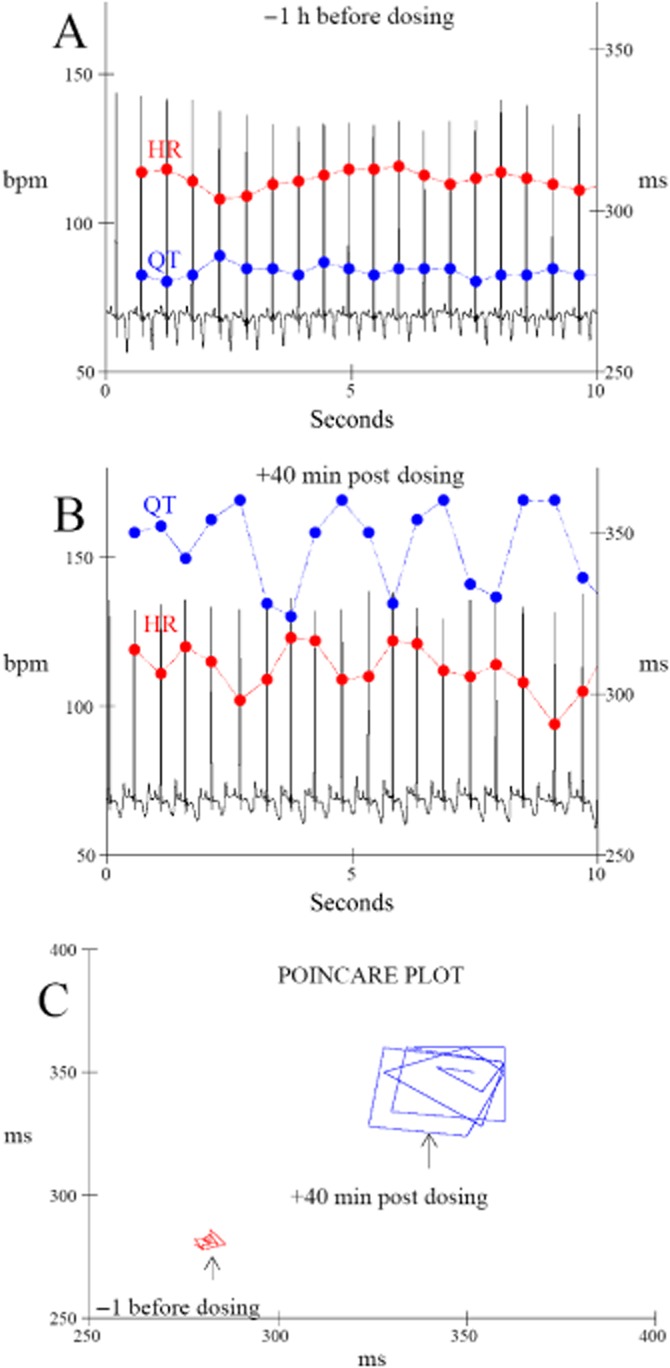
Dofetilide enhances QT interval HF oscillations through HR HF oscillation amplitude-dependent and -independent mechanisms
We built the relationship between the amplitudes of the QT interval oscillation and HF oscillations in HR (HF QT/HF HR relationship) using the same methodological approach as described earlier for the QT/RR relationship (Holzgrefe et al., 2014). Briefly, this analysis consists of sorting pairs of QT interval and HR HF oscillation amplitudes by increasing increments of 5 bpm. Then, mean QT interval HF oscillation amplitude was calculated for each 5 bpm increment. In vehicle-treated animals, this relationship revealed that the amplitude of the QT interval HF oscillation progressively increased with the amplitude of HF oscillations in HR (Figure 5A). Dofetilide had two distinct effects on this relationship (Figure 5B). First, dofetilide caused a shift of the whole relationship towards higher amplitude of HF oscillations in HR (horizontal shift). By prolonging this relationship towards greater HF oscillations in HR, dofetilide increases amplitude of QT interval HF oscillations. Furthermore, it enhanced amplitude of QT interval oscillations according to a mechanism independent of the amplitude of HF oscillations in HR (vertical shift) as it increased amplitude of QT interval HF oscillations for fixed values of amplitude of HF oscillations in HR. Similar findings were seen in beagle dogs (Supporting Information Fig. S6).
Figure 5.
(A) Relationship between the amplitudes of the QT interval oscillation and the HF oscillations in HR (HF QT/HF HR). (A) QT interval HF oscillation amplitude progressively increased with the amplitude of HF oscillations in HR in vehicle-treated animals. (B) Dofetilide (1 mg·kg−1, p.o.,) caused a horizontal shift (1) of the whole relationship towards higher amplitude of HF oscillations in HR. It also enhanced amplitude of QT interval oscillations according to a mechanism independent from amplitude of HF oscillations in HR (2: vertical shift). The relationship was built from all data collected between 1 and 10 h after dosing, that is the period during which dofetilide caused a marked QT prolongation and increase in HF oscillations in HR. Data are presented as mean values ± SEM; n = 6. **P ≤ 0.01, significantly different from vehicle.
