Abstract
Background and Purpose
Dehydroepiandrosterone (DHEA) is thought to be an anti-glucocorticoid hormone known to be fully functional in young people but deficient in aged humans. Our previous data suggest that DHEA not only counteracts the effect of cortisol on RACK1 expression, a protein required both for the correct functioning of immune cells and for PKC-dependent pathway activation, but also modulates the inhibitory effect of cortisol on LPS-induced cytokine production. The purpose of this study was to investigate the effect of DHEA on the splicing mechanism of the human glucocorticoid receptor (GR).
Experimental Approach
The THP1 monocytic cell line was used as a cellular model. Cytokine production was measured by specific elisa. Western blot and real-time RT-PCR were used, where appropriate, to determine the effect of DHEA on GRs, serine/arginine-rich proteins (SRp), and RACK1 protein and mRNA. Small-interfering RNA was used to down-regulate GRβ.
Key Results
DHEA induced a dose-related up-regulation of GRβ and GRβ knockdown completely prevented DHEA-induced RACK1 expression and modulation of cytokine release. Moreover, we showed that DHEA influenced the expression of some components of the SRps found within the spliceosome, the main regulators of the alternative splicing of the GR gene.
Conclusions and Implications
These data contribute to our understanding of the mechanism of action of DHEA and its effect on the immune system and as an anti-glucocorticoid agent.
Tables of Links
| TARGETS | |
|---|---|
| Nuclear hormone receptorsa | Enzymesb |
| Glucocorticoid receptor (GR) | PKCβ |
| LIGANDS | |
|---|---|
| Bombesin | Insulin-like growth factor (IGF-1) |
| Cortisol | LPS |
| DHEA | Testosterone |
| IL-8 (CXCL8) |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (
Introduction
One of the biggest challenges facing scientists nowadays is the study of age-related changes in human physiology in order to discriminate their negative effects. One relevant example is the metabolic dys-regulation caused by the imbalance between catabolic hormones (thyroid hormones and cortisol), which tend to remain stable with ageing, and anabolic hormones [testosterone, insulin-like growth factor-1 and dehydroepiandrosterone (DHEA)], which typically decrease with age (Maggio et al., 2010). In the past few years, we demonstrated that the imbalance between two of these hormones, cortisol and DHEA, is implicated in the decline in immune functions during ageing. In particular, we observed that cortisol and DHEA have opposite effects on the regulation of PKC activity involved in important immune processes such as cytokine release and lymphocyte proliferation (Corsini et al., 2002; Buoso et al., 2011). During ageing, the PKC-dependent signal transduction machinery is impaired mainly due to reduced expression of the scaffold protein known as receptor for activated C kinase 1 (RACK1) (Corsini et al., 1999; 2005,). RACK1 is a 36 kDa protein and the best-characterized member of the RACK family. RACK1 interacts with several isoforms of PKC, preferentially with PKCβ, to stabilize the active form of PKC and promote the translocation of the protein from the cytosol to the membrane compartments. The translocation is needed for PKC to exert its functions (Mochly-Rosen et al., 1991; 1995,). RACK1 is a highly conserved intracellular adaptor protein originally identified as the anchoring protein for activated PKC (Mochly-Rosen et al., 1991) and subsequently characterized as one of the most important scaffolding proteins in intracellular signalling (McCahill et al., 2002; Adams et al., 2011; Ron et al., 2013; Li and Xie, 2014), supporting a large number of binding partners and has a versatile role in assembling and dismantling complex signalling pathways from the cell membrane to the nucleus in health and disease.
We observed that physiological concentrations of cortisol exert an inhibitory effect on RACK1 expression through the presence of a glucocorticoid receptor (GR) binding consensus sequence [glucocorticoid-responsive element (GRE) sequence] on the promoter region of the human GNB2L1 gene (guanine nucleotide-binding protein β-2-like 1), which codes for RACK1 protein (Del Vecchio et al., 2009). With respect to the presence of the GRE sequence, we have recently shown that synthetic corticosteroids (e.g. prednisone, prednisolone, budesonide, betamethasone and methylprednisolone) are also able to reduce RACK1 expression. Synthetic corticosteroids are widely used for the treatment of many acute and chronic inflammatory conditions and modulate RACK1 expression at the transcriptional level. This supports the notion that RACK1 protein plays an important role in the immune system and in the anti-inflammatory effects of glucocorticoids (Corsini et al., 2014).
Conversely, treatment with DHEA could restore the levels of RACK1 protein both in vivo and in vitro (Corsini et al., 2002). The mechanism by which DHEA effectively contrasts the inhibitory effects of cortisol is not yet well established. In the context of the immune system, our hypothesis is that the anti-glucocorticoid effect of DHEA on RACK1 expression and the related PKC signalling could be due to the influence of DHEA on the expression of the β isoform of the human GR.
