Abstract
Background
Statin therapy in women without cardiovascular disease (CVD) is controversial given insufficient evidence for benefit. We analyzed sex-specific outcomes in JUPITER and synthesized the results with prior trials.
Methods and Results
JUPITER participants were 6,801 women ≥60 years and 11,001 men ≥50 years with high-sensitivity C-reactive protein ≥2mg/L and LDL cholesterol <130mg/dL randomized to rosuvastatin versus placebo. Meta-analysis studies were randomized placebo-controlled statin trials with predominantly or exclusively primary prevention women and sex-specific outcomes (20,147 women, >276 CVD events, mean age 63–69 years). Absolute CVD rates (per 100 person-years) in JUPITER women for rosuvastatin and placebo (0.57 and 1.04, respectively) were lower than men (0.88 and 1.54, respectively), with similar relative risk reduction in women (HR 0.54, 95% CI 0.37–0.80, P=0.002) and men (HR 0.58, 95% CI 0.45–0.73, P<0.001). In women, there was significant reduction in revascularization/unstable angina and non-significant reductions in other components of the primary endpoint. Meta-analysis of 13,154 women (240 CVD events, 216 total deaths) from exclusively primary prevention trials found significant reduction in primary CVD events with statins by a third (RR 0.63, 95% CI 0.49–0.82, P<0.001, P for heterogeneity 0.56) with a smaller non-significant effect on total mortality (RR 0.78, 95% CI 0.53–1.15, P=0.21, P for heterogeneity 0.20). Similar results were obtained for trials that were predominantly but not exclusively primary prevention.
Conclusions
JUPITER demonstrated that in primary prevention rosuvastatin reduced CVD events in women with similar relative risk reduction to that in men, a finding supported by meta-analysis of primary prevention statin trials.
Keywords: meta-analysis, prevention, lipids, women
The use of HMG-CoA reductase inhibitors (statins) in patients with manifest cardiovascular disease (CVD) is established, with similar benefit in women and men for relative risk reduction of approximately 20–30%, but statin use for primary prevention of CVD is controversial, particularly for women.1–3 Specifically, for primary prevention in men, prior meta-analyses showed significant reductions in coronary events with statins versus placebo, while in women the reduction was smaller and non-significant.4, 5 Prior to JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin), statins have not been found to reduce total or coronary mortality in women, men, or combined, for primary prevention.4–6 Moreover, the recent MEGA study (Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese) enrolled more women than men in large numbers, but the reduction in events was significant only in men.7
JUPITER was a multi-center randomized trial designed to assess the benefits and risks of statin therapy in apparently healthy individuals selected on the basis of elevated high-sensitivity C-reactive protein (hsCRP), a marker of higher cardiovascular risk, without a concomitant elevation in LDL cholesterol.8 We conducted a pre-specified sex-specific analysis in JUPITER comparing the efficacy and safety of rosuvastatin therapy in women versus men. We then performed an updated meta-analysis of statin therapy for primary prevention of CVD events and total mortality in women, with nearly twice the number of women included in the prior meta-analysis by Walsh and Pignone in 2004.4
METHODS
JUPITER study design and protocol
The JUPITER design has been previously published.8, 9 A total of 17,802 asymptomatic individuals (women ≥ 60 years, men ≥ 50 years) without prior history of coronary disease, stroke or diabetes and who had LDL cholesterol<130 mg/dL and hsCRP≥2.0 mg/L were randomized. Drugs that were exclusion criteria included current use of hormone therapy, previous or current use of lipid-lowering therapy, or immunosuppressant agents. Family history of premature coronary disease was defined as coronary disease in a first-degree relative, male <55 or female <65 years old. Metabolic syndrome and Framingham risk categories were defined according to ATP III guidelines.10
Follow-up included laboratory evaluations and structural interviews assessing outcomes and potential adverse events. Laboratory measurements for fasting lipids, high-sensitivity C-reactive protein, hepatic and renal function, fasting blood glucose levels, and hemoglobin A1c (HbA1c) were performed in a central laboratory. Estimated glomerular filtration rate was calculated from serum creatinine using the Modification of Diet in Renal Disease equation.11
Outcomes and adverse events
The trial was expected to last approximately 5 years, but on March 30, 2008, the Independent Data and Safety Monitoring Board terminated the trial early for benefit (after 1.9 year median follow-up, maximal follow-up 5 years). The primary endpoint of the JUPITER trial was a composite endpoint, defined as the combined end point of myocardial infarction, stroke, hospitalization for unstable angina, arterial revascularization, or cardiovascular death. Myocardial infarction, stroke, and cardiovascular death were confirmed according to standard criteria. Unstable angina was ischemic chest pain at rest or with minimal exertion occurring within the preceeding 48 hours, requiring hospitalization and presence of objective evidence of ischemia. Arterial revascularization was coronary artery bypass graft surgery, bypass grafting of any peripheral artery or carotid or the performance of at least 1 percutaneous transluminal intervention.
All reported primary endpoints that occurred through March 30, 2008 were adjudicated by an independent endpoint committee blinded to randomized treatment assignment. Adverse events were monitored and reported in a blinded manner until the date of the closeout visit and discontinuation of therapy.