HF oscillations contribute to arrhythmic properties of dofetilide in cynomolgus monkeys
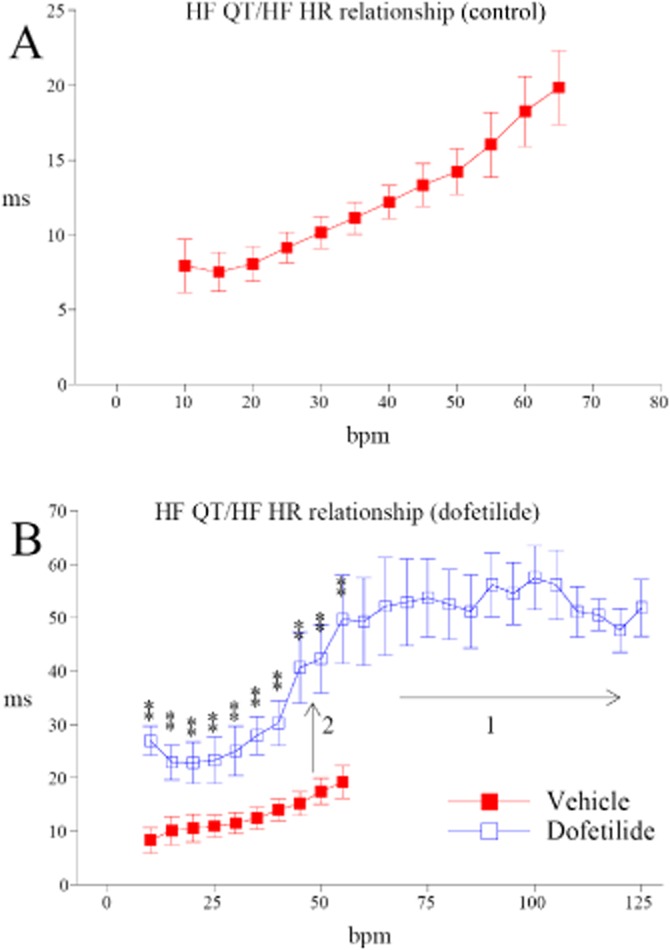
Dofetilide (1 mg·kg−1, p.o.) induces ventricular arrhythmias in all cynomolgus monkeys offering good conditions for evaluation of the role of HF oscillations on arrhythmic properties of this molecule. In dogs, dofetilide is also able to trigger ventricular arrhythmias, but not in all animals, making it more difficult to obtain valid statistical comparisons from small-sized groups. During the whole period of suppressed HF oscillations, hexamethonium prevented ventricular premature beats following dofetilide dosing in five out of six animals (Figure 6A), and in all animals for more severe ventricular arrhythmias (doublets, triplets, ventricular tachycardia episodes, Figure 6B). Moreover, four out of six animals exhibited TdP episodes with dofetilide, whereas no TdP was observed following dosing with dofetilide in the presence of hexamethonium. It should be pointed out that hexamethonium prevented dofetilide-induced arrhythmic events in a way independent of an elevation of HR levels. Conversely, hexamethonium slightly decreased HR in the presence of dofetilide during the first 4 h when compared with dofetilide alone, that is when the incidence of arrhythmias is already high with dofetilide (Supporting Information Fig. S7). We also noted that time course of STVQT changes were correlated with arrhythmia incidence in terms of start of occurrence and duration (Supporting Information Fig. S8). In the absence of autonomic blockade, dofetilide-induced TdP episodes were initiated by a premature ventricular beat that occurred just after a deceleration phase within HF oscillations in HR and just before the onset of the TdP episodes (Figure 6C). Taken together, these results highlight the role of HF autonomic oscillations and their importance for the mechanisms for triggering ventricular arrhythmias.
Figure 6.
Hexamethonium (HEX: 20 mg·kg−1, i.v.) prevented dofetilide-induced (DOF: 1 mg·kg−1, p.o.) ventricular arrhythmias during the whole period of suppression of HF oscillations in cynomolgus monkeys. Data are reported as individual total number of ventricular arrhythmic events counted in each animal (n = 6), N, number of arrhythmic event per animal. (A) Premature ventricular beats (PVB). (B) Doublets + triplets + ventricular tachycardia (VT) + TdP episodes. *P ≤ 0.05, **P ≤ 0.01, significantly different from dofetilide alone. (C) Example of HF oscillations in HR characterized by fast successive deceleration/acceleration phases preceding a PVB occurring just after a deceleration phase and preceding the onset of a TdP episode in a cynomolgus monkey (the same animal as Figure 1) dosed with dofetilide (1 mg·kg−1, p.o.).
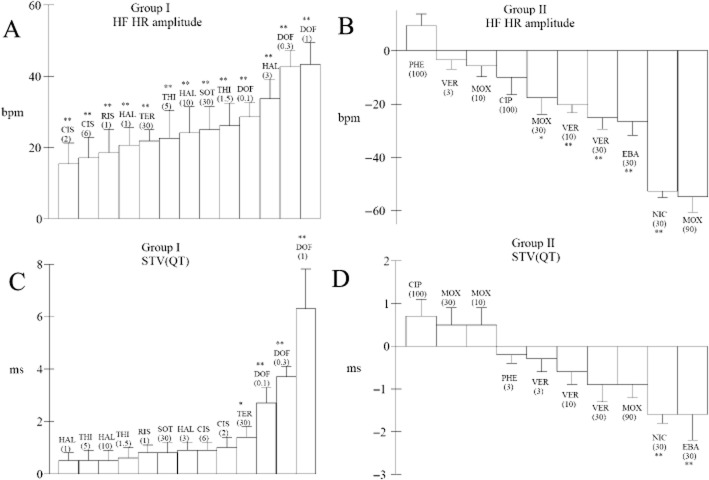
HR HF rhythm enhancement by several arrhythmic hERG blockers in beagle dogs
We retrospectively analysed data collected in our laboratory in beagle dogs with 13 other hERG channel blockers. This analysis shows that enhancements of endogenous HF oscillations in HR were also found in beagle dogs with dofetilide and with other hERG blockers such as cisapride, haloperidol, risperidone, DL sotalol, terfenadine and thioridazine (Figure 7A). On the other hand, the other hERG blockers had either no effect or reduced endogenous HF oscillations in HR in beagle dogs (Figure 7B). In parallel, few compounds were able to cause statistically significant increases in STVQT in the group of hERG blockers causing enhancement of HF oscillations in HR (dofetilide and terfenadine only, Figure 7C) while STVQT was rather decreased among the second group of hERG blockers, in particular with drugs causing the largest decrease in HF oscillations in HR (Figure 7D).