The GR belongs to a superfamily of nuclear receptors that function as ligand-dependent transcription factors and is responsible for the physiological and pharmacological actions of glucocorticoids (Evans, 1988). The human GR gene (NR3C1) is located on chromosome 5q31–32 and is composed of nine exons (Hollenberg et al., 1985; Theriault et al., 1989). Alternative splicing in exon 9 generates two homologous isoforms: GRα and GRβ (Oakley et al., 1996; 1999,). These two isoforms share the first 727 amino acids at their N-terminus but differ in their carboxyl terminus. As a result of these differences, GRβ lacks the ligand-binding domain and is unable to activate glucocorticoid-responsive genes (Oakley et al., 1996). However, there is evidence to suggest that GRβ itself affects gene expression and it may act as a dominant-negative regulator of GRα transcriptional activity (Bamberger et al., 1995; Oakley et al., 1999; Kino et al., 2009).
The mRNA splicing is mediated by the spliceosome. The spliceosome is partly composed of serine/arginine-rich proteins (SRp) that are a family of phylogenetically conserved and structurally related proteins. These proteins play many significant roles in the regulation of RNA processing and gene expression, ranging from transcription to translation (Zhong et al., 2009). Since its discovery, the SR protein family has expanded to include 12 proteins involved in pre-mRNA splicing (Ankö, 2014). Most importantly, SR proteins can positively or negatively regulate spliceosome function depending on their precise location, composition and post-translational modification (Sanford et al., 2003). Interestingly, data are emerging that establish an association between high levels of the GRβ isoform and altered SR protein expression. This imbalance is further implicated in a number of human diseases found to occur in different tissues (Xu et al., 2003; Zhu et al., 2007; Jain and Wordinger, 2012).
The purpose of this work was to investigate whether DHEA influences the alternative splicing of GR pre-mRNA by modulating specific SR proteins. Moreover, we are interested in studying whether variations in the GRβ/GRα ratio are involved in the anti-glucocorticoid effect of DHEA on RACK1 protein expression and function.
Methods
Cells
THP-1 cells (Istituto Zooprofilattico di Brescia, Brescia, Italy) were cultured in RPMI 1640 containing 10% heated-inactivated FBS, 2 mM L-glutamine, 0.1 mg·mL−1 streptomycin, 100 IU·mL−1 penicillin and 50 μM 2-mercaptoethanol. In all experiments involving treatment with glucocorticoids, cells were cultured in RPMI 1640 medium without phenol red supplemented with 10% charcoal stripped FBS. Details of times and concentrations are given in figures and table legends.
Cytokine production
Cytokine release (IL-8) was assessed in cell-free supernatants by a specific sandwich elisa, commercially available (R&D System, Minneapolis, MN, USA). Cell-free supernatants obtained by centrifugation at 1700× g for 5 min were stored at −80°C until measurements were performed. Results are expressed as % of control.
Real-time RT-PCR
For mRNA extraction, 2 × 106 cells were used. Total RNA was extracted using RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. QuantiTect reversion transcription kit and QuantiTect Syber Green PCR kit (Qiagen) were used for cDNA synthesis and gene expression analysis, respectively, following the manufacturer's specifications. GRα and GRβ primers were custom designed and synthesized by Primm (Milan, Italy) and the nucleotide primer sequences are found in Table 1. GAPDH primers were provided by Qiagen. GAPDH was used as endogenous reference and the quantification of the transcripts was performed using the ΔΔCT method (Livaka and Schmittgen, 2001).
Table 1.
The nucleotide primer sequences of GRα and GRβ primers
| Gene name | Forward primer sequence (5′ to 3′) | Reverse primer sequence (5′ to 3′) |
|---|---|---|
| NR3C1 (GRα) | GAAGGAAACTCCAGCCAGAACTG | GATGATTTCAGCTAACATCTCG |
| NR3C1 (GRβ) | CTTCCAGAACCATGGTAGCC | TACGAAACTCCACCCAAAGG |
Western blot analysis
The expression of RACK1, GRα and GRβ in cell homogenates was assessed by Western blot analysis. Briefly, cells (2 × 106) were treated with corticosteroids and DMSO as vehicle control for different times. Cells were then collected, washed once with PBS, centrifuged and lysed in 100 μL of homogenization buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100 and protease inhibitor mix). The protein content was measured using the Bradford method (Bradford, 1976). Western blotting samples were prepared mixing the cell lysate with sample buffer (125 mM Tris-HCl pH6, 8.4% SDS, 20% glycerol, 6% β-mercaptoethanol, 0.1% bromophenol) and denaturing at 95°C for 5 min. Equivalent amounts of extracted protein (20–30 μg) were electrophoresed into a 10% SDS-PAGE under reducing conditions. The proteins were then transferred to a PVDF membrane (Amersham, Little Chalfont, UK). The proteins were visualized using primary antibodies for RACK1 (1:1000), GRα (1:1000), GRβ (1:1000), SRp family (1:1000), SRp30c (1:1000) and β-actin (1:2000) and developed using enhanced chemiluminescence reagent (Pierce, Thermo Scientific, Rockford, IL, USA).
