Abstract
Background and Purpose
Medical therapy of lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) targets smooth muscle contraction in the prostate, or prostate growth. However, current therapeutic options are insufficient. Here, we investigated the role of Rac in the control of smooth muscle tone in human prostates and growth of prostate stromal cells.
Experimental Approach
Experiments were performed using human prostate tissues from radical prostatectomy and cultured stromal cells (WPMY-1). Expression of Rac was examined by Western blot and fluorescence staining. Effects of Rac inhibitors (NSC23766 and EHT1864) on contractility were assessed in the organ bath. The effects of Rac inhibitors were assessed by pull-down, cytotoxicity using a cell counting kit, cytoskeletal organization by phalloidin staining and cell growth using an 5-ethynyl-2′-deoxyuridine assay.
Key Results
Expression of Rac1–3 was observed in prostate samples from each patient. Immunoreactivity for Rac1–3 was observed in the stroma, where it colocalized with the smooth muscle marker, calponin. NSC23766 and EHT1864 significantly reduced contractions of prostate strips induced by noradrenaline, phenylephrine or electrical field stimulation. NSC23766 and EHT1864 inhibited Rac activity in WPMY-1 cells. Survival of WPMY-1 cells ranged between 64 and 81% after incubation with NSC23766 (50 or 100 μM) or EHT1864 (25 μM) for 24 h. NSC23766 and EHT1864 induced cytoskeletal disorganization in WPMY-1 cells. Both inhibitors impaired the growth of WPMY-1 cells.
Conclusions and Implications
Rac may be a link connecting the control of prostate smooth muscle tone with proliferation of smooth muscle cells. Improvements in LUTS suggestive of BPH by Rac inhibitors appears possible.
Tables of Links
| TARGETS | |
|---|---|
| GPCRsa | Enzymesb |
| α1-adrenoceptors | PAK1 |
| α1A-adrenoceptor | PAK2 |
| LIGANDS | |
|---|---|
| EGF | Phenylephrine |
| GTPγS | Rac1 |
| Noradrenaline | Rac2 |
| Phalloidin | Rac3 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
Prostate smooth muscle tone and prostate growth may be critically involved in the pathogenesis of lower urinary tract symptoms (LUTS), and are important targets for the treatment of LUTS (Hennenberg et al., 2014b). In patients with benign prostatic hyperplasia (BPH), enhanced α1-adrenoceptor-mediated prostate smooth muscle contraction and prostate enlargement may cause urethral obstruction and impairment of urethral flow either alone or together (Hennenberg et al., 2014b). Consequently, both components are important targets for medical therapy: while smooth muscle relaxation by treatment with α1-adrenoceptor antagonists (‘α1-blockers’) may cause an improvement in symptoms, application of 5α-reductase inhibibitors may reduce prostate size and the progression of BPH (Hennenberg et al., 2014a).
However, despite their wide use as therapeutics for LUTS, the benefits from α1-blockers are limited (Hennenberg et al., 2014b). In clinical studies, improvements in the LUTS suggestive of BPH by α1-blockers are only slightly different from the effects of placebos: while α1-blockers enhance the maximum flow rate (measured during micturition) by 15–40%, increases of up to 27% were reported in response to placebos (Hennenberg et al., 2014a,b,). Similarly, symptom scores assessed by internationally standardized questionnaires are reduced to 30–50% by α1-blockers, and to 10–34% by placebos (Hennenberg et al., 2014a,b,). Benefits from 5α-reductase inhibitors, another mainstay of medical therapy, are obvious as early as 3–6 months after commencement of the therapy (Hennenberg et al., 2014a). However, discontinuation of this treatment due to, for example, disappointing results and/or side effects may vary between medications and studies, ranging from 27 to 45% for 5α-reductase inhibitors (Nichol et al., 2009; Kruep et al., 2014). Adherence to α1-blocker therapy may be better, although discontinuation rates of up to 42% have been reported (Wilt et al., 2002; Nichol et al., 2009).
In fact, the medical therapy of LUTS is still a challenge, so alternative options are much needed. The development of new therapies requires an improved understanding of prostate smooth muscle contraction, and identification of critical targets for the control of prostate smooth muscle tone (Hennenberg et al., 2014a). Although it has been assumed that alterations in prostate smooth muscle tone are linked to changes in prostate growth, the molecular mechanisms connecting these two components with each other have still not been elucidated (Hennenberg et al., 2014b).
Rac is a small monomeric GTPase that is involved in the control of smooth muscle tone in the cardiovascular system and airways, and occurs in three different isoforms (Rac1–3) (Roscioni et al., 2011; Rahman et al., 2014). In addition, Rac promotes cell cycle progression in different smooth muscle cells, and hyperplastic growth in the cardiovascular system (Pelletier et al., 2005; Simeone-Penney et al., 2008; Wu et al., 2012). However, the role of Rac in the non-malignant prostate has not been addressed to date. Here, we examined the effects of two different Rac inhibitors, NSC23766 and EHT1864, on contractions of human prostate tissue and on the growth of non-malignant stromal cells.
Methods
Human prostate tissue
Human prostate tissues were obtained from patients undergoing radical prostatectomy for prostate cancer, but without previous transurethral resection of the prostate. The research was carried out in accordance with the Declaration of Helsinki of the World Medical Association, and has been approved by the ethics committee of the Ludwig Maximilian University, Munich, Germany. Tissues were taken from the periurethral zone, while most prostate tumours are located at the peripheral zone (Pradidarcheep et al., 2011; Shaikhibrahim et al., 2012). Tissue samples did not exhibit histological signs of neoplasia, cancer or inflammation. BPH is present in ca. 80% of patients with prostate cancer (Alcaraz et al., 2009; Orsted and Bojesen, 2013). Samples were taken immediately after prostatectomy and subsequent macroscopical examination by a pathologist. Organ bath studies were performed immediately after sampling, while samples for molecular analyses were shock frozen in liquid nitrogen and stored at −80°C.
Quantitative real-time PCR (RT-PCR)
RNA from frozen prostate tissues was isolated using the RNeasy Mini kit (Qiagen, Hilden, Germany). For isolation, 30 mg of tissue was homogenized using the FastPrep®-24 system with matrix A (MP Biomedicals, Illkirch-Graffenstaden, France). RNA concentrations were measured spectrophotometrically. Reverse transcription to cDNA was performed with 1 μg of isolated RNA using the Reverse Transcription System (Promega, Madison, WI, USA). RT-PCR for Rac1, Rac2, Rac3 and 18SrRNA was performed with a Roche Light Cycler (Roche, Basel, Switzerland) using primers provided by Qiagen (Hilden, Germany) as ready-to-use mixes, based on the RefSeq Accession numbers NM_006908 for Rac1, NM_002872 for Rac2, NM_005052 for Rac3 and X03205.1 for 18SrRNA. PCR reactions were performed in a volume of 25 μL containing 5 μL LightCycler® FastStart DNA MasterPlus SYBR Green I (Roche, Basel, Switzerland), 1 μL template, 1 μL primer and 18 μL water. Denaturation was performed for 10 min at 95°C, and amplification with 45 cycles of 15 s at 95°C followed by 60 s at 60°C. The specificity of primers and amplification was demonstrated by subsequent analysis of melting points, which revealed single peaks for each target. Results are expressed based on the number of cycles (Ct), at which the fluorescence signal exceeded a defined threshold.