Figure 7.
Maximum enhancement in the amplitude of HF oscillations in HR (HF HR) or changes in STVQT in two groups of hERG blockers tested at various dose levels in beagle dogs. (A) All hERG blockers of group I enhanced HR HF oscillation amplitude. (B) In the second group (II), hERG blockers rather caused a reduction in endogenous HF oscillations in HR. (C) Only two hERG blockers of the group I (dofetilide and terfenadine) were found to cause statistically significant increases in STVQT. (D) Compounds in group II causing the largest reduction of HF oscillations also induced the largest decrease in STVQT. All drugs were given orally: CIS, cisapride; CIP, ciprofloxacin; DOF; dofetilide; EBA, ebastine; HAL, haloperidol; MOX, moxifloxacin; NIC, nicardipine; PHE, phenytoin; RIS, risperidone; SOT, DL sotalol; TER, terfenadine; THI, thioridazine; VER, verapamil. Figures close to abbreviations indicate tested dose levels in mg·kg−1 p. o. Values were determined relative to vehicle at peak of effect. Data are presented as mean values ± SEM; n = 6 per compound. *P ≤ 0.05, **P ≤ 0.01, significantly different from vehicle.
Discussion
Autonomic origin of dofetilide-induced STVQT in healthy animals
Initially, beat-to-beat variability of repolarization was assessed from beat-to-beat STV derived from left ventricular monophasic action potential duration, that is STVLVMAPD, in anaesthetized chronic atrio-ventricular block dogs (Thomsen et al., 2004). Dofetilide was reported as increasing STVLVMAPD and causing TdP in this model. TdP occurrence was associated with an increase in STVLVMAPD. Later, STVQT was derived from QT interval in healthy conscious beagle dogs leading to the conclusion that this biomarker could be considered as better predictor than QTc prolongation for TdP liability (Lengyel et al., 2007). However, until now, no link between the ANS and STVQT has been described under conditions of QT prolongation by arrhythmic hERG blockers. On the other hand, STVAPD derived from action potential duration (APD) was found to be increased by arrhythmic hERG blockers in several models devoid of any interactions with the ANS such as isolated cardiomyocytes (Oros et al., 2010; Abi-Gerges et al., 2011) and isolated perfused heart (Hondeghem et al., 2001) leading to the conclusion that drug-induced increased STVAPD is a consequence of beat-to-beat repolarization instability because of particular electrophysiological properties (named thereafter electrophysiological instability). Our work does not call this concept into question, especially from those drawn from in vitro/ex vivo models. However, the data we present here demonstrate that in vivo STVQT increases by dofetilide in cynomolgus monkeys and beagle dogs are mainly dependent on autonomic HF oscillations in HR and do not simply reflect an erratic instability of the QT interval exclusively because of a source of electrophysiological instability. Worthy of note, we found a residual source of QT interval variability after autonomic blockade that can be attributed to the electrophysiological instability described from in vitro/ex vivo studies. Moreover, this electrophysiological instability may enhance the variability of QT interval dependent on the ANS. However, if sources of electrophysiological instability have a role, our results demonstrate that they require the presence of HF autonomic oscillations to induce changes in STVQT in healthy animals. Considering the frequency band of these oscillations, the HF parasympathetic oscillations of HR play a major role in hERG blocker-induced beat-to-beat ventricular repolarization variability.
Dofetilide enhances QT interval HF oscillations through HR HF oscillation amplitude-dependent and -independent mechanisms
Study of the relationship between HF oscillations in HR and QT interval oscillations demonstrated that QT interval oscillations physiologically increase with amplitude of HR oscillations. This relationship is not surprising as QT interval duration is highly dependent on HR, even during very short periods of 10 s (Holzgrefe et al., 2007). Consequently, important changes within these short periods of 10 s of beat-to-beat HR are expected to cause noticeable changes in beat-to-beat QT interval duration, that is in QT interval HF oscillations. Study of the relationship between QT interval oscillations and HF oscillations in HR demonstrates that the HF oscillations in HR were, by themselves, a source of beat-to-beat ventricular repolarization variability. Dofetilide has two distinct effects on this relationship between QT interval oscillations and HF oscillations in HR. First, it prolongs this relationship towards higher amplitude of HR oscillations, and consequently, causes an increase in amplitude of QT interval HF oscillations through this relationship. As these HR oscillations are driven by the ANS, these data demonstrate that dofetilide directly interacts with the autonomic control of HR. Second, dofetilide causes a vertical shift of QT interval oscillations towards higher amplitudes independently from amplitudes of the HF oscillations in HR. This vertical shift cannot be simply attributed to QT prolongation properties as, moxifloxacin, another hERG blocker devoid of effect on ventricular repolarization STV in chronic AV block dogs (Thomsen et al., 2006) or in cynomolgus monkeys (Supporting Information Fig. S9), was unable to cause such vertical shift despite marked QT prolongation properties (Supporting Information Fig. S10). These data support the hypothesis that the mechanism of this effect is likely to be a consequence of an interaction between a second source of variability and the endogenous QT interval variability generated by the ANS. As previously proposed, this second source of variability could be the beat-to-beat ventricular repolarization electrophysiological instability, visible with dofetilide under autonomic blockade. This interaction could result from this source of beat-to-beat ventricular repolarization instability with autonomic HR oscillations throughout the dependence of QT interval towards HR. Reciprocally, our results also show that QT interval changes during HR oscillations in dofetilide treated animals may influence subsequent beat-to-beat HR values and elicit correcting HR adaptation, causing, in turn, an increase in QT interval variability through rate-dependent mechanisms. This latter mechanism involving correcting HR adaptation is in accordance with abnormalities of QT/RR relationship restitution described first by Fossa (2008) with arrhythmic hERG blockers.