Transcription factors (elisa-based transcription factor binding assay)
Nuclear extracts were prepared as described by Schreiber et al. (1989). Briefly, after treatment, 4 × 106 cells were resuspended in 0.4 mL of hypotonic lysis buffer (10 mM HEPES, pH 7.8, 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 0.1 mM PMSF). Cells were incubated on ice for 15 min and then 25 μL of a 10% Nonidet P-40 solution was added. The cells were mixed for 15 s and then centrifuged for 30 s at 17 000× g Pelleted nuclei were suspended in 50 μL of buffer C (50 mM HEPES, pH 7.8, 50 mM KCl, 300 mM NaCl, 10% glycerol, 1 mM DTT, 0.1 mM EDTA, 0.1 mM PMSF) mixed for 20 min and centrifuged for 5 min at 17 000× g. The supernatants represent the nuclear extracts. Protein concentrations were measured using a commercial kit (Bio-Rad, Richmond, CA, USA). Nuclear extracts were used to assess GR translocation using a colorimetric elisa-based format (McCue and Freed, 2004). Briefly, 96-well plates were coated with oligonucleotides containing the consensus binding sequence (5′-AGAACACCCTCCGGAAGCACA-3′) for the GRE present in the GNB2L1 promoter region (Del Vecchio et al., 2009). The DNA oligonucleotides were diluted in Reacti-Bind DNA coating solution (Pierce) at 2 μg·mL−1 and plates were coated overnight at room temperature. Unbound DNA was washed with washing buffer, 100 μL per well. Nuclear extracts (5 μg per well) were incubated in the wells to allow the transcription factor to bind its sequence. Unbound proteins were washed away, and primary antibodies specific for GRα and GRβ were added (Abcam). Following incubation with HRP-conjugated secondary antibodies and substrate, colour intensity was measured using a standard μL plate reader. Results are expressed as % of control or as GRβ/GRα ratio.
Statistical analysis
All experiments were repeated at least three times, with representative results shown. Data are expressed as mean ± SD. Statistical analysis was performed using InStat software version 3.0a (GraphPad Software, La Jolla, CA, USA). Statistical differences were determined using anova followed by a multiple comparison test as indicated in the legends. Effects were designated as significant if P < 0.05.
Chemicals
DHEA, cortisol and bombesin were obtained from Sigma-Aldrich (St. Louis, MO, USA). They were dissolved in DMSO at concentration of 50 mM and frozen (−20°C) in stock aliquots. Stock aliquots were diluted at a final concentration in culture media at the time of use (final concentration of DMSO in culture medium <0.1%). LPS from Escherichia coli serotype 0127:B8 was obtained from Sigma (St. Louis, MO, USA). Cell culture media and all supplements were from Sigma. Mouse anti-human RACK1 monoclonal antibody (610177) and mouse monoclonal anti-β-actin (612656) were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Rabbit polyclonal anti-GRα (ab3580) and mouse monoclonal anti-GRβ (ab130227) were acquired from Abcam (Cambridge, UK). Mouse monoclonal anti-SRp family (MABE126) was bought from Merck Millipore (Darmstadt, Germany) and rabbit polyclonal anti-SRp30c (sc-134036) were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Host specific peroxidase conjugated IgG secondary antibody (31460) was purchased from Thermo Scientific. Electrophoresis reagents came from Bio-Rad. All reagents were purchased at the highest purity available.
Results and discussion
Effect of DHEA on GRα and GRβ expression
Evidence present in literature suggests that DHEA can counteract several adverse effects of glucocorticoid in different tissues (Kalimi et al., 1994; Ferrari et al., 2001). Its mechanism of action and physiological implications are still not fully understood. In the context of the immune system, we hypothesized that DHEA exerts its anti-glucocorticoid activity by modulating GRβ expression and antagonizing the function of GRα. Firstly, we investigated the effects of DHEA on the expression of GRα and GRβ both at the protein and at mRNA levels. THP-1 cells (106 mL−1) were treated for 16–18 h with increasing concentrations of DHEA (1, 10, 100 and 1000 nM) or with DMSO as vehicle control (<0.1% final concentration). GRα and GRβ protein expressions were assessed by Western blot using a specific antibody for each isoform and normalized to β-actin. Results are expressed as % of control. As shown in Figure 1A, DHEA induced an up-regulation of GRβ protein levels in a dose-dependent manner, with a significant effect at 10 and 100 nM. These concentrations of DHEA did not affect the levels of GRα expression (Figure 1B). Also, these two concentrations represent, respectively, physiological and pharmacological concentrations of DHEA (Baulieu et al., 2000; Oka et al., 2007).
Figure 1.