Western blot analysis
Frozen prostate tissues were homogenized in a buffer containing 25 mM Tris/HCl, 10 μM PMSF, 1 mM benzamidine and 10 μg·mL−1 leupeptine hemisulfate, using the FastPrep-24 system with matrix A (MP Biomedicals). After centrifugation (20 000× g, 4 min), supernatants were assayed for protein concentration using the Dc-Assay kit (Biorad, Munich, Germany) and boiled for 10 min with SDS sample buffer (Roth, Karlsruhe, Germany). Samples of WPMY-1 cells were prepared as described later. Samples of prostate homogenates (20 μg per lane), WPMY-1 cells (40 μg per lane), or from pull-down assays (20 μL per lane) were subjected to SDS-PAGE, and proteins were blotted on Protran® nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Membranes were blocked with PBS containing 5% milk powder (Roth, Karlsruhe, Germany) overnight, and incubated with rabbit anti-Rac1 (sc-217), rabbit anti-Rac2 (sc-96), rabbit anti-Rac3 (ab124943) (Abcam, Cambridge, UK), rabbit anti-RhoA (sc-179), rabbit anti-p21-activated kinase (PAK) 1 antibody (2602) (Cell Signaling, Ipswich, MA, USA), rabbit anti-phospho-PAK1 (Thr423)/PAK2 (Thr401) (2601) (Cell Signaling), goat anti-myosin light chain (MLC) (MYL9) (sc-34487), rabbit anti-phospho-MYL9 (Thr18/Ser19) (sc-12896-R), mouse anti-α1A-adrenoceptor (sc-100291), rabbit anti-TH (sc-14007), mouse anti-pan-cytokeratin (sc-8018), mouse anti-calponin 1/2/3 (sc-136987), mouse anti-prostate-specific antigen (PSA) (sc-7316), or mouse anti-β-actin antibody (sc-47778) (if not stated otherwise, all compounds were obtained from Santa Cruz Biotechnology, Santa Cruz, CA, USA). Subsequently, membranes were washed with PBS containing 0.1% Tween 20 (PBS-T), incubated with secondary biotinylated goat anti-rabbit, horse anti-mouse, or horse anti-goat IgG (BA-1000, BA-2000, BA-9500) (Vector Laboratories, Burlingame, CA, USA), washed again with PBS-T, incubated with Avidin and biotinylated HRP from the ‘Vectastain ABC kit’ (Vector Laboratories) both diluted 1:200 in PBS, and washed again with PBS-T. Finally, blots were developed with enhanced chemiluminescence (ECL) using ECL Hyperfilm (GE Healthcare, Freiburg, Germany).
Immunofluorescence
Human prostate specimens, embedded in optimal cutting temperature compound, were snap-frozen in liquid nitrogen and kept at −80°C. Sections (8 μm) were cut in a cryostat and collected on microscope slides (Superfrost®, Gerhard Menzel GmbH, Braunschweig, Germany). Sections were post-fixed in methanol at −20°C and blocked in 1% BSA before incubation with primary antibody over night at room temperature. For double labelling, the following primary antibodies were used (all from Santa Cruz Biotechnology): rabbit anti-Rac1 (sc-217), rabbit anti-Rac2 (sc-96), rabbit anti-Rac3 (ab124943) (Abcam), mouse anti-pan-cytokeratin (sc-8018), mouse anti-calponin 1/2/3 (sc-136987). Binding sites were visualized using Cy3- and Cy5-conjugated secondary antibodies (goat anti-mouse, AP124C, Millipore, Billerica, MA, USA; goat anti-rabbit, ab6564, Abcam). Nuclei were counterstained with DAPI (Invitrogen, Camarillo, CA, USA). Immunolabelled sections were analysed using a laser scanning microscope (Leica SP2, Wetzlar, Germany). Fluorescence was recorded with separate detectors. Control stainings without primary antibodies did not yield any signals.
Tension measurements
Prostate strips (6 × 3 × 3 mm) were mounted in 10 mL aerated (95% O2 and 5% CO2) tissue baths (Föhr Medical Instruments, Seeheim, Germany), containing Krebs–Henseleit solution (37°C, pH 7.4). Preparations were stretched to 4.9 mN and left to equilibrate for 45 min. In the initial phase of the equilibration period, spontaneous decreases in tone were usually observed. Therefore, tension was adjusted three times during the equilibration period, until a stable resting tone (4.9 mN) was attained. After the equilibration period, maximum contraction induced by 80 mM KCl was assessed. Subsequently, chambers were washed three times with Krebs–Henseleit solution for a total of 30 min. Cumulative concentration-response curves for noradrenaline or phenylephrine were created after addition of Rac inhibitors, or solvent (ethanol for NSC23766, water for EHT1864). Similarly, frequency-response curves induced by electrical field stimulation (EFS) were created after addition of inhibitors or solvent. EFS simulates action potentials, resulting in the release of neurotransmitters, including noradrenaline. Inhibitors or solvent were applied 30 min before obtaining concentration- or frequency-response curves. For calculation of agonist- or EFS-induced contractions, tensions were expressed as a % of KCl-induced contractions, as this may correct for different ratios of stromal/epithelial content among the different prostate samples.
Cell culture
WPMY-1 cells are an immortalized cell line obtained from non-malignant human prostate stroma. Cells were obtained from American Type Culture Collection (Manassas, VA, USA), and kept in RPMI 1640 (Gibco, Carlsbad, CA, USA) supplemented with 10% FCS and 1% penicillin/streptomycin at 37°C with 5% CO2. Before addition of NSC23766 (100 μM) EHT1864 (100 μM), the medium was changed to an FCS-free medium. At the end of the experiment, cells were placed under a microscope and pictures were taken using the AxioCam (Zeiss, Oberkochen, Germany). For Western blot analysis, cells were lysed using RIPA buffer (Sigma-Aldrich, St. Louis, MO, USA), and removed from flasks after a 15 min incubation on ice. Cell debris were removed by centrifugation (10 000× g, 10 min, 4°C), and different aliquots of supernatants were either subjected to protein determination, or boiled with SDS sample buffer.
Pull-down assays and phosphorylation assessments
Assays were performed using the Rac or Rho pull-down activation assay kit (BK035 or BK036) (Cytoskeleton, Denver, CO, USA), according to the manufacturer's instructions, and by Western blot analysis as described above. For pull-down assays and PAK phosphorylation, WPMY-1 cells were grown in T75 flasks. After 48 h, the medium was changed to FCS-free medium. After 24 h, NSC23766 (final concentration 100 μM), EHT1864 (100 μM), or solvent (DMSO) was added to the serum-starved cells. One hour later, GTPγS was added with a final concentration of 100 μM. After 2 h, cells were stimulated for 3 min with EGF (50 ng mL−1 final concentration). Subsequently, cells were lysed using lysis buffer from the kit, and removed from flasks after 15 min of incubation on ice. Cell debris was removed by centrifugation (10 000× g, 10 min, 4°C), and supernatants were directly subjected to pull-down assays and protein determination. Other aliquots of these samples were not subjected to pull-down assays, but to Western blot analysis for phospho-PAK, total PAK, or (total) Rac1 and RhoA. For assessment of MLC phosphorylation, NSC23766 (100 μM), EHT1864 (100 μM), or solvent (DMSO) was added to serum-starved cells, followed by addition of noradrenaline (30 μM final concentration). Fifteen minutes later, cells were lysed and subjected to Western blot analysis as described earlier. Pull-down assays and phosphorylation analyses were each repeated in a preliminary series of three independent experiments.
Cytotoxicity assay
Cytotoxicity of Rac inhibitors was assessed using the Cell Counting Kit-8 (CCK-8) (Sigma-Aldrich). Cells were grown in 96-well plates (20 000 cells per well) for 24 h, before NSC23766, EHT1864, or solvent were added at the indicated concentrations (25–100 μM). Subsequently, cells were grown for the indicated periods (24, 48, 72 h). Separate controls were performed for each period. At the end of this period, 10 μL of [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt from CCK-8 was added, and absorbance in each well was measured at 450 nm after incubation for 2 h at 37°C. Each setting was repeated in a preliminary series of three independent experiments.
Phalloidin staining
For fluorescence staining with phalloidin, cells were grown on Lab-Tek Chamber slides (Thermo Fisher, Waltham, MA, USA). Staining was performed using 100 μM FITC-labelled phalloidin (Sigma-Aldrich, Munich, Germany), according to the manufacturer's instruction. Labelled cells were analysed using a laser scanning microscope (Leica SP2, Wetzlar, Germany). Each setting was repeated in a preliminary series of three independent experiments.