Applying to Coumel's triangle
The increase in HF oscillations in HR reflects an enhancement of alternate cycles of very short-term deceleration and acceleration processes over very short periods of less than 10 s. Classically, HF rhythms of HR are dependent on alternate withdrawal and activation of the parasympathetic pathway. Oscillatory short-long-short (SLS) cycle length sequences were described as preceding the onset of premature ventricular beats and TdP episodes in patients with acquired prolonged ventricular repolarization (Locati et al., 1995), as we found in cynomolgus monkeys with dofetilide. Such pro-arrhythmic property of SLS sequences is still routinely used in the clinic in patients with unexplained syncopes or rhythm disorders using electrical programmed stimulations (Wellens et al., 1985). Likewise, acceleration from an initial slow rate was also reported to facilitate early after-depolarization in canine myocardial M cells and Purkinje fibres under hERG blockade via a mechanism involving intracellular calcium loading (Burashnikov and Antzelevitch, 1998). All these findings demonstrated that fast deceleration and acceleration processes constitute sources of triggering of ventricular arrhythmias. Interestingly, QT prolongation induced by hERG blockers was proposed to constitute a substrate for occurrence of premature ventricular beats in particular during acceleration phases (Fossa, 2008). Increases in HR HF rhythms have also been described in patients with congenital LQT (Shamsuzzaman et al., 2003). Independently from this latter study, STVQT was found to be increased in patients with LQTs (Hinterseer et al., 2009). Taken together, our results and already published findings strongly support that an enhancement of fast deceleration and acceleration processes carried by endogenous HR parasympathetic oscillations could constitute a primary trigger factor leading secondarily to creation of favourable conditions for expression of cellular trigger factors such as re-entries or early after-depolarizations because of confluence of impaired repolarization in a context of QT prolongation.
In other words, these data support the concept of Coumel's triangle of arrhythmogenesis. To develop life-threatening arrhythmia such as TdP, arrhythmic hERG channel blockers would require (i) an electrophysiological substrate, that is APD prolongation and instability (APDi); (ii) a primary trigger factor, that is enhancement of HF oscillations in HR; and (iii) a modulator factor, the ANS, which modulates both APD and endogenous HF oscillations. The combination of these three ingredients leads to the increase of ventricular repolarization STV (STVQT), therefore creating favourable conditions for expression of cellular triggering factors such as re-entries or early after-depolarizations. Figure 8 proposes an adaptation of Coumel's triangle as applied to dofetilide in cynomolgus monkeys that also serves to summarise the previous discussions.
Figure 8.
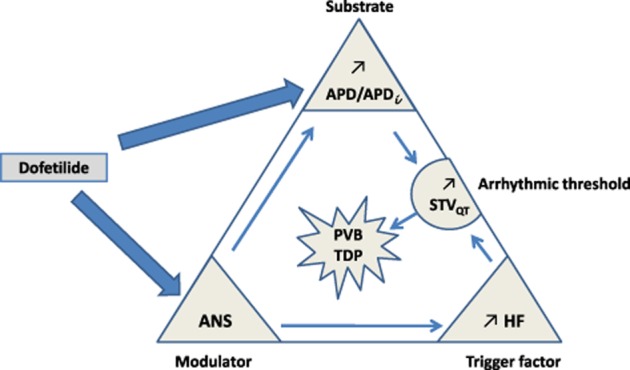
Applying Coumel's triangle of arrhythmogenesis to the actions of dofetilide. To develop life-threatening arrhythmia such as TdP, this agent would require (i) an electrophysiological substrate, that is APD prolongation and APDi; (ii) a primary trigger factor, that is enhancement of HR HF oscillations; and (iii) a modulator factor, the ANS which modulates both APD and endogenous HF oscillations. The combination of these three ingredients leads to increasing of ventricular repolarization STV (STVQT), so creating favourable conditions for expression of cellular triggering factors such as re-entries or early after depolarizations. In the context of these drug-induced arrhythmias, STVQT is a biomarker of the arrhythmic threshold above which probability for arrhythmia occurrence is high. PVB, premature ventricular beats.
Predictive value of HF oscillations in HR
We found that all hERG blockers able to enhance endogenous HF oscillations in HR in beagle dogs (group I) were compounds already reported as causing TdP (Redfern et al., 2003; Vieweg et al., 2008). In the second group of hERG blockers, only moxifloxacin was described as responsible for TdP at an extremely low incidence (Haverkamp et al., 2012). Consequently, this biomarker may be of relevance in safety assessment, in particular for new drug candidates exhibiting hERG-blocking properties. However, many mechanisms are susceptible to enhance endogenous HF parasympathetic oscillations in HR, such as baroreflex activation, central effects and parasympathomimetic properties. Consequently, taken alone, this biomarker cannot thus be considered as a specific surrogate for TdP liability. On the other hand, an enhancement of HF oscillations in HR could be considered as an early and sensitive surrogate for TdP liability when associated with evidence of hERG-blocking properties, such as QT prolongation. This way of indicating TdP liability is more sensitive than STVQT assessment. Indeed, enhancement of HF oscillations in HR was easily detected with several arrhythmic hERG blockers, sometimes at low dose levels like thioridazine or with low TdP incidence arrhythmic drugs such as risperidone (Vieweg et al., 2008), while increases in STVQT reached statistically significant levels only with a few drugs (dofetilide and terfenadine) in healthy animals. Finally, analysis of HF QT oscillations amplitude in clinical studies exploring QT variability in patients with long-QT syndrome (Hinterseer et al., 2009) could offer interesting opportunities to check whether this biomarker is more sensitive than STVQT in human to reveal increased QT variability issues.