Meta-analysis methods
We performed a review of peer-reviewed publications that were identified through searches of MEDLINE through July 2009. Bibliographies from these references were also reviewed. Criteria used for study selection included randomized placebo-controlled statin trials that included predominantly or exclusively primary prevention individuals with mean follow-up of >1 year and with sex-specific clinical outcomes on CVD or total mortality. Other criteria used were English language and validity based on the venue of publication. For this analysis, we included primary prevention trials that included women with diabetes. Three trials (Air Force/Texas Coronary Atherosclerosis Prevention Study [AFCAPS/TexCAPS], MEGA, and JUPITER) were considered exclusively primary prevention, whereas 2 other trials (The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack [ALLHAT-LLT] and The Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm [ASCOT-LLA]) were considered predominantly primary prevention because they included approximately 15% prior CVD. Two other trials, the Heart Protection Study (HPS) and Pravastatin in eldery individuals at risk of vascular disease (PROSPER) included a substantial number of women without known CVD but did not report sex-specific outcomes for these women. We repeated the meta-analysis including these 2 trials.
Statistical analysis
Statistical analyses were performed using SAS software, version 8.2, and STATA software, version 10.1. For the JUPITER trial, all analyses were performed separately in women and men as pre-specified in the trial design. Wilcoxon 2 sample tests for continuous variables and Chi-square tests for categorical variables were used to compare the distribution of risk factors and levels of biomarkers across the two randomized treatment arms in sex-specific analyses.
Statistical tests for outcomes were performed according to intention-to-treat. The exposure time was calculated as the time from randomization to occurrence of the primary endpoint or the date of death, last study visit, withdrawal, loss to follow-up, or March 30, 2008, whichever came first. Absolute event rates were calculated per 100-person years. Cox proportional hazard models were used to calculated the hazard ratios (HRs) and 95% confidence intervals (CIs). The NNT was computed based on absolute 4-year values projected over an average 5-year period.12 P-values for heterogeneity of treatment effect for outcomes between women and men were obtained from likelihood ratio tests in Cox models that included treatment assignment, sex, and the interaction term. All P-values were two-tailed.
For the meta-analysis, 5 studies met the selection criteria.8, 13–16 2 × 2 tables were constructed for the statin and placebo arms for CVD, the primary endpoint of the meta-analysis. These predominantly included myocardial infarction, angina/revascularization, stroke, and CVD death, with some of the trials including peripheral vascular events8, 13 and one trial including ischemic congestive heart failure.13 Two trials included only myocardial infarction and coronary death.14, 15 Four trials reported the number of cases in the statin arm and placebo arm except for ALL-HAT,14 and hence the total number of events could only be reported as greater than the number of events in these 5 trials (i.e >276 CVD events). Four of the 5 trials included sex-specific data on total mortality,8, 14, 16, 17 and separate 2 × 2 tables were constructed for total mortality. Summary relative risks (RRs) were obtained from random-effects regression models. Tests for heterogeneity between studies and an estimator of between studies variance were also obtained, with plots for the individual and pooled estimates.
Drs. Mora, Glynn, and Ridker had full access to the data and takes responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
JUPITER baseline characteristics
A total of 6,801 women were randomized (3,426 allocated to rosuvastatin; 3,375 to placebo), compared with 11,001 men (5,475 to rosuvastatin; 5,526 to placebo). Screen failure rates were 80.5% and 79.6% in women and men, respectively. Table 1 shows baseline characteristics of participants. Women were older than men (median age 68 and 63 years, respectively), which reflected the sex-specific age entry criterion (women ≥60 years, men ≥50 years), and were less likely to be white. Women were heavier and had more prevalent hypertension and metabolic syndrome, but they smoked much less than men. Similar rates of aspirin use were noted in women and men.
Table 1.
Baseline characteristics of women and men in the JUPITER trial
| Women N=6,801 |
Men N=11,001 |
P Value | |
|---|---|---|---|
| Age, y | 68.0 (65.0–73.0) | 63.0 (58.0–70.0) | <0.001 |
| Current smoking | 7.6 | 21.0 | <0.001 |
| Hypertension | 62.7 | 54.1 | <0.001 |
| Race/ethnic group, % | |||
| White | 61.7 | 77.1 | |
| Black | 15.9 | 10.4 | <0.001 |
| Hispanic | 18.9 | 8.8 | |
| Other or unknown | 3.5 | 3.6 | |
| Body mass index, kg/m2 | 29.2 (25.7–33.2) | 27.9 (25.1–31.2) | <0.001 |
| Metabolic syndrome | 46.7 | 38.7 | <0.001 |
| Family history of premature CHD | 12.2 | 11.1 | 0.02 |
| Aspirin use | 16.4 | 16.8 | 0.51 |
| hsCRP, mg/L | 4.6 (3.1–7.7) | 4.1 (2.7–6.8) | <0.001 |
| LDL cholesterol, mg/dL | 109 (96–120) | 108 (93–119) | <0.001 |
| HDL cholesterol, mg/dL | 54 (46–66) | 45 (38–55) | <0.001 |
| Triglycerides, mg/dL | 118 (88–163) | 118 (84–174 | 0.64 |
| Total cholesterol, mg/dL | 192 (175–205) | 182 (165–195) | <0.001 |
| Glucose, mg/dL | 93 (87–101) | 95 (88–102) | <0.001 |
| HbA1c, % | 5.8 (5.5–6.0) | 5.6 (5.4–5.9) | <0.001 |
| Glomerular filtration rate, ml/min/1.73 m2 of BSA | 66.8 (58.6–77.0) | 77.4 (66.8–88.4) | <0.001 |
hsCRP: high-sensitivity C-reactive protein; HbA1c: hemoglobin A1c; BSA: body surface area.