Cell proliferation assay
WPMY-1 cells were plated at a density of 50 000 per well in a 16-well chambered coverslip (Thermo Scientific, Waltham, MA, USA). After 24 h, cells were treated with NSC23766 (100 μM) or EHT1864 (25 μM) in FCS-free medium. After a further 24, 48 or 72 h, the medium was changed to a 10 mM 5-ethynyl-2′-deoxyuridine (EdU) solution in FCS-free medium containing Rac inhibitors. After 20 h, cells were fixed with 3.7% formaldehyde. EdU incorporation was determined using the ‘EdU-Click 555’ cell proliferation assay (Baseclick, Tutzing, Germany) according to the manufacturer's instructions. In this assay, incorporation of EdU into DNA is assessed by detection with fluorescing 5-carboxytetramethylrhodamine (5-TAMRA). Counterstaining of all nuclei was performed with DAPI. Cells were analysed by flourescence microscopy (excitation: 546 nm; emission: 479 nm). Each setting was repeated in a preliminary series of three independent experiments.
Drugs and nomenclature
NSC23766 and EHT1864 are structurally unrelated inhibitors of Rac GTPases (Gao et al., 2004; Akbar et al., 2006; Shutes et al., 2007). Stock solutions (10 mM) were prepared with ethanol (NSC23766) or water (EHT1864) for organ bath experiments, and kept at −20°C until use. For cell culture experiments, NSC23766 and EHT1864 were dissolved in DMSO, so that one DMSO control was used for both inhibitors. Phenylephrine ((R)-3-[-1-hydroxy-2-(methylamino)ethyl]phenol) is a selective agonist for α1-adrenoceptors (Michel and Vrydag, 2006). Aqueous stock solutions of phenylephrine and noradrenaline (10 mM) were freshly prepared before each experiment. NSC23766 and EHT1864 were obtained from Tocris (Bristol, UK), phenylephrine and noradrenaline were obtained from Sigma (Munich, Germany).
Statistical analysis
Data are presented as means ± SEM with the indicated number (n) of experiments. Student's two-tailed t-test was used for paired or unpaired observations. P-values <0.05 were considered statistically significant.
Results
Expression of Rac1–3 in prostate tissue
Expression of mRNAs for Rac1, Rac2 and Rac3 was detected in prostate samples from all the patients investigated (n = 7) (Figure 1A). Average Ct was 24.6 ± 1.97 for Rac1, 25.3 ± 0.98 for Rac2, and 25.1 ± 0.47 for Rac3, while the housekeeping gene 18SrRNA was detectable with an average Ct of 14.9 ± 0.26.
Figure 1.
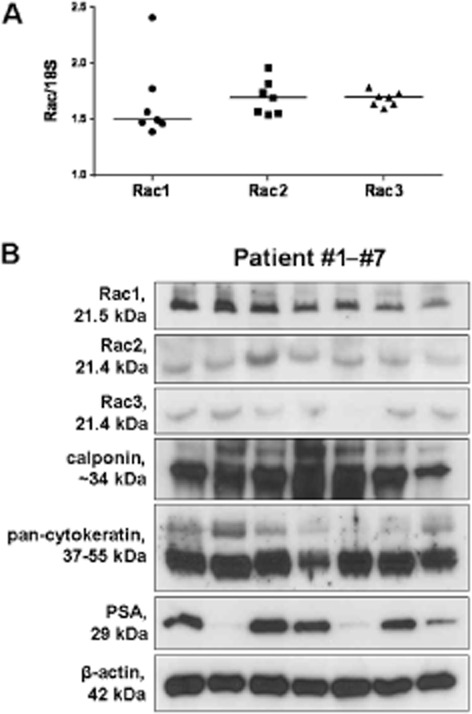
RT-PCR and Western blot analysis of human prostate tissues. (A) Detection of mRNA of Rac isoforms 1–3 by RT-PCR in periurethral prostate tissues from n = 7 patients. Expression of Rac is referred to as expression of 18SrRNA. Shown are values for each sample (ratios of Ct values), and the median for each target. Note: the higher the value, the lower the expression of the target. (B) Periurethral prostate tissues from n = 7 patients were subjected to Western blotting using antibodies for Rac isoforms 1–3, calponin (smooth muscle marker), pan-cytokeratin (marker for epithelium/glands), PSA (marker for BPH), and β-actin (housekeeping, loading control).
Western blot analysis of human prostate tissues revealed bands with the expected sizes of Rac1 (21.5 kDa), Rac2 (21.4 kDa) and Rac3 (21.4 kDa) (Figure 1B). Although their intensity varied slightly, these bands were obtained with each prostate sample (Figure 1B). Similarly, bands for the smooth muscle marker, calponin, and the epithelial marker, pan-cytokeratin, were obtained with slightly varying intensity, but from all samples (Figure 1B). The content of PSA varied greatly, while the intensity of β-actin bands was similar between all samples (Figure 1B).
After fluorescence staining of prostate sections with an anti-Rac1 antibody, immunoreactivity was mostly observed in the stroma, and to lower degree in the glands (Figure 2). Rac1 staining colocalized with immunoreactivity for calponin, which was completely confined to the stroma (Figure 2). In contrast, no colocalization was observed with pan-cytokeratin, which was completely confined to glands (Figure 2). Staining with an anti-Rac2 antibody revealed a similar pattern, with high immunoreactivity and calponin colocalization in the stroma, but weak immunoreactivity and a lack of pan-cytokeratin colocalization in the glands (Figure 2). In contrast, immunoreactivity after staining with an anti-Rac3 antibody was strongest in glandular epithelial cells, where it colocalized with pan-cytokeratin, while it was weaker, but still colocalizing with calponin in the stroma (Figure 2).
Figure 2.
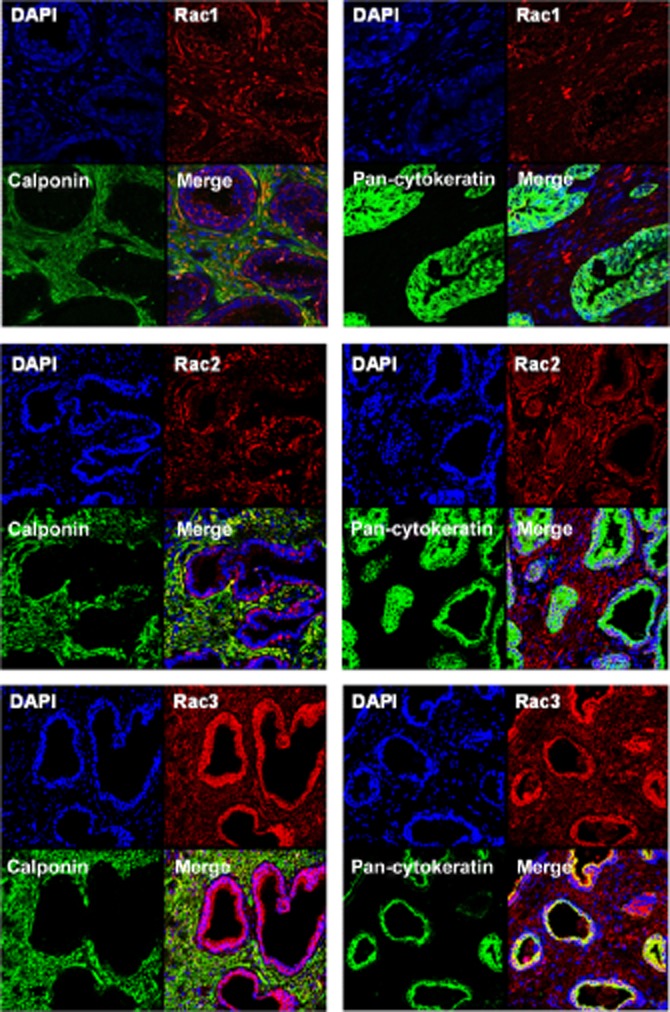
Fluorescence staining of human prostate tissue. Tissues were double-labelled using isoform-specific antibodies for Rac isoforms 1–3, and calponin (left panels) or pan-cytokeratin (right panels). Yellow colour indicates colocalization of immunoreactivities. Shown are representative stainings from a series of tissues from n = 6 patients, with similar results.
Effects of Rac inhibitors on contraction of prostate tissue
In the organ bath, noradrenaline and the α1-adrenoceptor agonist, phenylephrine, induced concentration-dependent contractions of human prostate strips (Figure 3). These were significantly reduced by application of NSC23766 (100 μM) (Figure 3). EFS induced frequency-dependent contractions, which were also significantly reduced by NSC23766 (Figure 3). Likewise, application of EHT1864 (100 μM) caused significant inhibitions of noradrenaline-, phenylephrine- and EFS-induced contractions of prostate strips (Figure 3).