In conclusion, the present work offers a new insight by demonstrating a link between the arrhythmic profile of dofetilide and interaction with cardiac autonomic control. This concept could be very helpful as it may allow refinement of the current preclinical safety pharmacology strategy in this area.
Acknowledgments
We acknowledge the contribution of D Bouard, A Contamine and C Roubinet for their technical assistance. Jerome Thireau holds a CNRS position and was supported by Fondation de France (Project SYNAPTOCARD N°201300038586).
Glossary
- ANS
autonomic nervous system
- APD
action potential duration
- EAG
ether-a-go-go
- ERG
ether-a-go-go related gene
- GLP
Good Laboratory Practices
- hERG
human ether-a-go-go related gene
- HF
high-frequency
- HR
heart rate
- LQTS
long QT syndrome
- SLS
short-long-short
- STV
short-term variability
- TdP
Torsades de pointes
Author contributions
Research studies were designed by P. C. Experiments were conducted by S. J., C. L.-B. and A. M. Data analysis and interpretation were performed by P. C. The paper was written by P. C., J. T. and J. Y. L. G., and was critically evaluated by all authors. J. T. proposed application to Coumel's triangle.
Conflict of interest
The authors state no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Typical example of determination of HF oscillations amplitude. Amplitude of HF oscillations is calculated from the difference between maximum and minimum HR values noted within a 10 s sequence, that is the maximum period of HF rhythms (0.1 Hz). The same principle is followed for determination of QT interval HF oscillations amplitude.
Figure S2 Regression curves between changes in beat-to-beat QTn or QTn−1 interval versus HR (HRn) drawn from the typical example of the Figure 1B (dofetilide, 1 mg·kg−1, p.o.) in cynomolgus monkeys. This figure shows a better correlation between QT intervals (QTn−1) preceding subsequent HR values than concomitant beat-to-beat QT intervals (QTn) and HR (HRn) values. This analysis supports that changes in QT interval might influence subsequent beat-to-beat HR values and elicit correcting HR adaptation in dofetilide-treated (1 mg·kg−1, p.o.) animals. This possible mechanism could contribute towards the enhancement of HF HR oscillations.
Figure S3 Regression curves between changes in STVQT and HF QT interval rhythms power (A, HF QTp), HF QT interval oscillations amplitude (B, HF QTa), QT interval (C, QT) and QT interval corrected by Holzgrefe's probabilistic method (D, QTcH). This figure shows that changes in STVQT recorded for 12 h after dosing with dofetilide (1 mg·kg−1, p.o.) were more closely correlated to changes in HF QT interval rhythms power and HF QT interval oscillations amplitude than with QT or QTc interval changes. Correlations curves were drawn from mean changes calculated in relation to vehicle over the period ranging from 1 to 12 h after dosing with dofetilide. R, regression coefficient; P, significance level.
Figure S4 (A) Hexamethonium (20 mg·kg−1, i.v.) had moderate effects on HR related to suppression of cardiac autonomic control of HR, that is lowering HR during the daylight period and inhibition of the nocturnal bradycardia during the first hours of the night period. (B) QT interval was increased in parallel to lowering HR. Filled squares, vehicle; open squares, hexamethonium alone. Data are presented as mean values ± SEM (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle).
Figure S5 Effects of autonomic blockade by hexamethonium (20 mg·kg−1, i.v., H) on dofetilide-induced HR and QT interval variability in beagle dogs. (A) Hexamethonium alone fully abolished endogenous HR HF oscillations for 4 h when compared with vehicle (V). During this period, this ganglioplegic agent markedly reduced dofetilide (1 mg·kg−1, p.o., D) induced increases in the HF oscillations in HR (B), QT interval HF oscillations (C) and STVQT (D). (E) a residual QT interval variability (STVQT) insensitive to autonomic blockade was still visible in the presence of dofetilide plus hexamethonium versus hexamethonium alone. (F) On the other hand, dofetilide did not cause any further beat-to-beat HR variability (STVHR) under autonomic blockade when compared with hexamethonium alone. Data are presented as mean values ± SEM (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle or hexamethonium alone).
Figure S6 (A) Relationship between QT interval and HR HF oscillations amplitudes (HF QT/HF HR) in vehicle beagle dogs (filled squares) over a 24 h period: QT interval HF oscillation amplitude progressively increased with HR interval HF oscillation amplitude in vehicle-treated animals. (B) dofetilide (open squares) caused a horizontal shift (1) of the whole relationship towards higher amplitude of HF oscillations in HR. It also amplified amplitude of QT interval oscillations according to a mechanism independent from amplitude of HF oscillations in HR (2: vertical shift). The relationship was built from all data collected between 2 and 6 h after dosing, that is the period during which dofetilide caused a marked QT prolongation and increase in HF oscillations in HR. Data are presented as mean values ± SEM (n = 6, **P ≤ 0.01, when compared with vehicle).