Values are median (25th–75th percentile) or %. P values were obtained from Wilcoxon 2 sample tests.
Women had higher concentrations of hsCRP (4.6 vs 4.1 mg/L, respectively, P<0.001), even though they were not on hormone therapy. Women and men had similar baseline LDL cholesterol (109 and 108 mg/dL, respectively), although the small LDL cholesterol difference was statistically significant. HDL cholesterol was higher in women by ~10 mg/dL, and hence total cholesterol was also higher by ~10 mg/dL. Small yet statistically significant differences were also noted for fasting glucose (lower in women) and HbA1c (lower in men). Women had lower glomerular filtration rates (~10 ml.min/1.73 m2 of body surface area) compared with men.
JUPITER changes in lipids and hsCRP
At 12-month follow-up, the median change in hsCRP concentrations in women was −1.8 mg/L (39%) with rosuvastatin and −0.6 mg/L (13%) with placebo, which were similar to the reductions in men (Table 2). In women, the median change in LDL cholesterol was −51 mg/dL (47%) on rosuvastatin and +4 mg/dL (4%) on placebo, with similar changes in men. HDL cholesterol increased by 3 mg/dL and 1 mg/dL in women on rosuvastatin and placebo, respectively. Triglycerides were reduced by 17 mg/dL (14%) in women on rosuvastatin with no change on placebo, and similarly for men. Total cholesterol was higher in women at baseline and remained higher during follow-up, but the reduction was similar in women and men.
Table 2.
Lipid and other laboratory measurements during follow-up in JUPITER
| Baseline | 12 months | Change from baseline to 12 months | P* | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Women | Rosuvastatin | Placebo | Rosuvastatin | Placebo | Rosuvastatin | Placebo | |
| hsCRP, mg/L | 4.6 (3.1, 7.7) | 4.6 (3.1, 7.6) | 2.5 (1.4, 4.8) | 4.1 (2.4, 7.0) | −1.8 (−3.6, −0.6) | −0.6 (−2.2, 0.8) | <0.0001 |
| LDL cholesterol, mg/dL | 109 (97, 120) | 109 (95, 120) | 55 (44, 73) | 112 (97, 127) | −51 (−65, −27) | 4 (−7, 17) | <0.0001 |
| HDL cholesterol, mg/dL | 54 (46, 66) | 54 (46, 66) | 58 (48, 70) | 55 (46, 67) | 3 (−2, 8) | 1 (−4, 6) | <0.0001 |
| Triglycerides, mg/dL | 118 (88, 162) | 117 (88, 164) | 98 (76, 131) | 118 (89, 161) | −17 (−44, 3) | −1 (−23, 21) | <0.0001 |
| Total cholesterol, mg/dL | 192 (175, 205) | 192 (175, 205) | 138 (121, 163) | 196 (177, 215) | −51 (−68, −27) | 4 (−9, 19) | <0.0001 |
| Men | |||||||
| hsCRP, mg/L | 4.0 (2.7, 6.6) | 4.1 (2.7, 6.8) | 2.1 (1.2, 4.1) | 3.2 (1.8, 5.8) | −1.7 (−3.4, −0.4) | −0.8 (−2.5, 0.8) | <0.0001 |
| LDL cholesterol, mg/dL | 107 (92, 119) | 108 (92, 118) | 55 (44, 71) | 108 (92, 123) | −49 (−62, −29) | 3.0 (−9, 15) | <0.0001 |
| HDL cholesterol, mg/dL | 46 (38, 55) | 45 (38, 55) | 49 (41, 59) | 47 (39, 56) | 3 (−2, 8) | 1 (−3, 5) | <0.0001 |
| Triglycerides, mg/dL | 118 (83, 176) | 118 (84, 172) | 100 (73, 142) | 119 (86, 172) | −16 (−50, 7) | 2 (−26, 27) | <0.0001 |
| Total cholesterol, mg/dL | 182 (165, 196) | 182 (166, 195) | 129 (113, 151) | 185 (166, 203) | −50 (−66, −28) | 3 (−9, 17) | <0.0001 |
Values are median (25th,75th percentile).
P value comparing rosuvastatin versus placebo for the change from baseline to 12 months.
JUPITER Outcomes
As shown in Table 3, the absolute rates (per 100 person-years) of the primary endpoint in rosuvastatin and placebo were lower in women (0.56 and 1.04, respectively) than men (0.88 and 1.54, respectively), but the relative risk reduction with rosuvastatin was similar and statistically significant in both women (hazard ratio 0.54, 95% CI 0.37–0.80, P=0.002) and men (0.58, 95% CI 0.45–0.73, P<0.001. The P-value for a treatment-by-sex interaction using a sex-stratified Cox proportional hazard model was non-significant (P=0.80). The HR and 95% CI for the ratio of the relative hazards for treatment in women and men for the primary endpoint were 0.94 (0.60–1.49).