Figure 3.
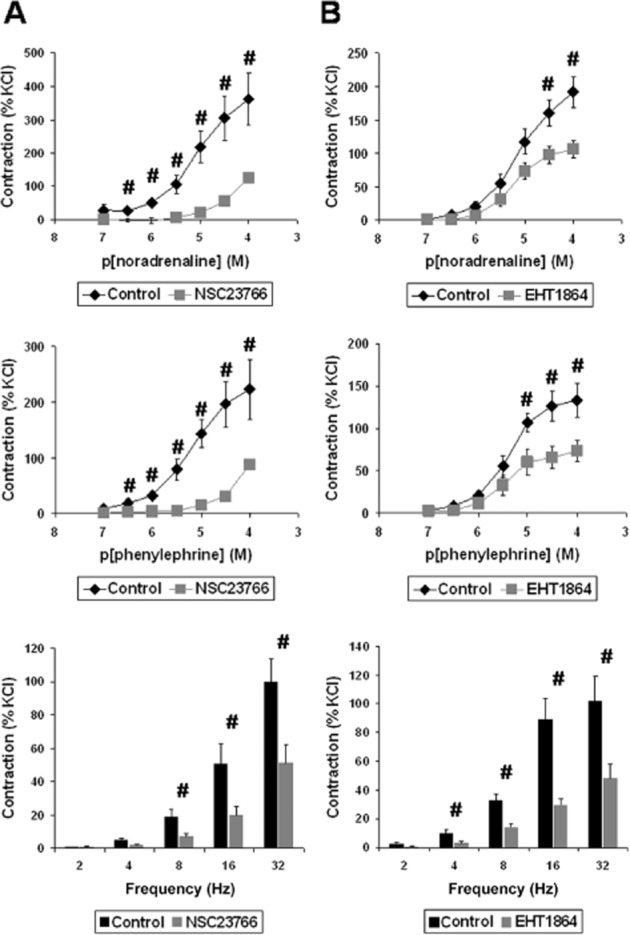
Inhibition of prostate contraction by the Rac inhibitors, NSC23766 (A) and EHT1864 (B). In an organ bath, human prostate strips were exposed for 30 min to NSC23766 (100 μM) or solvent (ethanol) as a control for NSC23766, or for 30 min to EHT1864 (100 μM) or solvent (water) as a control for EHT1864. Subsequently, concentration–response curves for noradrenaline or the α1-adrenoceptor agonist phenylephrine were constructed, as well as EFS-induced frequency–response curves. Data are means (±SEM) from experiments with prostate tissues from n = 5 (noradrenaline/NSC23766), n = 9 (phenylephrine/NSC23766), n = 8 (EFS/NSC23766), n = 10 (noradrenaline/EHT1864), n = 7 (phenylephrine/EHT1864), or n = 6 (EFS/EHT1864) patients (#P < 0.05).
Characterization of WPMY-1 cells
Western blot analysis of WPMY-1 cells revealed intense bands for calponin and α1A-adrenoceptors, which are important features of prostate smooth muscle cells (preliminary series of three independent experiments) (Figure 4A). In contrast, bands for the epithelial marker, pan-cytokeratin, and for the marker for catecholaminergic nerves, TH were virtually missing (Figure 4A). Bands for all three Rac isoforms, for PAK1, and for β-actin were detectable in all samples (Figure 4A).
Figure 4.
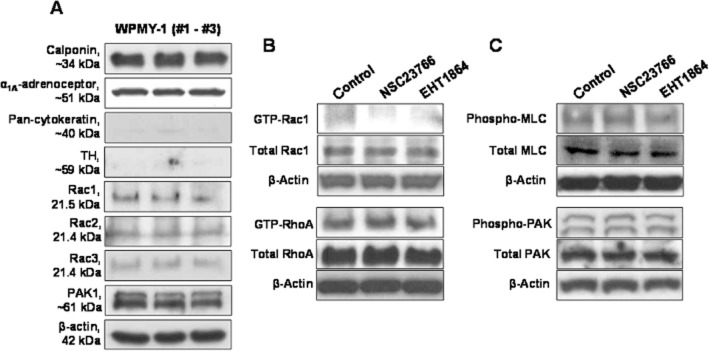
WPMY-1 cells: characterization (A), and effects of Rac inhibitors on Rac1 and RhoA activity (B) and on MLC and PAK phosphorylation (C). (A) Western blot analysis of three independent samples was performed using antibodies for different markers and α1A-adrenoceptors, for Rac isoforms 1–3, for PAK1, and β-actin. (B) Cells were treated with NSC23766 (100 μM, 1 h), EHT1864 (100 μM, 1h), or solvent (DMSO, 1 h), and subsequently subjected to pull-down assays for assessment of Rac1 or RhoA activity. (C) Cells were treated with NSC23766 (100 μM, 1 h), EHT1864 (100 μM, 1h), or solvent (DMSO, 1 h), and subsequently subjected to Western blot analysis using phospho-specific and non-phospho-specific MLC and PAK antibodies. Shown are representative blots from three independent experiments, with similar results.
Effects of Rac inhibitors on Rac and RhoA activities
Activities of Rac and RhoA were examined by pull-down assays in a preliminary series of three independent experiments for each GTPase. Active Rac1 and RhoA were detectable by pull-down assays in WPMY-1 cells (Figure 4B). Incubation with NSC23766 (100 μM) or EHT1864 (25 μM) for 1 h reduced the content of active, GTP-loaded Rac in WPMY-1 cells, while the total content of Rac1 was similar in inhibitor- and solvent-treated cells (Figure 4B). In contrast, incubation of WPMY-1 cells with NSC23766 (100 μM) or EHT1864 (100 μM) for 1 h did not affect the content of active, GTP-loaded RhoA or of total RhoA (Figure 4B).
Effects of Rac inhibitors on MLC and PAK phosphorylation
Western blot analyses using phospho- and non-phospho-specific antibodies suggested that incubation with NSC23766 (100 μM) or EHT1864 (100 μM) for 1 h neither affected the content of phospho-MLC or total MLC, nor of phospho-PAK or total PAK (preliminary series of three independent experiments) (Figure 4C).
Assessment of cytotoxicity of Rac inhibitors in WPMY-1 cells
Survival of WMPY-1 cells after incubation with different concentrations of NSC23766 (50, 100 μM) or EHT1864 (25, 100 μM) for different periods (24, 48, 72 h) was assessed using a CCK assay, by preliminary series of three independent experiments for each setting. Twenty-four hours after incubation of cells with 50 or 100 μM NSC23766, or with 25 μM EHT1864, survival ranged between 64 and 81% (Figure 5). Forty-eight or 72 h after incubation of cells with NSC23766 or EHT1864, both inhibitors dose-dependently reduced survival, with a maximum survival of 52% (NSC23766 50 μM, 48 h), and a minimum survival of 1% (EHT1864 100 μM, 48 and 72 h) (Figure 5).
Figure 5.