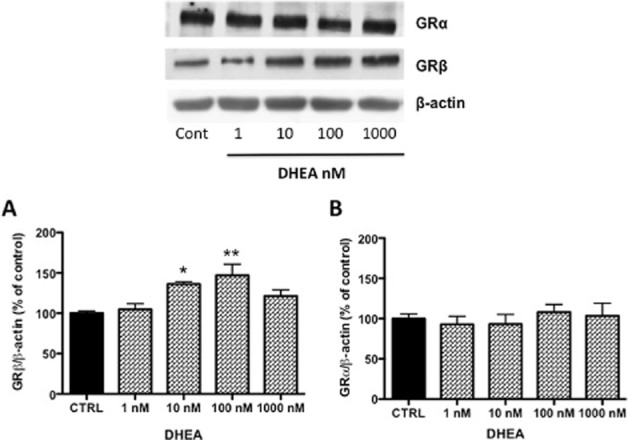
DHEA effect on GR protein expression. THP-1 cells were incubated for 16 h in a medium containing vehicle or DHEA at different concentrations as indicated. Cellular extracts were examined by Western blot using a specific antibodies against the GRβ (A) and GRα (B) isoforms. β-Actin expression was detected to normalize the samples. The image is a representative Western blot result. Each value in the graph represents the mean ± SD of three independent experiments. Statistical analysis was performed with Dunnett's multiple comparison test with *P < 0.05, **P < 0.01 versus CTRL (control; vehicle-treated cells).
GR mRNA expression was evaluated by real-time PCR. GAPDH was used as housekeeping gene and results are expressed as 2−ΔΔCt (Figure 2). We performed two experiments at two different times (4 and 18 h after DHEA treatment) in order to establish if the effect of DHEA on GR gene expression is time-dependent. In particular, we focused our attention mainly on the variation of the GRβ/GRα ratio. As shown in Figure 2C, DHEA induced an increase in the GRβ/GRα ratio mainly due to an up-regulation of GRβ mRNA (Figure 2A). This up-regulation was not time-dependent but dose-related, confirming our Western blot results.
Figure 2.
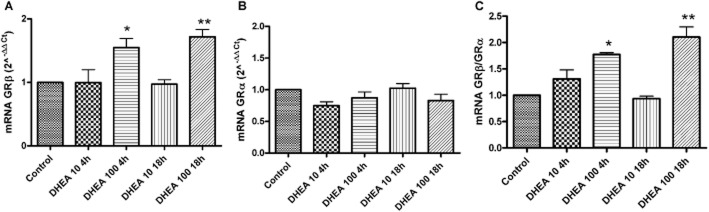
DHEA modulated GRα and GRβ mRNA expression. THP-1 cells were treated for 4 or 18 h with DHEA at two different concentrations (10 and 100 nM). The effect on mRNA levels was evaluated by real-time PCR in three independent experiments using GAPDH as an endogenous reference. Each value represents the mean ± SD of GRβ (A) and GRα (B) mRNA levels and GRβ/GRα ratio (C). Statistical analysis was performed with Dunnett's multiple comparison test with *P < 0.05; **P < 0.01 versus control (vehicle-treated cells).
Effect of GRβ silencing on RACK1 expression and cytokine release
Previous evidence suggested that DHEA could modulate RACK1 protein levels via a transcriptional mechanism that does not involve a direct interaction with the promoter region of the RACK1 gene (Corsini et al., 2002). Furthermore, prolonged DHEA exposure counteracts the effects of cortisol by restoring RACK1 levels and cytokine production without pharmacologically competing with cortisol for binding to the GR (Müller et al., 2004; Buoso et al., 2011). To evaluate whether the effects of DHEA effects are related to its modulation of GRβ as postulated before, we silenced the expression of GRβ with a specific small-interfering (si) RNA. Forty-eight hours after transfection of this siRNA, GRβ protein levels were significantly suppressed compared with controls as shown in Figure 3A. This effect was GRβ specific as GRβ siRNA did not alter GRα expression (Figure 3B). GRβ knock-down completely blocked the effect of DHEA on RACK1 protein expression; the levels of RACK1 protein were markedly reduced in the absence of GRβ in comparison with samples without siRNA (Figure 3C). Moreover, in the presence of this GRβ siRNA, DHEA failed to stimulate LPS-induced cytokine release (Figure 3D). These results suggest that the effect of DHEA is driven by a modulation of GRβ expression and activity. Interestingly, the silencing of GRβ still influenced RACK1 expression and IL-8 release in control group, reinforcing the idea that GRβ is a dominant-negative regulator of GRα activity.
Figure 3.
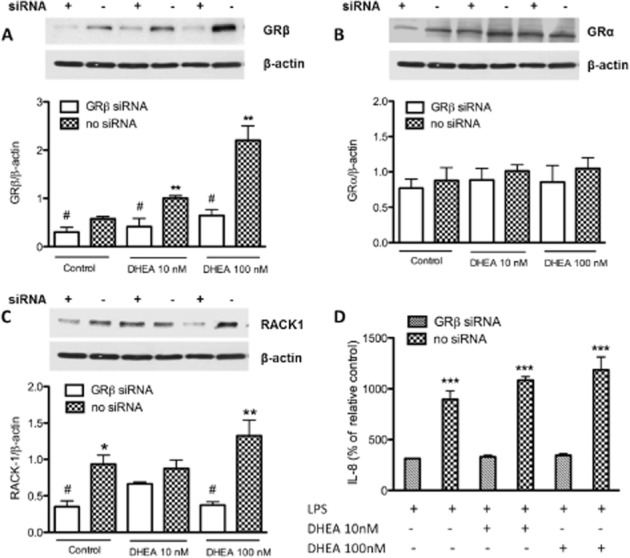
The silencing of GRβ affected the effect of DHEA on protein expression and cytokine production. THP-1 cells were transfected for 48 h with siRNA for the GRβ isoform. Transfection was carried out using Lipofectamine 2000 as detailed in Methods. After 48 h cells were treated for 16 h with DHEA (10 and 100 nM) or DMSO as a vehicle control. Cellular extracts were examined by Western blot using specific antibodies, respectively, against GRβ isoform (A), GRα (B) and RACK1 protein (C). β-Actin expression was detected to normalize the samples. The images are representative of Western blot results. After DHEA treatment, cells were exposed to LPS (0.1 μg·mL−1) and IL-8 release was evaluated 24 h later in culture supernatants by elisa (D). Each value in the graph represents the mean ± SD of three independent experiments. Statistical analysis was performed with Tukey's multiple comparison test with **P < 0. 01, ***P < 0. 001 versus silenced control (GRβ siRNA) and with #P < 0. 05 versus no silenced control.