Table 3.
Outcomes among women and men in JUPITER
| Rosuvastatin Women N=3,426 Men N=5,475 |
Placebo Women N=3,375 Men N=5,526 |
Hazard Ratio (95% CI) |
P | P for Heterogeneity | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. | Rate per 100 p-y | No. | Rate per 100 p-y | ||||
| Women | |||||||
| Primary endpoint | 39 | 0.56 | 70 | 1.04 | 0.54 (0.37–0.80) | 0.002 | 0.80 |
| Nonfatal MI | 8 | 0.12 | 14 | 0.21 | 0.56 (0.24–1.33) | 0.18 | 0.24 |
| Any MI | 10 | 0.14 | 18 | 0.27 | 0.54 (0.25–1.18) | 0.11 | 0.60 |
| Nonfatal stroke | 18 | 0.26 | 21 | 0.31 | 0.84 (0.45–1.58) | 0.59 | 0.04 |
| Any stroke | 18 | 0.26 | 23 | 0.34 | 0.77 (0.42–1.42) | 0.40 | 0.09 |
| Arterial revascularization | 8 | 0.12 | 29 | 0.43 | 0.27 (0.12–0.59) | 0.0003 | 0.04 |
| Arterial revascularization or hospitalization for unstable angina | 8 | 0.12 | 33 | 0.49 | 0.24 (0.11–0.51) | <0.0001 | 0.01 |
| MI, stroke, or confirmed death from cardiovascular causes | 36 | 0.52 | 48 | 0.71 | 0.73 (0.48–1.13) | 0.16 | 0.06 |
| Venous thromboembolism | 12 | 0.17 | 16 | 0.24 | 0.74 (0.35–1.55) | 0.42 | 0.43 |
| Death from any cause | |||||||
| Death on known date | 57 | 0.78 | 69 | 0.95 | 0.81 (0.57–1.16) | 0.25 | 0.99 |
| Any death | 60 | 0.82 | 77 | 1.07 | 0.77 (0.55–1.06) | 0.12 | 0.74 |
| Men | |||||||
| Primary endpoint | 103 | 0.88 | 181 | 1.54 | 0.58 (0.45–0.73) | <0.0001 | |
| Nonfatal MI | 14 | 0.12 | 48 | 0.40 | 0.29 (0.16–0.54) | <0.0001 | |
| Any MI | 21 | 0.18 | 50 | 0.42 | 0.42 (0.26–0.71) | 0.0006 | |
| Nonfatal stroke | 12 | 0.10 | 37 | 0.31 | 0.33 (0.17–0.63) | 0.0003 | |
| Any stroke | 15 | 0.13 | 41 | 0.34 | 0.37 (0.21–0.67) | 0.0005 | |
| Arterial revascularization | 63 | 0.54 | 102 | 0.86 | 0.63 (0.46–0.86) | 0.003 | |
| Arterial revascularization or hospitalization for unstable angina | 68 | 0.58 | 110 | 0.93 | 0.63 (0.46–0.85) | 0.002 | |
| MI, stroke, or confirmed death from cardiovascular causes | 47 | 0.40 | 109 | 0.92 | 0.44 (0.31–0.61) | <0.0001 | |
| Venous thromboembolism | 22 | 0.19 | 44 | 0.37 | 0.51 (0.30–0.85) | 0.008 | |
| Death from any cause | |||||||
| Death on known date | 133 | 1.07 | 166 | 1.32 | 0.81 (0.65–1.02) | 0.07 | |
| Any death | 138 | 1.11 | 170 | 1.35 | 0.82 (0.66–1.03) | 0.08 | |
MI: myocardial infarction. P for heterogeneity of treatment effect for outcomes between women and men were obtained from likelihood ratio tests that included treatment assignment, sex, and the interaction term.
When the components of the composite primary endpoint were analyzed, the HR for each component favored rosuvastatin therapy for both women and men, with some sex differences noted. Women had significant reduction in revascularization/unstable angina (HR 0.24, 95% CI 0.11–0.51), which was greater in magnitude than for men (HR 0.63, 95% CI 0.46–0.85), P for heterogeneity 0.01. Women had non-significant reductions in other components of the primary endpoint, and less of a reduction in nonfatal stroke compared with men (P for heterogeneity 0.04). The HR for all cause death was similarly reduced for women (HR 0.77, 95% CI 0.55–1.06) and men (HR 0.82, 95% CI 0.66–1.03). Although this did not reach statistical significance in either sex separately, it was significant when combined (P=0.02).
The 5-year NNT to prevent 1 primary endpoint was calculated at 36 in women, 22 in men, and 25 when combined.
JUPITER adverse events
The occurrence of serious adverse events was similar by sex (Table 4). Specifically, the rates of muscle disorders or myopathy were similar in women and men, regardless of treatment assignment. Death from cancer was examined due to prior reports of possibly increased rates of cancer-related death in women treated with statins,18 but we found no significant difference in cancer death in women.