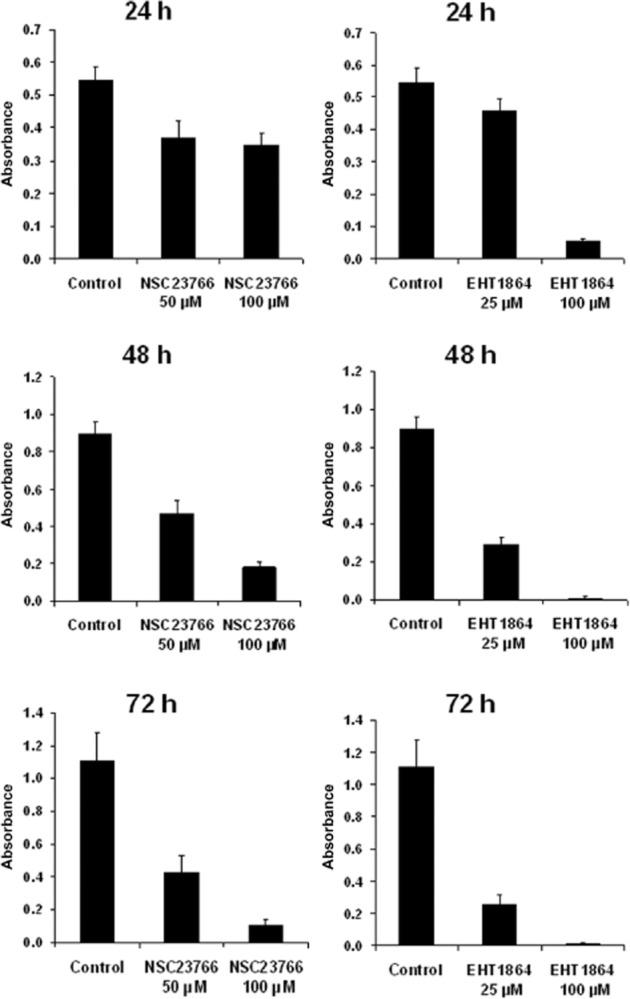
Assessment of cytotoxicity of Rac inhibitors in WPMY-1 cells. Cells were treated with different concentrations of NSC23766 (50, 100 μM) or EHT1864 (25, 100 μM), or solvent (DMSO) for different periods (24, 48 or 72 h), and subjected to the CCK assay for assessment of survival. Absorbance was measured at 450 nm; lower values indicate lower survival. Shown are means ± SEM from three independent experiments for each setting.
Effects of Rac inhibitors on the organization of actin filaments in WPMY1-1 cells
The organization of actin filaments was examined by phalloidin staining, which was assessed in a preliminary series of three independent experiments for each setting. In cells without application of inhibitors (controls), actin filaments were arranged in bundles, which determined the shape of the cells (Figure 6A and B). The shape of control cells was characterized by many long and thin protrusions, containing bundles of actin filaments (Figure 6). Treatment of cells with 50 μM NSC23766 for 24 h induced a partial breakdown of this filament organization (Figure 6A). Treatment of cells with 100 μM NSC23766, or with 25 or 100 μM EHT1864 for 24 or 48 h induced a complete breakdown of the cytoskeleton, characterized by a disorganization of the filament arrangement, and of the cellular shape (Figure 6A and B). Thus, on application of inhibitors, protrusions became shorter and thicker, resulting in a rounded cell shape (Figure 6A and B).
Figure 6.
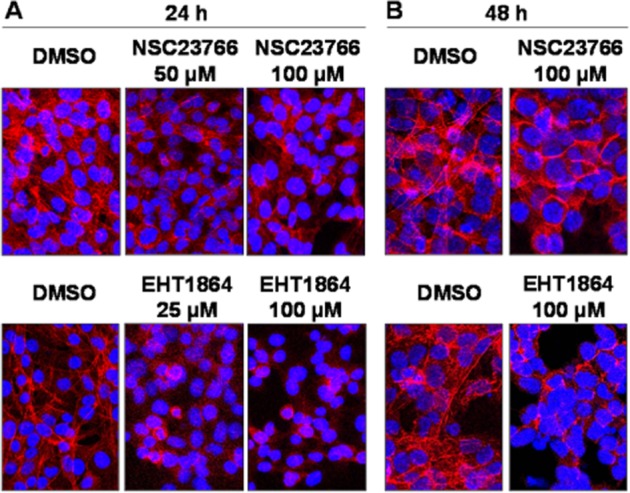
Phalloidin staining of WPMY-1 cells. Cells were treated with different concentrations of NSC23766 (50, 100 μM) or EHT1864 (25, 100 μM), or solvent (DMSO) for different periods (24 or 48 h), and subsequently stained with FITC-labelled phalloidin (red fluorescence) and DAPI (blue fluorescence). Shown are representative stainings from three independent experiments.
Effects of Rac inhibitors on growth of WPMY-1 cells
The proliferation of cells was assessed using an EdU assay (preliminary series of three independent experiments for each setting), and referred to the total number of WPMY-1 cells being visualized by DAPI. After application of NSC23766 (100 μM) for 24, 48 or 72 h, or after application of EHT1864 (25 μM), the number of cells showing proliferation declined compared with controls without inhibitors (Figure 7). This decline may be progressive, as the number of proliferating cells was lower after 72 h of incubation than 24 h after incubation (Figure 7). In contrast, the number of control cells proliferating was similar 24, 48 and 72 h after incubation (Figure 7).
Figure 7.
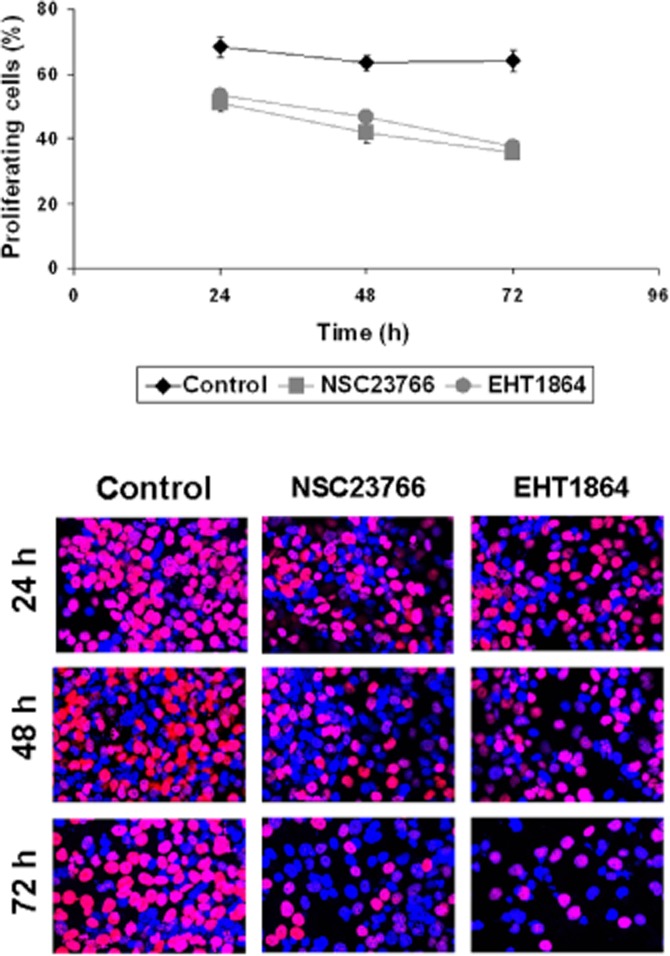
EdU assay in WPMY-1 cells. Cells were treated with NSC23766 (100 μM), EHT1864 (25 μM), or solvent (DMSO) for 24, 48 or 72 h, and subsequently subjected to the EdU assay. Proliferation is indicated by red nuclei (pink in overlay) in EdU staining. The quantified results (means ± SEM), and representative stainings are shown from three independent experiments.
Discussion
In patients with BPH, prostate smooth muscle tone and prostate growth may contribute to LUTS either alone or together (Hennenberg et al., 2014b). Both may impair urethral flow, causing bladder outlet obstruction (BOO). Contraction of prostate smooth muscle, which may be enhanced in BPH, is to a large extent mediated by α1-adrenoceptors (Andersson et al., 1997; Michel and Vrydag, 2006; Hennenberg et al., 2014b). Consequently, smooth muscle relaxation by application of α1-blockers, and inhibition of prostate growth by 5α-reductase inhibitors are the main options for pharmacological therapy of LUTS suggestive of BPH (Hennenberg et al., 2014a). However, their effects are limited or even insufficient (Hennenberg et al., 2014b). New options require an improved understanding of smooth muscle contraction and growth in the prostate, and identification of new targets (Hennenberg et al., 2014a).
Besides Rac, the superfamily of small monomeric GTPases comprises at least 25 members, including RhoA, amongst others (Takai et al., 2001; Wennerberg and Der, 2004). While the role of RhoA in smooth muscle contraction in different organs, including the prostate, has been intensively studied (Christ and Andersson, 2007; Hennenberg et al., 2014b), the role of Rac in the control of smooth muscle tone is just emerging. Recent studies suggested that Rac mediates relaxation of airway smooth muscle, while Rac mediates contraction of vascular smooth muscle (Roscioni et al., 2011; Rahman et al., 2014). This prompted us to investigate the functions of Rac in the human prostate.