DHEA counteracted the cortisol-induced binding of GRα to the RACK1 promoter region.
The ability of GRβ to antagonize the function of GRα is related to various mechanisms, including competition for GRE binding, squelching for transcriptional co-regulators and formation of an inactive GRβ/GRα heterodimer (Oakley et al., 1996; Charmandari et al., 2005). To examine whether GRβ exerts its dominant-negative activity by modulating the isoform ratio of GR binding to GREs, we performed an elisa-based transcription factor binding assay (see Methods for details). The assay was performed using oligonucleotides containing the GRE sequence present in the promoter region of GNB2L1 (Del Vecchio et al., 2009). THP-1 cells were treated for different times (30, 60 and 120 min) with physiological concentrations of cortisol (0.1 and 0.5 μM) to establish the optimum treatment time (data not shown). Nuclear extracts of THP-1 were used to assess GRβ and GRα binding to the GRE domain (Figure 4A and B). As shown in Figure 4C the GRβ/GRα ratio was constant over time in the presence of cortisol alone and was comparable with the control. If cells were treated for 16–18 h with DHEA (10 and 100 nM) and then stimulated with cortisol (0.1 and 0.5 μM), a significant increase in the GRβ/GRα binding ratio was observed with respect to cells treated with cortisol alone (Figure 4C).
Figure 4.
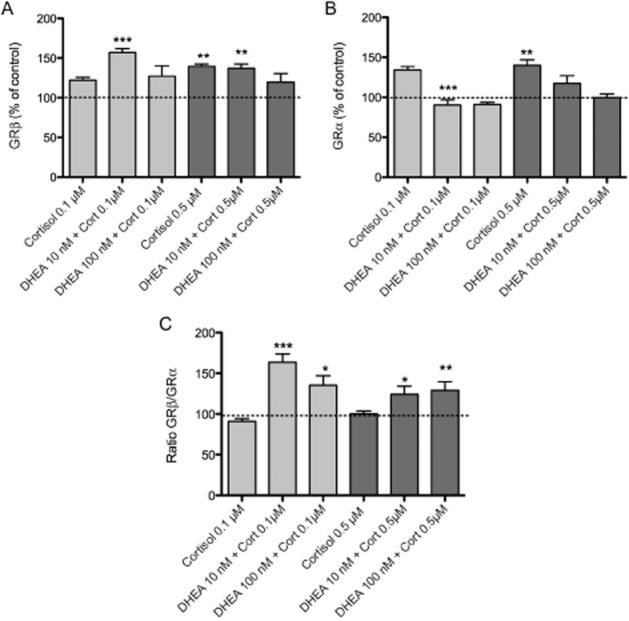
DHEA reduced cortisol-induced GRα binding to the GRE present in the GNB2L1 promoter. THP-1 cells were treated for 18 h with DHEA (10 and 100 nM) or DMSO as vehicle control. Cortisol (0.1 and 0.5 μM) was then added for 1 h. Nuclear extracts of THP-1 were used to evaluate the binding of GRβ (A) and GRα (B) to the GRE domain. GRβ/GRα binding ratio has been calculated on these results (C). Each value represents the mean ± SD of three to five independent experiments. Statistical analysis was performed with Turkey's multiple comparison test with *P < 0.05, **P < 0.01, ***P < 0.001 versus control.