Figure S7 (A) Time course of arrhythmic events induced by dofetilide alone (1 mg·kg−1, p.o., red circles) versus dofetilide in hexamethonium (20 mg·kg−1, i.v., blue circles) treated cynomolgus monkeys. Data are reported as number of arrhythmic events (premature ventricular beats, doublets, triplets, ventricular tachycardia and TdP episodes). (B) Time course of HR changes induced by dofetilide alone (1 mg·kg−1, p.o., red circles) versus dofetilide in hexamethonium-treated (20 mg·kg−1, i.v., blue circles) animals. The analysed period of 6 h corresponds to the duration of effects of hexamethonium on HF HR oscillations amplitude and the period, during which incidence of arrhythmias was analysed referring to the Figure 6 of the main paper. This figure shows that hexamethonium prevented dofetilide-induced arrhythmic events in a way independent of an elevation of HR levels. Conversely, hexamethonium slightly decreased HR in the presence of dofetilide during the first 4 h when compared with dofetilide alone, that is when the incidence of arrhythmias is already high with dofetilide. Data are presented as median values (A: n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with dofetilide alone, non-parametric Mann–Whithney U-test) and as mean values ± SEM (B: n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with dofetilide alone).
Figure S8 Time course of STVQT changes (left axis, blue circles) versus arrhythmic events (right axis, black circles) induced by dofetilide (1 mg·kg−1, p.o.) in cynomolgus monkeys. This figure shows that dofetilide-induced arrhythmic events started to occur as soon as STVQT began to increase. Moreover, arrhythmic events disappeared 12 h after dosing when STVQT returned to baseline. Data are presented as median values for arrhythmia incidence (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle, non-parametric Mann–Whithney U-test) and mean values ± SEM for STVQT (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle). No arrhythmia was seen in vehicle-treated animals.
Figure S9 (A) Moxifloxacin (90 mg·kg−1, p.o.) had no effect on HR (A) in cynomolgus monkeys. It caused a large QTc prolongation (B, probabilistic method) and a moderate lowering of HF oscillations in HR (C). Moxifloxacin did not change STVQT (D). Filled squares: vehicle. Open squares: moxifloxacin. Data are presented as mean values ± SEM (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle).
Figure S10 Moxifloxacin (90 mg·kg−1, p.o.) had no effect on the relationship between amplitudes of the QT interval and the HF oscillations in HR in cynomolgus monkeys. The relationship was built from all data collected between 2 and 12 h after dosing, that is the period during which moxifloxacin caused a marked and stable QT prolongation. Data are presented as mean values ± SEM (n = 6). P > 0.05, when compared with vehicle.
References
- Abi-Gerges N, Valentin JP, Pollard CE. Dog left ventricular midmyocardial myocytes for assessment of drug-induced delayed repolarization: short-term variability and proarrhythmic potential. Br J Pharmacol. 2011;159:77–92. doi: 10.1111/j.1476-5381.2009.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Gerges N, Holkham H, Jones EM, Pollard CE, Valentin JP, Robertson GA. hERG subunit composition determines differential drug sensitivity. Br J Pharmacol. 2011;164:419–432. doi: 10.1111/j.1476-5381.2011.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C. Ionic, molecular, and cellular bases of QT-interval prolongation and torsade de pointes. Europace. 2007;9:4–15. doi: 10.1093/europace/eum166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Acceleration-induced action potential prolongation and early afterdepolarizations. J Cardiovasc Electrophysiol. 1998;9:934–948. doi: 10.1111/j.1540-8167.1998.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Champeroux P, Ouillé A, Martel E, Fowler JS, Maurin A, Jude S, et al. Interferences of the autonomic nervous system with drug induced QT prolongation: a point to consider in non-clinical safety studies. J Pharmacol Toxicol Methods. 2010;61:251–263. doi: 10.1016/j.vascn.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Champeroux P, Martel E, Jude S, Laigot C, Laveissière A, Weyn-Marotte AA, et al. Power spectral analysis of heart rate variability in cynomolgus monkeys in safety pharmacology studies: comparative study with beagle dogs. J Pharmacol Toxicol Methods. 2013;68:166–174. doi: 10.1016/j.vascn.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Coumel P. Cardiac arrhythmias and the autonomic nervous system. J Cardiovasc Electrophysiol. 1993;4:338–355. doi: 10.1111/j.1540-8167.1993.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Coumel P. Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 1996;7:999–1007. doi: 10.1111/j.1540-8167.1996.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA, et al. The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther. 2013;139:213–248. doi: 10.1016/j.pharmthera.2013.04.008. [DOI] [PubMed] [Google Scholar]
- De Ponti F, Cavalli A, Recanatini M, Montanaro N. Safety of non-antiarrhythmic drugs that prolong the QT interval or induce Torsade de pointes: an overview. Drug Saf. 2002;25:263–286. doi: 10.2165/00002018-200225040-00004. [DOI] [PubMed] [Google Scholar]
- Di Veroli GY, Davies MR, Zhang H, Abi-Gerges N, Boyett MR. hERG inhibitors with similar potency but different binding kinetics do not pose the same proarrhythmic risk: implications for drug safety assessment. J Cardiovasc Electrophysiol. 2014;25:197–207. doi: 10.1111/jce.12289. [DOI] [PubMed] [Google Scholar]