Table 4.
Monitored adverse events, measured laboratory values, and other reported events during follow-up in JUPITER
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Rosuvastatin N=3,426 |
Placebo N=3,375 |
P | Rosuvastatin N=5,475 |
Placebo N=5,526 |
P | |
| Monitored adverse events | ||||||
| Any serious adverse event | 503 (7.7) | 481 (7.4) | 0.61 | 849 (7.6) | 896 (7.9) | 0.31 |
| Muscular weakness, stiffness, or pain | 552 (8.9) | 509 (8.3) | 0.24 | 869 (8.1) | 866 (7.9) | 0.77 |
| Myopathy | 5 (0.07) | 4 (0.06) | 0.76 | 5 (0.04) | 5 (0.04) | 0.99 |
| Rhabdomyolysis | 0 | 0 | – | 1 (0.01) | 0 | 0.32 |
| Newly diagnosed cancer | 100 (1.4) | 94 (1.4) | 0.74 | 198 (1.7) | 220 (1.8) | 0.32 |
| Death from cancer | 12 (0.2) | 17 (0.2) | 0.33 | 23 (0.2) | 41 (0.3) | 0.03 |
| Gastrointestinal disorder | 724 (12.0) | 734 (12.5) | 0.54 | 1,029 (9.8) | 977 (9.0) | 0.13 |
| Renal disorder | 166 (2.4) | 135 (2.0) | 0.09 | 369 (3.2) | 345 (2.9) | 0.29 |
| Bleeding | 99 (1.4) | 106 (1.5) | 0.54 | 159 (1.3) | 169 (1.4) | 0.63 |
| Hepatic disorder | 57 (0.8) | 63 (0.9) | 0.53 | 159 (1.3) | 123 (1.0) | 0.02 |
| Laboratory values | ||||||
| Creatinine, >100% increase from baseline | 6 (0.09) | 6 (0.09) | 0.98 | 10 (0.08) | 4 (0.03) | 0.10 |
| Glomerular filtration rate at 12 mo, ml/min/1.73 m2 | 64.1 (56.1–71.6) | 64.2 (56.6–70.9) | 0.66 | 71.0 (63.6–79.7) | 70.5 (62.7–79.4) | 0.006 |
| Alanine aminotransferase >3x ULN on consecutive visits | 3 (0.04) | 5 (0.07) | 0.47 | 20 (0.16) | 12 (0.10) | 0.15 |
| HbA1c at 24 mo, % | 5.9 (5.7–6.2) | 5.9 (5.6–6.1) | <0.0001 | 5.9 (5.6–6.1) | 5.8 (5.6–6.0) | <0.0001 |
| Glucose at 24 mo, mg/dL | 96 (89–104) | 95 (88–104) | 0.37 | 99 (92–107) | 99 (91–108) | 0.18 |
| Other events | ||||||
| Newly diagnosed diabetes (physician-reported) | 108 (1.5) | 71 (1.0) | 0.008 | 162 (1.4) | 145 (1.2) | 0.29 |
| Hemorrhagic stroke | 3 (0.04) | 3 (0.04) | 0.99 | 3 (0.02) | 6 (0.05) | 0.32 |
Values are number of events (rates per 100 person-years) or median (25th–75th percentile)
While both women and men treated with rosuvastatin had higher HbA1c at 12 months, a higher incidence of physician-reported diabetes was observed in women treated with rosuvastatin vs placebo (1.53 vs 1.03 per 100 person-years, respectively, HR 1.49, 95% CI 1.11–2.01, P=0.008) compared with men (1.36 vs 1.20 per 100 person-years, respectively, HR 1.14, 95% CI 0.91–1.43, P=0.24). The test for heterogeneity of diabetes by sex was not-significant (P for heterogeneity=0.16).
JUPITER sex-specific subgroups
Subgroup analysis showed reduction in the primary endpoint for both women and men (Figure 1). Two borderline significant interactions were noted for women, while for men there was no significant interaction for any subgroup. First, in the 829 women who reported a family history of premature coronary disease, there was a greater proportional reduction in the primary endpoint compared to the women without, although both were significant (HRs 0.20, 95% CI 0.06–0.69 and 0.63, 95% CI 0.41–0.96, respectively, P for interaction 0.07). Men with and without family history of premature coronary disease had similar proportional reduction in the primary endpoint. Second, women without the metabolic syndrome appeared to have greater reduction with rosuvastatin than those with the metabolic syndrome (HRs 0.35, 95% CI 0.19–0.65, and 0.77, 95% CI 0.46–1.30, respectively, P for interaction 0.06).
Figure 1.