RT-PCR demonstrated the expression of all three Rac isoforms (Rac1–3) in prostate tissues. In our Western blot analyses and fluorescence stainings, we observed immunoreactivity for each isoform in each prostate sample. The variation in Rac immunoreactivities was quite low, while the content of PSA (marker for BPH) strongly varied among patients. Thus, Rac1–3 may be constitutively expressed, and does not depend on the degree of BPH, reflecting the importance of Rac as a principle regulator of cellular functions in the prostate. For our study, we used tissues from the periurethral area. Most prostate tumours are located in the peripheral zone (Pradidarcheep et al., 2011; Shaikhibrahim et al., 2012), so our samples were non-malignant. Almost all patients undergoing radical prostatectomy show BPH (Alcaraz et al., 2009; Orsted and Bojesen, 2013), although to varying degrees. This was reflected by the variations in PSA levels in Western blot, as PSA is a suitable marker for BPH (Levitt and Slawin, 2007). Comparisons with non-hyperplastic tissue were not possible, because such tissues were unavailable. Expression of all three Rac isoforms was also observed in WPMY-1 cells. This cell line was derived from the non-malignant stroma of a human prostate, where the major cell type are smooth muscle cells. Our characterization by Western blot analysis demonstrated the expression of calponin and α1A-adrenoceptors in WPMY-1 cells, which are important features of prostate smooth muscle cells. In contrast, the markers for epithelial cells and catecholaminergic neurons, pan-cytokeratin and TH, were virtually missing in these cells. This pattern suggests that WPMY-1 cells are prostate smooth muscle cells, or at least strongly resemble prostate smooth muscle cells.
NSC23766 and EHT1864 are structurally different compounds, but both are specific inhibitors for Rac GTPases. Rac inhibition by NSC23766 is accomplished by inhibition of Rac-specific guanosine nucleotide exchange factors (Gao et al., 2004; Akbar et al., 2006). The selectivity of NSC23766 may be higher for Rac1 than for Rac2 or Rac3, while it does not inhibit RhoA or CDC42 (Gao et al., 2004; Akbar et al., 2006). EHT1864 is specific for Rac GTPases, but quite unselective for different Rac isoforms (Rac1–3) (Shutes et al., 2007). Our preliminary results from pull-down assays suggest the inhibition of Rac1 by both inhibitors in prostate stromal cells, while the activity of RhoA remained unaffected by NSC23766 or EHT1864. The isoform selectivity of both inhibitors is probably too low to allow conclusions about isoform-specific contributions from our organ bath experiments. Of note, inhibition of contractions by noradrenaline, phenylephrine and EFS in our organ bath experiments was quite strong, as it mostly ranged from 40 to 90%. This degree of inhibition is almost comparable with α1-blockers, which ranges from 30 to 80% for human prostate tissues (Michel and Vrydag, 2006).
We assume that this inhibition was at least partially caused by disruption of actin filaments. In WPMY-1 cells, this may occur in response to NSC23766 and EHT1864, as suggested by our phalloidin stainings. Polymerization of actin to filaments, the precise organization of filaments to bundles, and their attachment to membranes are important prerequisites for contraction of smooth muscle cells (Hennenberg et al., 2014b). Disorganization of filaments similar to that in our inhibitor-treated prostate stromal cells may result in loss of contractility. In fact, it has been suggested by numerous studies and for different cell types that Rac1 promotes procontractile actin dynamics, and is crucial for actin-dependent processes such as migration (Machesky and Hall, 1997; Pelletier et al., 2005; Bristow et al., 2009; Tsang et al., 2012). Similar to our WPMY-1 cells, Rac inhibition prevented the formation of cell protrusions in other cells (Tovell et al., 2012). Whether further mechanisms are involved in NSC23766- and EHT1864-mediated inhibition of prostate smooth muscle contraction may be the subject of further studies. Definitively, our findings strongly suggest a critical role for Rac in prostate smooth muscle contraction.
We assume that such a breakdown of cytoskeletal organization by NSC23766 and EHT1864 is responsible for the inhibition of contraction of prostate tissues in the organ bath. In fact, the correct assembly and attachment of the cytoskeleton may be critical for smooth muscle contraction in the prostate, in addition to MLC phosphorylation (Walther et al., 2012; Hennenberg et al., 2014b; Kunit et al., 2014). It has been previously suggested that regulation of smooth muscle tone and of cytoskeletal organization by Rac requires Rac-mediated PAK phosphorylation, crosstalk with RhoA or MLC phosphorylation are also other mechanisms (Vidal et al., 2002; Roscioni et al., 2011; Weidemann et al., 2013). Our preliminary findings from three independent experiments suggest that Rac regulates contraction of prostate smooth muscle independently of MLC phosphorylation, namely by organization of actin filaments. Thereby, the mechanisms of Rac-dependent regulation of prostate smooth muscle contraction may differ from mechanisms in non-prostatic smooth muscle. In fact, the role of Rac for regulation of smooth muscle tone and its underlying mechanisms may vary in different organs (Li et al., 2011; Roscioni et al., 2011; Rahman et al., 2014). While Rac promotes relaxation of airway smooth muscle, it promotes contraction of vascular smooth muscle by mechanisms interfering with MLC phosphorylation (Roscioni et al., 2011; Rahman et al., 2014). Finally, PAK regulation by Rac in the human prostate will probably occur, as suggested by pull-down assays using a recombinant PAK-based Rac interaction partner, but appears to be phosphorylation-independent.
Besides prostate smooth muscle contraction, NSC23766 and EHT1864 may impair growth of WPMY-1 cells, as suggested by our preliminary series of cell culture experiments. Rac-mediated proliferation has been reported for different cell types, including smooth muscle cells from different organs, and malignant prostate cells. Rac promotes proliferation of prostate cancer cells, and growth of prostate tumours (Qin et al., 2009; Goc et al., 2012; Kato et al., 2013; Zins et al., 2013). Rac-mediated proliferation has been reported for airway, bladder and vascular smooth muscle cells (Beier et al., 2008; Simeone-Penney et al., 2008; Toumaniantz et al., 2010; Wu et al., 2012; Jiang et al., 2014). Interestingly, it has been suggested that Rac may be involved in hyperplastic growth and differentiation in the cardiovascular system, including cardiac hypertrophy, neointimal formation, vascular remodelling and angiogenesis (Sawada et al., 2010; Vettel et al., 2012; Elnakish et al., 2013; Staiculescu et al., 2013; Talukder et al., 2013). Therefore, it is tempting to speculate that Rac-mediated proliferation of prostate stromal cells may be involved in BPH.
In the light of these findings, we assume that Rac may be a link, connecting the control of smooth muscle tone with the regulation of growth in the prostate. The coupling of prostate smooth tone with growth has been proposed previously, although the underlying molecular mechanisms still remain unclear (Hennenberg et al., 2014b). Of note, similar connections between the regulation of cytokinesis and contractility may exist in other cell types. In fact, both processes need precise and correct organization of the cytoskeleton and actin filaments (Zhou et al., 2010). The mitotic spindle, which is required for proper cell division, contains cytoskeletal components including actin (Zhou et al., 2010). It has been proposed that the organization of cytokinesis depends on cytoskeletal regulation by Rac (Zhou et al., 2010). This concept may apply to prostate smooth muscle cells, as our findings suggested cytoskeletal disorganization in response to Rac inhibitors in WPMY-1 cells. In addition, the correct assembly and attachment of the cytoskeleton is required for contraction (Hennenberg et al., 2014b). Thus, both processes may underlie the control by common regulators, such as Rac; Rac may regulate contractility and cell cycle by organizing the filaments.
Together, our findings suggest that Rac inhibitors interfere with both components of BOO, namely prostate smooth muscle contraction and prostate growth. In vivo studies are required to find out whether Rac inhibitors inhibit prostate growth, or induce improvements in LUTS suggestive of BPH. In patients with BPH, medical treatment for improvement of LUTS should increase maximum urinary flow rate (Qmax) and reduce symptom scores (‘international prostate symptom score’) (Oelke et al., 2013). An inhibition of prostate growth or reduction in prostate volume takes longer to translate into improvements in LUTS, but may reduce the progression of BPH, the risk of complications (e.g. acute urinary retention), or the need for invasive interventions (Oelke et al., 2013). At present, contraction and growth may be targeted by different compounds (α1-blockers, 5α-reductase inhibitors). A single compound, targeting both components of BOO at once is not yet available for treatment of LUTS. Based on our findings, both may be expected from Rac inhibitors.