- Fossa AA. The impact of varying autonomic states on the dynamic beat-to-beat QT–RR and QT–TQ interval relationships. Br J Pharmacol. 2008;154:1508–1515. doi: 10.1038/bjp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp W, Kruesmann F, Fritsch A, van Veenhuyzen D, Arvis P. Update on the cardiac safety of moxifloxacin. Curr Drug Saf. 2012;7:149–163. doi: 10.2174/157488612802715735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterseer M, Beckmann BM, Thomsen MB, Pfeufer A, Dalla Pozza R, Loeff M, et al. Relation of increased short-term variability of QT interval to congenital long-QT syndrome. Am J Cardiol. 2009;103:1244–1248. doi: 10.1016/j.amjcard.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Holzgrefe H, Ferber G, Champeroux P, Gill M, Honda M, Greiter-Wilke A, et al. Preclinical QT safety assessment: cross-species comparisons and human translation from an industry consortium. J Pharmacol Toxicol Methods. 2014;69:61–101. doi: 10.1016/j.vascn.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Holzgrefe HH, Cavero I, Gleason CR. Analysis of the nonclinical telemetered ECG: impact of logging rate and RR bin width in the dog and cynomolgus monkey. J Pharmacol Toxicol Methods. 2007;56:34–42. doi: 10.1016/j.vascn.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
- Huffaker SJ, Chen J, Nicodemus KK, Sambataro F, Yang F, Mattay V, et al. A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat Med. 2009;15:509–518. doi: 10.1038/nm.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhardt A, Denjoy I, Guicheney P. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012;5:1044–1052. doi: 10.1161/CIRCEP.111.962027. [DOI] [PubMed] [Google Scholar]
- Lengyel C, Varró A, Tábori K, Papp JG, Baczkó I. Combined pharmacological block of I(Kr) and I(Ks) increases short-term QT interval variability and provokes Torsades de pointes. Br J Pharmacol. 2007;151:941–951. doi: 10.1038/sj.bjp.0707297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locati EH, Maison-Blanche P, Dejode P, Cauchemez B, Coumel P. Spontaneous sequences of onset of Torsade de pointes in patients with acquired prolonged repolarization: quantitative analysis of Holter recordings. J Am Coll Cardiol. 1995;25:1564–1575. doi: 10.1016/0735-1097(95)00100-i. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalos L, Varkevisser R, Jonsson MK, Houtman MJ, Beekman JD, van der Nagel R, et al. Comparison of the IKr blockers moxifloxacin, dofetilide and E-4031 in five screening models of pro-arrhythmia reveals lack of specificity of isolated cardiomyocytes. Br J Pharmacol. 2012;165:467–478. doi: 10.1111/j.1476-5381.2011.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omichi C, Momose Y, Kitahara S. Congenital long QT syndrome presenting with a history of epilepsy: misdiagnosis or relationship between channelopathies of the heart and brain? Epilepsia. 2010;51:289–292. doi: 10.1111/j.1528-1167.2009.02267.x. [DOI] [PubMed] [Google Scholar]
- Oros A, Houtman MJ, Neco P, Gomez AM, Rajamani S, Oosterhoff P, et al. Robust anti-arrhythmic efficacy of verapamil and flunarizine against dofetilide-induced TdP arrhythmias is based upon a shared and a different mode of action. Br J Pharmacol. 2010;161:162–175. doi: 10.1111/j.1476-5381.2010.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podrid PJ, Fuchs T, Candinas R. Role of the sympathetic nervous system in the genesis of ventricular arrhythmia. Circulation. 1990;82:103–113. [PubMed] [Google Scholar]
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and Torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman AS, Ackerman MJ, Kara T, Lanfranchi P, Somers VK. Sympathetic nerve activity in the congenital long-QT syndrome. Circulation. 2003;107:1844–1847. doi: 10.1161/01.CIR.0000066284.34258.59. [DOI] [PubMed] [Google Scholar]
- Shi W, Wymore RS, Wang HS, Pan Z, Cohen IS, McKinnon D, et al. Identification of two nervous system-specific members of the erg potassium channel gene family. J Neurosci. 1997;17:9423–9432. doi: 10.1523/JNEUROSCI.17-24-09423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MB, Verduyn SC, Stengl M, Beekman JD, de Pater G, van Opstal J, et al. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004;110:2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Beekman JD, Attevelt NJ, Takahara A, Sugiyama A, Chiba K, et al. No proarrhythmic properties of the antibiotics Moxifloxacin or Azithromycin in anaesthetized dogs with chronic-AV block. Br J Pharmacol. 2006;149:1039–1048. doi: 10.1038/sj.bjp.0706900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012;92:1393–1478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- Vieweg WV, Hasnain M, Hancox JC, Baranchuk A, Digby GC, Kogut C, et al. Risperidone, QTc interval prolongation, and torsade de pointes: a systematic review of case reports. Psychopharmacology (Berl) 2008;228:515–524. doi: 10.1007/s00213-013-3192-8. [DOI] [PubMed] [Google Scholar]
- Wellens HJ, Brugada P, Stevenson WG. Programmed electrical stimulation of the heart in patients with life-threatening ventricular arrhythmias: what is the significance of induced arrhythmias and what is the correct stimulation protocol? Circulation. 1985;72:1–7. doi: 10.1161/01.cir.72.1.1. [DOI] [PubMed] [Google Scholar]
- Yang T, Chun YW, Stroud DM, Mosley JD, Knollmann BC, Hong C, et al. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation. 2014;130:224–234. doi: 10.1161/CIRCULATIONAHA.113.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamorano-León JJ, Yañez R, Jaime G, Rodriguez-Sierra P, Calatrava-Ledrado L, Alvarez-Granada RR, et al. KCNH2 gene mutation: a potential link between epilepsy and long QT-2 syndrome. J Neurogenet. 2012;26:382–386. doi: 10.3109/01677063.2012.674993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Typical example of determination of HF oscillations amplitude. Amplitude of HF oscillations is calculated from the difference between maximum and minimum HR values noted within a 10 s sequence, that is the maximum period of HF rhythms (0.1 Hz). The same principle is followed for determination of QT interval HF oscillations amplitude.
Figure S2 Regression curves between changes in beat-to-beat QTn or QTn−1 interval versus HR (HRn) drawn from the typical example of the Figure 1B (dofetilide, 1 mg·kg−1, p.o.) in cynomolgus monkeys. This figure shows a better correlation between QT intervals (QTn−1) preceding subsequent HR values than concomitant beat-to-beat QT intervals (QTn) and HR (HRn) values. This analysis supports that changes in QT interval might influence subsequent beat-to-beat HR values and elicit correcting HR adaptation in dofetilide-treated (1 mg·kg−1, p.o.) animals. This possible mechanism could contribute towards the enhancement of HF HR oscillations.