Characterization of SR family profile in THP-1 cells
As suggested before, DHEA might alter the effects of cortisol by modulating the expression of splicing factors. Among the splicing factors, the arginine/serine-rich proteins are the most prevalent involved in the alternative splicing of the GR. They are, therefore, the most likely candidates for gene expression regulation by DHEA. To confirm this hypothesis, we verified the pattern of expression of the SR protein family in our cellular model. The first preliminary set of experiments was conducted on THP-1 cell lysates and with an antibody that recognizes the main nuclear SRp proteins and several other proteins related to the SR family. The results showed that many of these proteins are expressed in THP-1 cells and recognized by the antibody (Figure 5). A series of bands appeared to be regulated by bombesin or DHEA treatment and are indicated in the figure by an asterisk. Bombesin treatment was included because it has been demonstrated that this 14 amino acid neuropeptide can interfere with the splicing of GR by modulating the expression of SR proteins. In particular, bombesin causes an up-regulation of the SRp30c isoform (Zhu et al., 2007). In Figure 5, among the proteins modulated by bombesin or DHEA treatment, we have specifically indicated the one that, according to its molecular weight, most likely represents SRp30. The 30 kDa band could contain one or more of SRp30a, SRp30b or SRp30c. Of these three candidates, SRp30c has been predominantly found to direct alternative splicing of GR gene in neutrophils (Xu et al., 2003). By using a specific antibody for the single SRp30c isoform, we showed that both bombesin and DHEA can induce, in a concentration-dependent manner, an increase in SRp30c protein levels (Figure 6). Our observations, although at present limited to one of the various components of the splicing factors, support others in the scientific literature and strongly suggest a specific role for the SRp30c subspecies in increasing the alternative splicing of GRβ mRNA and protein expression (Xu et al., 2003; Zhu et al., 2007) following DHEA treatment in THP-1 cells.
Figure 5.
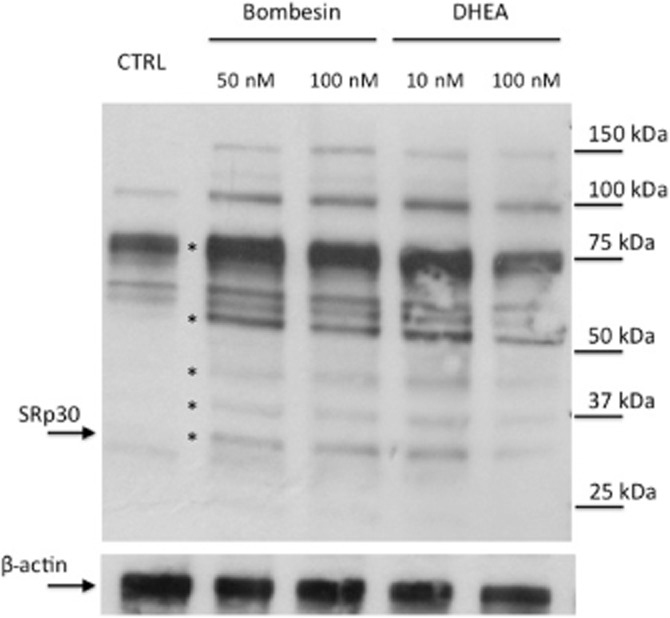
SRp family profile in THP-1 cells and effect of DHEA and bombesin. THP-1 cells were treated for 18 h with DHEA (10 and 100 nM) or bombesin (50 and 100 nM). Cellular extracts were examined by Western blot using an antibody reactive towards the whole family of SR proteins. The antibody recognizes the major isoforms of the family and a number of other related proteins belonging to the SR family. β-Actin was detected to normalize the samples. Bands that appear to be regulated by bombesin or DHEA treatment are indicated by asterisks with SRp30 indicated by an arrow on the left. Molecular weights of protein markers are indicated on the right side.
Figure 6.

Effect of on SRp30c protein levels. THP-1 cells were treated for 18 h with DHEA (10 and 100 nM) or bombesin (50 and 100 nM). Cellular extracts were examined by Western blot using a specific antibody against SRp30c. β-Actin was detected to normalize the samples. The inset shows a representative Western blot and in the graph the results are quantified and each value bar represents the mean ± SD of three independent experiments. Statistical analysis was performed with Dunnett's multiple comparison test with *P < 0. 05 and **P < 0.01 versus CTRL (vehicle-treated cells).
Conclusions
Over the past few years, we have focused our attention on the effects that the age-related imbalance between DHEA and cortisol has on the functions of the immune system (Corsini et al., 2002; 2005; 2014,,; Del Vecchio et al., 2009; Buoso et al., 2011). The complexity of hormonal activity makes it difficult to identify a unique clinical strategy to counteract this kind of change. Thus, it became very important to understand the molecular mechanism involved in the consequences of an imbalance in the hormonal equilibrium. DHEA is an adrenal hormone synthesized both from cholesterol and de novo in the brain, leading to its additional designation as a neuroactive neurosteroid (Baulieu and Robel, 1998; Compagnone and Mellon, 2000). It is described as the ‘elixir of youth’ for its anti-ageing properties, anti-obesity, anti-diabetic and immune-promoting effects (Ebeling and Koivisto, 1994; Loria et al., 1996; Williams, 2000). However, its mechanism of action and physiological implications are not fully understood.