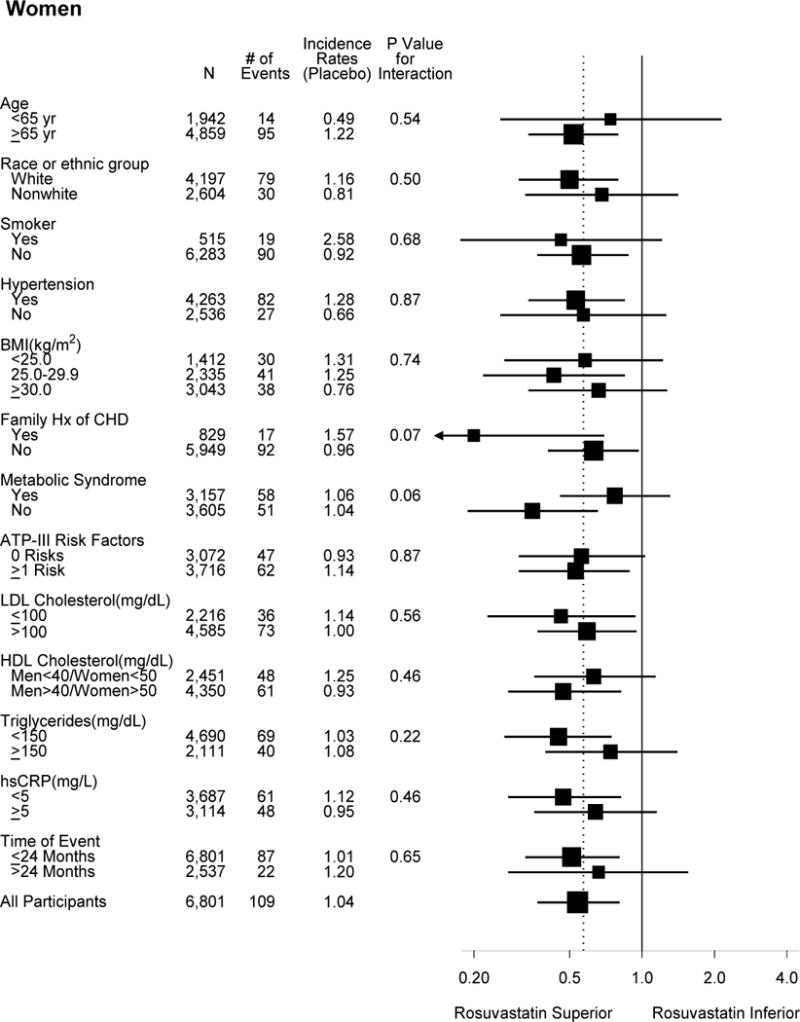
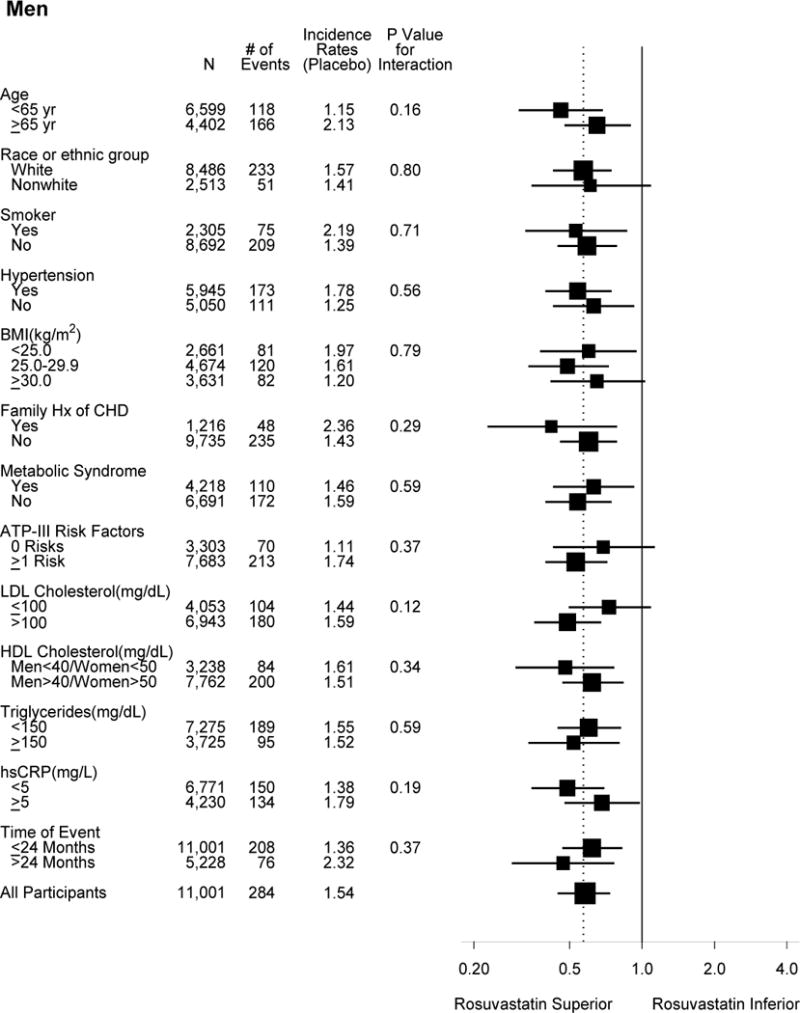
Effects of rosuvastatin on the primary composite endpoint according to baseline characteristics. The dashed overall line indicates the overall hazard ratio for the entire cohort (men and women combined).