Figure S3 Regression curves between changes in STVQT and HF QT interval rhythms power (A, HF QTp), HF QT interval oscillations amplitude (B, HF QTa), QT interval (C, QT) and QT interval corrected by Holzgrefe's probabilistic method (D, QTcH). This figure shows that changes in STVQT recorded for 12 h after dosing with dofetilide (1 mg·kg−1, p.o.) were more closely correlated to changes in HF QT interval rhythms power and HF QT interval oscillations amplitude than with QT or QTc interval changes. Correlations curves were drawn from mean changes calculated in relation to vehicle over the period ranging from 1 to 12 h after dosing with dofetilide. R, regression coefficient; P, significance level.
Figure S4 (A) Hexamethonium (20 mg·kg−1, i.v.) had moderate effects on HR related to suppression of cardiac autonomic control of HR, that is lowering HR during the daylight period and inhibition of the nocturnal bradycardia during the first hours of the night period. (B) QT interval was increased in parallel to lowering HR. Filled squares, vehicle; open squares, hexamethonium alone. Data are presented as mean values ± SEM (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle).
Figure S5 Effects of autonomic blockade by hexamethonium (20 mg·kg−1, i.v., H) on dofetilide-induced HR and QT interval variability in beagle dogs. (A) Hexamethonium alone fully abolished endogenous HR HF oscillations for 4 h when compared with vehicle (V). During this period, this ganglioplegic agent markedly reduced dofetilide (1 mg·kg−1, p.o., D) induced increases in the HF oscillations in HR (B), QT interval HF oscillations (C) and STVQT (D). (E) a residual QT interval variability (STVQT) insensitive to autonomic blockade was still visible in the presence of dofetilide plus hexamethonium versus hexamethonium alone. (F) On the other hand, dofetilide did not cause any further beat-to-beat HR variability (STVHR) under autonomic blockade when compared with hexamethonium alone. Data are presented as mean values ± SEM (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle or hexamethonium alone).
Figure S6 (A) Relationship between QT interval and HR HF oscillations amplitudes (HF QT/HF HR) in vehicle beagle dogs (filled squares) over a 24 h period: QT interval HF oscillation amplitude progressively increased with HR interval HF oscillation amplitude in vehicle-treated animals. (B) dofetilide (open squares) caused a horizontal shift (1) of the whole relationship towards higher amplitude of HF oscillations in HR. It also amplified amplitude of QT interval oscillations according to a mechanism independent from amplitude of HF oscillations in HR (2: vertical shift). The relationship was built from all data collected between 2 and 6 h after dosing, that is the period during which dofetilide caused a marked QT prolongation and increase in HF oscillations in HR. Data are presented as mean values ± SEM (n = 6, **P ≤ 0.01, when compared with vehicle).
Figure S7 (A) Time course of arrhythmic events induced by dofetilide alone (1 mg·kg−1, p.o., red circles) versus dofetilide in hexamethonium (20 mg·kg−1, i.v., blue circles) treated cynomolgus monkeys. Data are reported as number of arrhythmic events (premature ventricular beats, doublets, triplets, ventricular tachycardia and TdP episodes). (B) Time course of HR changes induced by dofetilide alone (1 mg·kg−1, p.o., red circles) versus dofetilide in hexamethonium-treated (20 mg·kg−1, i.v., blue circles) animals. The analysed period of 6 h corresponds to the duration of effects of hexamethonium on HF HR oscillations amplitude and the period, during which incidence of arrhythmias was analysed referring to the Figure 6 of the main paper. This figure shows that hexamethonium prevented dofetilide-induced arrhythmic events in a way independent of an elevation of HR levels. Conversely, hexamethonium slightly decreased HR in the presence of dofetilide during the first 4 h when compared with dofetilide alone, that is when the incidence of arrhythmias is already high with dofetilide. Data are presented as median values (A: n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with dofetilide alone, non-parametric Mann–Whithney U-test) and as mean values ± SEM (B: n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with dofetilide alone).
Figure S8 Time course of STVQT changes (left axis, blue circles) versus arrhythmic events (right axis, black circles) induced by dofetilide (1 mg·kg−1, p.o.) in cynomolgus monkeys. This figure shows that dofetilide-induced arrhythmic events started to occur as soon as STVQT began to increase. Moreover, arrhythmic events disappeared 12 h after dosing when STVQT returned to baseline. Data are presented as median values for arrhythmia incidence (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle, non-parametric Mann–Whithney U-test) and mean values ± SEM for STVQT (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle). No arrhythmia was seen in vehicle-treated animals.
Figure S9 (A) Moxifloxacin (90 mg·kg−1, p.o.) had no effect on HR (A) in cynomolgus monkeys. It caused a large QTc prolongation (B, probabilistic method) and a moderate lowering of HF oscillations in HR (C). Moxifloxacin did not change STVQT (D). Filled squares: vehicle. Open squares: moxifloxacin. Data are presented as mean values ± SEM (n = 6, *P ≤ 0.05, **P ≤ 0.01, when compared with vehicle).
Figure S10 Moxifloxacin (90 mg·kg−1, p.o.) had no effect on the relationship between amplitudes of the QT interval and the HF oscillations in HR in cynomolgus monkeys. The relationship was built from all data collected between 2 and 12 h after dosing, that is the period during which moxifloxacin caused a marked and stable QT prolongation. Data are presented as mean values ± SEM (n = 6). P > 0.05, when compared with vehicle.