Here we demonstrated how the ability of GRβ to act as a dominant-negative inhibitor of GRα function could underlie the anti-glucocorticoid effect of DHEA on RACK1 protein expression. We showed that DHEA treatment induced a dose-dependent up-regulation of GRβ without affecting GRα expression and that GRβ knockdown completely prevented the effects of DHEA on RACK1 and cytokine release. The molecular basis for the selective increase in GRβ still remains unclear. In this context, it is certainly important to consider the alternative splicing machinery represented by the spliceosome and by its components, such as the snRNPs and the SRps (Wahl et al., 2009). SR proteins are not part of the core spliceosomal complex, but they have essential functions during spliceosome assembly and activation and they are emerging as one of the master regulators of gene expression. Several studies have established a close correlation between high levels of the GRβ isoform and an altered expression of SR proteins (Xu et al., 2003; Zhu et al., 2007; Zhong et al., 2009; Yan et al., 2010). Different levels and/or activities of these SR proteins and their tissue specificity are still largely an open question. In the present study, we analysed the pattern of expression of the SR protein family in our cellular model and focused our attention on SRp30c. We demonstrated that DHEA treatment induced an overexpression of SRp30c, suggesting that modulation of the spliceosome proteins involved in GR mRNA splicing can be a significant mechanism for regulation of glucocorticoid activities. Further studies are needed to elucidate the splicing mechanism and the full ensemble of proteins specifically involved.
In conclusion, a greater understanding of the role of the GR isoforms and their heterogeneity in the cellular response is a relevant issue that should be examined more in depth. This topic is also of particular interest when considering the importance of GR in the pathophysiology of human disorders and its utility for therapeutic manipulation with drugs.
Acknowledgments
This project was supported by Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN2009). We are grateful to Caterina Selva for reading and editing the manuscript.
Glossary
- DHEA
dehydroepiandrosterone
- GNB2L1
guanine nucleotide-binding protein β-2-like 1
- GR
glucocorticoid receptor
- GRE
glucocorticoid-responsive element
- SR protein
serine/arginine-rich protein
- RACK1
receptor for activated C kinase 1
Author contributions
M. R. and E. C. designed the study; A. P., B. M., J. O., M. M. S., A. D. and V. G. conducted the experiments; A. P. analysed the data; A. P., M. R. and E. C. wrote and edited the manuscript.
Conflict of interest
All authors declare not having any financial nor personal association with any of the individuals or organizations that could have inappropriately influenced the submitted work.
References
- Adams DR, Ron D, Kiely PA. RACK1, A multifaceted scaffolding protein: structure and function. Cell Commun Signal. 2011;6:9–22. doi: 10.1186/1478-811X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear hormone receptors. Br J Pharmacol. 2013a;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankö ML. Regulation of gene expression programmes by serine–arginine rich splicing factors. Semin Cell Dev Biol. 2014;32:11–21. doi: 10.1016/j.semcdb.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Natl Acad Sci U S A. 1998;95:4089–4091. doi: 10.1073/pnas.95.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge study to asociobiomedical issue. Proc Natl Acad Sci U S A. 2000;97:4279–4284. doi: 10.1073/pnas.97.8.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buoso E, Lanni C, Molteni E, Rousset F, Corsini E, Racchi M. Opposing effects of cortisol and dehydroepiandrosterone on the expression of the receptor for Activated C Kinase 1: implications in immunosenescence. Exp Gerontol. 2011;46:877–883. doi: 10.1016/j.exger.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Chrousos GP, Ichijo T, Bhattacharyya N, Vottero A, Souvatzoglou E, et al. The human glucocorticoid receptor (hGR) beta isoform suppresses the transcriptional activity of hGRalpha by interfering with formation of active coactivator complexes. Mol Endocrinol. 2005;19:52–64. doi: 10.1210/me.2004-0112. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Corsini E, Battaini F, Lucchi L, Marinovich M, Racchi M, Govoni S, et al. A defective protein kinase C anchoring system underlying age-associated impairment in TNF-α production in rat macrophages. J Immunol. 1999;163:3468–3473. [PubMed] [Google Scholar]
- Corsini E, Lucchi L, Meroni M, Racchi M, Solerte B, Fioravanti M, et al. In vivo dehydroepiandrosterone restores age-associated defects in protein kinase C signal transduction pathway and related functional responses. J Immunol. 2002;168:1753–1758. doi: 10.4049/jimmunol.168.4.1753. [DOI] [PubMed] [Google Scholar]
- Corsini E, Racchi M, Sinforiani E, Lucchi L, Viviani B, Rovati GE, et al. Age-related decline in RACK-1 expression in human leukocytes is correlated to plasma levels of dehydroepiandrosterone. J Leukoc Biol. 2005;77:247–256. doi: 10.1189/jlb.0504268. [DOI] [PubMed] [Google Scholar]