Any application in vivo requires that medications are safe. Here, we assessed the cytotoxicity of NSC23766 and EHT1864 in WPMY-1 cells; our preliminary findings suggested cytotoxicity in response to high concentrations and prolonged incubation periods. At lower concentrations and shorter exposure, cytotoxicity appeared to be remarkably low. Although cytotoxicity of both inhibitors may be additionally relevant in non-prostatic cells and may limit clinical use, NSC23766 and EHT1864 have been recently applied repeatedly in animal models, where no severe side effects became apparent (Desire et al., 2005; Kawarazaki et al., 2012; Zhang et al., 2013; Hwaiz et al., 2014; Liao et al., 2014; Yoshida et al., 2014).
Conclusions
Rac may be a link connecting the control of prostate smooth muscle tone with proliferation of smooth muscle cells. Improvements in LUTS suggestive of BPH by Rac inhibitors appears possible, which may be addressed in the future by in vivo studies.
Acknowledgments
We thank Prof Dr E. Noessner and her co-workers for support with immunofluorescence microscopy. We thank Prof Dr Kirchner (Institute of Pathology, Ludwig Maximilian University, Munich) and his co-workers Dr V. Mai and Dr C. Faber for providing the tissue samples from prostates. This study was supported by grants from the Deutsche Forschungsgemeinschaft (grants HE 5825/2-1, and GR 3333/2-1), and from the Friedrich-Baur-Stiftung (73/13).
Glossary
- BOO
bladder outlet obstruction
- BPH
benign prostatic hyperplasia
- CCK-8
Cell Counting Kit-8
- ECL
enhanced chemiluminescence
- EdU
5-ethynyl-2′-deoxyuridine
- EFS
electrical field stimulation
- EHT1864
5-(5-(7-(trifluoromethyl)quinolin-4-ylthio)pentoxyl)-2-(morpholinomethyl)-4H-pyran-4-one dihydrochloride
- LUTS
lower urinary tract symptoms
- MLC
myosin light chain
- NSC23766
N6-[2-[[4-(diethylamino)-1-methylbutyl]amino]-6-methyl-4-pyrimidinyl]-2-methyl-4,6-quinolinediamine trihydrochloride
- PAK
p21-activated kinase
- PBS-T
PBS containing 0.1% Tween 20
- PSA
prostate-specific antigen
- RT-PCR
real-time PCR
- 5-TAMRA
5-carboxytetramethylrhodamine
Authors' contributions
M. H. created the research design. Y. W., T. K., A. C., B. R., A. S., F. S., R. W., C. L., C. G. S., C. G. and M. H. were involved in acquisition of data. Y. W., T. K. and M. H. analysed the data. Y. W. C. L. and M. H. interpreted the data. Y. W. and M. H. drafted the paper. T. K., A. C., B. R., A. S., F. S., R. W., C. L., C. G. S. and C. G. critically revised the paper. All authors approved the submitted and final version.
Conflict of interest
None.
References
- Akbar H, Cancelas J, Williams DA, Zheng J, Zheng Y. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol. 2006;406:554–565. doi: 10.1016/S0076-6879(06)06043-5. [DOI] [PubMed] [Google Scholar]
- Alcaraz A, Hammerer P, Tubaro A, Schroder FH, Castro R. Is there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature review. Eur Urol. 2009;55:864–873. doi: 10.1016/j.eururo.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE, Lepor H, Wyllie MG. Prostatic alpha 1-adrenoceptors and uroselectivity. Prostate. 1997;30:202–215. doi: 10.1002/(sici)1097-0045(19970215)30:3<202::aid-pros9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Beier I, Dusing R, Vetter H, Schmitz U. Epidermal growth factor stimulates Rac1 and p21-activated kinase in vascular smooth muscle cells. Atherosclerosis. 2008;196:92–97. doi: 10.1016/j.atherosclerosis.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Bristow JM, Sellers MH, Majumdar D, Anderson B, Hu L, Webb DJ. The Rho-family GEF Asef2 activates Rac to modulate adhesion and actin dynamics and thereby regulate cell migration. J Cell Sci. 2009;122(Pt 24):4535–4546. doi: 10.1242/jcs.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ GJ, Andersson KE. Rho-kinase and effects of Rho-kinase inhibition on the lower urinary tract. Neurourol Urodyn. 2007;26(6 Suppl):948–954. doi: 10.1002/nau.20475. [DOI] [PubMed] [Google Scholar]
- Desire L, Bourdin J, Loiseau N, Peillon H, Picard V, De Oliveira C, et al. RAC1 inhibition targets amyloid precursor protein processing by gamma-secretase and decreases Abeta production in vitro and in vivo. J Biol Chem. 2005;280:37516–37525. doi: 10.1074/jbc.M507913200. [DOI] [PubMed] [Google Scholar]
- Elnakish MT, Moldovan L, Khan M, Hassanain HH, Janssen PM. Myocardial Rac1 exhibits partial involvement in thyroxin-induced cardiomyocyte hypertrophy and its inhibition is not sufficient to improve cardiac dysfunction or contractile abnormalities in mouse papillary muscles. J Cardiovasc Pharmacol. 2013;61:536–544. doi: 10.1097/FJC.0b013e31828d4b9d. [DOI] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc A, Abdalla M, Al-Azayzih A, Somanath PR. Rac1 activation driven by 14-3-3zeta dimerization promotes prostate cancer cell-matrix interactions, motility and transendothelial migration. PLoS ONE. 2012;7:e40594. doi: 10.1371/journal.pone.0040594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennenberg M, Stief CG, Gratzke C. Pharmacology of the lower urinary tract. Indian J Urol. 2014a;30:181–188. doi: 10.4103/0970-1591.126903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennenberg M, Stief CG, Gratzke C. Prostatic alpha1-adrenoceptors: new concepts of function, regulation, and intracellular signaling. Neurourol Urodyn. 2014b;33:1074–1085. doi: 10.1002/nau.22467. [DOI] [PubMed] [Google Scholar]
- Hwaiz R, Rahman M, Zhang E, Thorlacius H. Rac1 regulates platelet shedding of CD40L in abdominal sepsis. Lab Invest. 2014;94:1054–1063. doi: 10.1038/labinvest.2014.92. [DOI] [PubMed] [Google Scholar]
- Jiang D, Li D, Cao L, Wang L, Zhu S, Xu T, et al. Positive feedback regulation of proliferation in vascular smooth muscle cells stimulated by lipopolysaccharide is mediated through the TLR 4/Rac1/Akt pathway. PLoS ONE. 2014;9:e92398. doi: 10.1371/journal.pone.0092398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Fujita Y, Nakane K, Mizutani K, Terazawa R, Ehara H, et al. CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine. 2013;64:251–257. doi: 10.1016/j.cyto.2013.06.313. [DOI] [PubMed] [Google Scholar]
- Kawarazaki W, Nagase M, Yoshida S, Takeuchi M, Ishizawa K, Ayuzawa N, et al. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol. 2012;23:997–1007. doi: 10.1681/ASN.2011070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruep EJ, Phillips E, Hogue S, Eaddy M. Early symptom improvement and discontinuation of 5-alpha-reductase inhibitor (5ARI) therapy in patients with benign prostatic hyperplasia (BPH) Ann Pharmacother. 2014;48:343–348. doi: 10.1177/1060028013514213. [DOI] [PubMed] [Google Scholar]
- Kunit T, Gratzke C, Schreiber A, Strittmatter F, Waidelich R, Rutz B, et al. Inhibition of smooth muscle force generation by focal adhesion kinase inhibitors in the hyperplastic human prostate. Am J Physiol Renal Physiol. 2014;307:F823–F832. doi: 10.1152/ajprenal.00011.2014. [DOI] [PubMed] [Google Scholar]
- Levitt JM, Slawin KM. Prostate-specific antigen and prostate-specific antigen derivatives as predictors of benign prostatic hyperplasia progression. Curr Urol Rep. 2007;8:269–274. doi: 10.1007/s11934-007-0072-y. [DOI] [PubMed] [Google Scholar]
- Li T, Yang G, Xu J, Zhu Y, Liu L. Regulatory effect of Rac1 on vascular reactivity after hemorrhagic shock in rats. J Cardiovasc Pharmacol. 2011;57:656–665. doi: 10.1097/FJC.0b013e318215e21d. [DOI] [PubMed] [Google Scholar]