There was no significant interaction in women stratified by Framingham risk scores or by HDL cholesterol. Few events occurred in women with Framingham risk scores <5% (N=2618, 15 events). Women with Framingham risk scores of 5 to 10% (N=2,525) and >10% (N=1,646) had similar and significant proportional reduction in events (HRs 0.44, 95% CI 0.22–0.89, and 0.57, 95% CI 0.34–0.97). The absolute event rates (per 100 person-years) were lower in women with scores 5 to 10% (0.42 in rosuvastatin and 0.96 in placebo) compared with women with scores >10% (1.28 and 2.23, respectively). Similar results were found in men stratified by Framingham risk scores, although men had higher absolute rates. The event rates were also low for women younger than 65 years old, although there was no significant interaction by age. Both men younger and older than 65 years benefited from rosuvastatin therapy. Similar findings were observed using an alternative age cut-point of 70 years.
Meta-analysis results
Compared with placebo, statin therapy in women significantly reduced CVD by about one third in exclusively primary prevention trials (Figure 2A). The summary RR for the 3 trials was 0.63 (0.49–0.82), P<0.001, P for heterogeneity 0.56. When trials that included predominantly primary prevention were analyzed together with the exclusively primary prevention trials, the summary RR was similar but not statistically significant, 0.79 (95% CI 0.59–1.05), P=0.11, P for heterogeneity=0.053 (Figure 2B). When we included HPS and PROSPER together with the other 5 trials (data not shown), the summary RR was unchanged (0.82, 95% CI 0.69–0.98), P=0.03.
Figure 2.
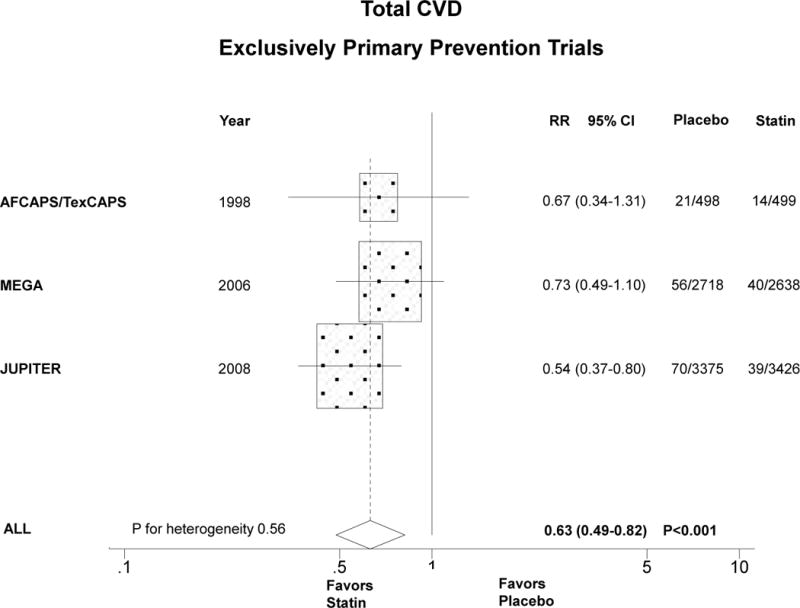
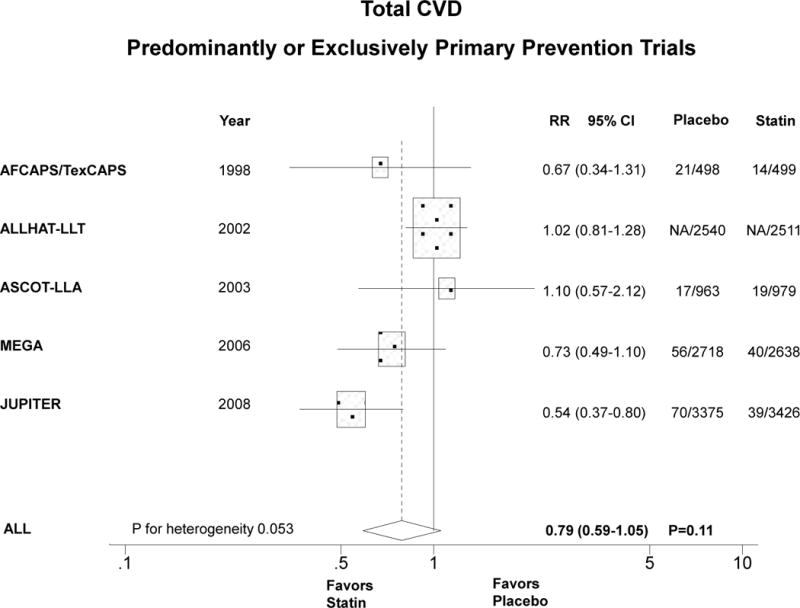
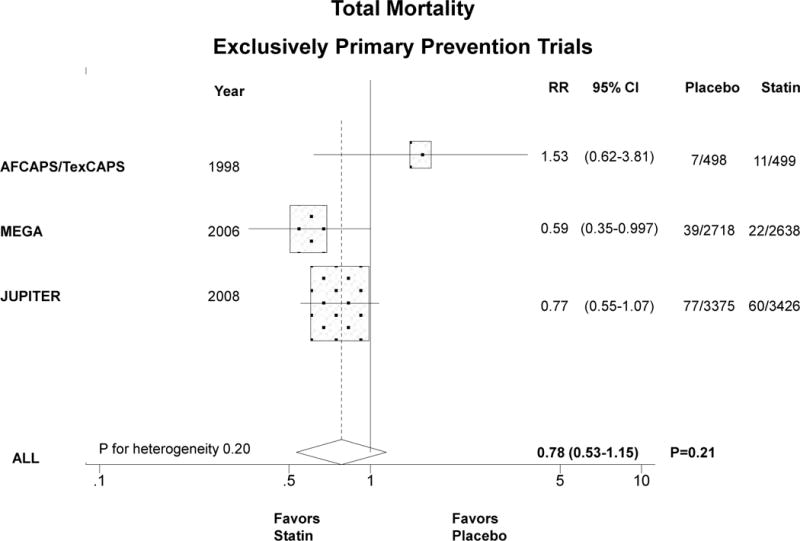
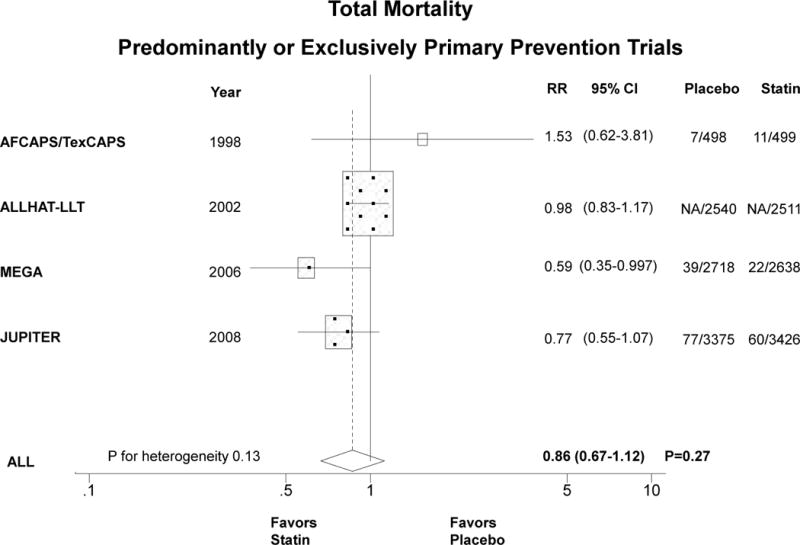
Relative risk of allocation to statin compared with placebo in women in relation to CVD in exclusively primary prevention trials (panel A) and predominantly or exclusively primary prevention trials (panel B). Similarly for total mortality (panels C and D). The size of the squares is proportional to the number of events. Mean age, % diabetic, and statin dose were, respectively, as follows: AFCAPS/TexCAPS 63 years, 3%, lovastatin 20–40 mg/day; ALLHAT-LLA 66 years, 35%, pravastatin 20–40 mg/day; ASCOT-LLA 63 years, 24%, atorvastatin 10 mg/day; MEGA 60 years, 18%, pravastatin 10–20 mg/day; JUPITER 69 years, 0%, rosuvastatin 20 mg/day.