- Corsini E, Pinto A, Galbiati V, Viviani B, Galli C, Marinovich M, et al. Corticosteroids modulate the expression of the PKC-anchoring protein RACK-1 and cytokine release in THP-1 cells. Pharmacol Res. 2014;81:10–16. doi: 10.1016/j.phrs.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Del Vecchio I, Zuccotti A, Pisano F, Canneva F, Lenzken SC, Rousset F, et al. Functional mapping of the promoter region of the GNB2L1 human gene coding for RACK1 scaffold protein. Gene. 2009;430:17–29. doi: 10.1016/j.gene.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Ebeling P, Koivisto VA. Physiological importance of dehydroepiandrosterone. Lancet. 1994;343:1479–1481. doi: 10.1016/s0140-6736(94)92587-9. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari E, Cravello L, Muzzoni B, Casarotti D, Paltro M, Solerte SB, et al. Age-related changes of the hypothalamic-pituitary-adrenal axis: pathophysiological correlates. Eur J Endocrinol. 2001;144:319–329. doi: 10.1530/eje.0.1440319. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Wordinger RJ. Spliceosome protein (SRp) regulation of glucocorticoid receptor isoforms and glucocorticoid response in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012;53:857–866. doi: 10.1167/iovs.11-8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Antiglucocorticoid effects of dehydroepiandrosterone (DHEA) Mol Cell Biochem. 1994;131:99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- Kino T, Manoli I, Kelkar S, Wang Y, Su YA, Chrousos GP. Glucocorticoid receptor (GR) beta has intrinsic, GRalpha-independent transcriptional activity. Biochem Biophys Res Commun. 2009;381:671–675. doi: 10.1016/j.bbrc.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Xie D. RACK1, a versatile hub in cancer. Oncogene. 2014 doi: 10.1038/onc.2014.127. . doi: 10.1038/onc.2014.127. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Livaka KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loria RM, Padgett DA, Huynh PN. Regulation of the immune response by dehydroepiandrosterone and its metabolites. J Endocrinol. 1996;150(Suppl):S209–S220. [PubMed] [Google Scholar]
- Maggio M, Cattabiani C, Lauretani F, Ferrucci L, Luci M, Valenti G, et al. The concept of multiple hormonal dysregulation. Acta Biomed. 2010;81:19–29. [PMC free article] [PubMed] [Google Scholar]
- McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- McCue J, Freed B. Measuring lymphocyte transcription factors activity by ELISA. Curr Protoc Toxicol. 2004:5. doi: 10.1002/0471140856.tx1805s22. Unit 18: [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci U S A. 1991;88:3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D, Smith BL, Chen CH, Disatnik MH, Ron D. Interaction of protein kinase C with RACK1, a receptor for activated C-kinase: a role in beta protein kinase C mediated signal transduction. Biochem Soc Trans. 1995;23:596–600. doi: 10.1042/bst0230596. [DOI] [PubMed] [Google Scholar]
- Müller C, Cluzeaud F, Pinon GM, Rafestin-Oblin M, Morfin R. Dehydroepiandrosterone and its 7-hydroxylated metabolites do not interfere with the transactivation and cellular trafficking of the glucocorticoid receptor. J Steroid Biochem Mol Biol. 2004;92:469–476. doi: 10.1016/j.jsbmb.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties and putative function. J Biol Chem. 1996;271:9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor beta isoform. Specificity and mechanisms of action. J Biol Chem. 1999;274:27857–27866. doi: 10.1074/jbc.274.39.27857. [DOI] [PubMed] [Google Scholar]
- Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, et al. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res. 2007;74:377–387. doi: 10.1016/j.cardiores.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–D1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Adams DR, Baillie GS, Long A, O'Connor R, Kiely PA. RACK1 to the future – a historical perspective. Cell Commun Signal. 2013;11:53. doi: 10.1186/1478-811X-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JR, Longman D, Caceres JF. Multiple roles of the SR protein family in splicing regulation. Progress in molecular subcell. Biology. 2003;31:33–58. doi: 10.1007/978-3-662-09728-1_2. [DOI] [PubMed] [Google Scholar]
- Schreiber EP, Matthias MM, Schaffner W. Rapid detection of octameric binding proteins with mini-extract prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419–6422. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault A, Boyd E, Harrap SB, Hollenber SM, Connor JM. Regional chromosomal assignment of the human glucocorticoid receptor gene to 5q31. Hum Genet. 1989;83:289–291. doi: 10.1007/BF00285175. [DOI] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Williams JR. The effects of dehydroepiandrosterone on carcinogenesis, obesity, the immune system, and aging. Lipids. 2000;35:325–331. doi: 10.1007/s11745-000-0529-7. [DOI] [PubMed] [Google Scholar]
- Xu Q, Leung DY, Kisich KO. Serine-arginine-rich protein p30 directs alternative splicing of glucocorticoid receptor pre-mRNA to glucocorticoid receptor beta in neutrophils. J Biol Chem. 2003;278:27112–27118. doi: 10.1074/jbc.M300824200. [DOI] [PubMed] [Google Scholar]
- Yan XB, Tang CH, Huang Y, Fang H, Yu ZQ, Wu LM, et al. Alternative splicing in exon 9 of glucocorticoid receptor pre-mRNA is regulated by SRp40. Mol Biol Rep. 2010;37:1427–1433. doi: 10.1007/s11033-009-9529-z. [DOI] [PubMed] [Google Scholar]
- Zhong XY, Wang P, Han J, Rosenfeld MG, Fu XD. SR proteins in vertical integration of gene expression from transcription to RNA processing to translation. Mol Cell. 2009;35:1–10. doi: 10.1016/j.molcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Gong JY, Goodman OB. Bombesin attenuates pre-mRNA splicing of glucocorticoid receptor by regulating the expression of serine-arginine protein p30c (SRp30c) in prostate cancer cells. Biochim Biophys Acta. 2007;1773:1087–1094. doi: 10.1016/j.bbamcr.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