- Liao J, Ye Z, Huang G, Xu C, Guo Q, Wang E. Delayed treatment with NSC23766 in streptozotocin-induced diabetic rats ameliorates post-ischemic neuronal apoptosis through suppression of mitochondrial p53 translocation. Neuropharmacology. 2014;85:508–516. doi: 10.1016/j.neuropharm.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147(Suppl. 2):S88–S119. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol MB, Knight TK, Wu J, Barron R, Penson DF. Evaluating use patterns of and adherence to medications for benign prostatic hyperplasia. J Urol. 2009;181:2214–2221. doi: 10.1016/j.juro.2009.01.033. , discussion 2221-2212. [DOI] [PubMed] [Google Scholar]
- Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118–140. doi: 10.1016/j.eururo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Orsted DD, Bojesen SE. The link between benign prostatic hyperplasia and prostate cancer. Nat Rev Urol. 2013;10:49–54. doi: 10.1038/nrurol.2012.192. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier S, Julien C, Popoff MR, Lamarche-Vane N, Meloche S. Cyclic AMP induces morphological changes of vascular smooth muscle cells by inhibiting a Rac-dependent signaling pathway. J Cell Physiol. 2005;204:412–422. doi: 10.1002/jcp.20308. [DOI] [PubMed] [Google Scholar]
- Pradidarcheep W, Wallner C, Dabhoiwala NF, Lamers WH. Anatomy and histology of the lower urinary tract. Handb Exp Pharmacol. 2011;202:117–148. doi: 10.1007/978-3-642-16499-6_7. [DOI] [PubMed] [Google Scholar]
- Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J, et al. Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene. 2009;28:1853–1863. doi: 10.1038/onc.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Davis B, Lovdahl C, Hanumaiah VT, Feil R, Brakebusch C, et al. The small GTPase Rac1 is required for smooth muscle contraction. J Physiol. 2014;592(Pt 5):915–926. doi: 10.1113/jphysiol.2013.262998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscioni SS, Maarsingh H, Elzinga CR, Schuur J, Menzen M, Halayko AJ, et al. Epac as a novel effector of airway smooth muscle relaxation. J Cell Mol Med. 2011;15:1551–1563. doi: 10.1111/j.1582-4934.2010.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada N, Li Y, Liao JK. Novel aspects of the roles of Rac1 GTPase in the cardiovascular system. Curr Opin Pharmacol. 2010;10:116–121. doi: 10.1016/j.coph.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikhibrahim Z, Lindstrot A, Ellinger J, Rogenhofer S, Buettner R, Perner S, et al. The peripheral zone of the prostate is more prone to tumor development than the transitional zone: is the ETS family the key? Mol Med Rep. 2012;5:313–316. doi: 10.3892/mmr.2011.647. [DOI] [PubMed] [Google Scholar]
- Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol Chem. 2007;282:35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
- Simeone-Penney MC, Severgnini M, Rozo L, Takahashi S, Cochran BH, Simon AR. PDGF-induced human airway smooth muscle cell proliferation requires STAT3 and the small GTPase Rac1. Am J Physiol Lung Cell Mol Physiol. 2008;294:L698–L704. doi: 10.1152/ajplung.00529.2007. [DOI] [PubMed] [Google Scholar]
- Staiculescu MC, Galinanes EL, Zhao G, Ulloa U, Jin M, Beig MI, et al. Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc Res. 2013;98:428–436. doi: 10.1093/cvr/cvt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Talukder MA, Elnakish MT, Yang F, Nishijima Y, Alhaj MA, Velayutham M, et al. Cardiomyocyte-specific overexpression of an active form of Rac predisposes the heart to increased myocardial stunning and ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2013;304:H294–H302. doi: 10.1152/ajpheart.00367.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toumaniantz G, Ferland-McCollough D, Cario-Toumaniantz C, Pacaud P, Loirand G. The Rho protein exchange factor Vav3 regulates vascular smooth muscle cell proliferation and migration. Cardiovasc Res. 2010;86:131–140. doi: 10.1093/cvr/cvp387. [DOI] [PubMed] [Google Scholar]
- Tovell VE, Chau CY, Khaw PT, Bailly M. Rac1 inhibition prevents tissue contraction and MMP mediated matrix remodeling in the conjunctiva. Invest Ophthalmol Vis Sci. 2012;53:4682–4691. doi: 10.1167/iovs.11-8577. [DOI] [PubMed] [Google Scholar]
- Tsang SM, Brown L, Gadmor H, Gammon L, Fortune F, Wheeler A, et al. Desmoglein 3 acting as an upstream regulator of Rho GTPases, Rac-1/Cdc42 in the regulation of actin organisation and dynamics. Exp Cell Res. 2012;318:2269–2283. doi: 10.1016/j.yexcr.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettel C, Wittig K, Vogt A, Wuertz CM, El-Armouche A, Lutz S, et al. A novel player in cellular hypertrophy: Giβγ/PI3K-dependent activation of the RacGEF TIAM-1 is required for alpha(1)-adrenoceptor induced hypertrophy in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2012;53:165–175. doi: 10.1016/j.yjmcc.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Vidal C, Geny B, Melle J, Jandrot-Perrus M, Fontenay-Roupie M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood. 2002;100:4462–4469. doi: 10.1182/blood.V100.13.4462. [DOI] [PubMed] [Google Scholar]
- Walther S, Strittmatter F, Roosen A, Heinzer F, Rutz B, Stief CG, et al. Expression and alpha1-adrenoceptor regulation of caldesmon in human prostate smooth muscle. Urology. 2012;79:745 e5–745 e745. doi: 10.1016/j.urology.2011.10.053. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Breyer J, Rehm M, Eckardt KU, Daniel C, Cicha I, et al. HIF-1alpha activation results in actin cytoskeleton reorganization and modulation of Rac-1 signaling in endothelial cells. Cell Commun Signal. 2013;11:1–6. doi: 10.1186/1478-811X-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it) J Cell Sci. 2004;117(Pt 8):1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- Wilt TJ, Howe W, MacDonald R. Terazosin for treating symptomatic benign prostatic obstruction: a systematic review of efficacy and adverse effects. BJU Int. 2002;89:214–225. [PubMed] [Google Scholar]
- Wu T, Chen L, Wei T, Wang Y, Xu F, Wang K. Effect of cyclic hydrodynamic pressure-induced proliferation of human bladder smooth muscle through Ras-related C3 botulinum toxin substrate 1, mitogen-activated protein kinase kinase 1/2 and extracellular regulated protein kinases 1/2. Int J Urol. 2012;19:867–874. doi: 10.1111/j.1442-2042.2012.03043.x. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ishizawa K, Ayuzawa N, Ueda K, Takeuchi M, Kawarazaki W, et al. Local mineralocorticoid receptor activation and the role of Rac1 in obesity-related diabetic kidney disease. Nephron Exp Nephrol. 2014;126:16–24. doi: 10.1159/000358758. [DOI] [PubMed] [Google Scholar]
- Zhang S, Rahman M, Song L, Herwald H, Thorlacius H. Targeting Rac1 signaling inhibits streptococcal M1 protein-induced CXC chemokine formation, neutrophil infiltration and lung injury. PLoS ONE. 2013;8:e71080. doi: 10.1371/journal.pone.0071080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Kee YS, Poirier CC, Jelinek C, Osborne J, Divi S, et al. 14-3-3 coordinates microtubules, Rac, and myosin II to control cell mechanics and cytokinesis. Curr Biol. 2010;20:1881–1889. doi: 10.1016/j.cub.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zins K, Lucas T, Reichl P, Abraham D, Aharinejad S. A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice. PLoS ONE. 2013;8:e74924. doi: 10.1371/journal.pone.0074924. [DOI] [PMC free article] [PubMed] [Google Scholar]