The summary RR for the 3 exclusively primary prevention trials (N=13,154 women, 216 deaths) that reported sex-specific total mortality was 0.78 (95% CI 0.53–1.15), P=0.21 (Figure 2C). When all trials that reported sex-specific mortality outcomes in predominantly or exclusively primary prevention women were included, the summary RR was similar (Figure 2D). HPS and PROSPER did not report sex-specific mortality data in the primary prevention arms.
DISCUSSION
In the JUPITER trial, statin treatment of apparently healthy individuals with elevated hsCRP and low LDL cholesterol (women ≥60 years and men ≥50 years) resulted in similar and significant proportional reduction in the primary endpoint for both women (46%, P=0.002) and men (42%, P<0.001). Absolute event rates were lower in women, even though women were older and generally had more cardiovascular risk factors than men. There was no significant heterogeneity of treatment effect by sex for the primary composite endpoint or all cause mortality. In this updated meta-analysis of statin therapy for primary prevention in women, statin allocation yielded a significant relative risk reduction in CVD by one third, similar to prior results seen in men and in secondary prevention women. Statin allocation had a smaller, non-significant reduction in total mortality for primary prevention in women.
In JUPITER, there were sex differences noted in two components of the primary endpoint, with women having significantly more reduction compared with men in revascularization/unstable angina, and men having more reduction in stroke. Subgroup analysis suggested that women with a family history of premature coronary disease may benefit more from rosuvastatin therapy that those without, while in men the benefit was similar for those with and without a family history. Women and men with Framingham risk scores <5%, as well as women <65 years old, had low event rates, although there was no suggestion of heterogeneity by categories of Framingham risk in either women or men.
The JUPITER findings demonstrate for the first time that the proportional cardiovascular benefit from rosuvastatin therapy for primary prevention was similar and significant in women and men who were selected for therapy based on an elevated hsCRP level. JUPITER differs from prior statin trials in using elevated levels of hsCRP as an entry criterion, while previous statin trials selected participants mostly based on dyslipidemia. HsCRP has been shown to identify asymptomatic women and men who are at increased risk of CVD events independent of their LDL cholesterol.9 This underscores the importance of selecting individuals with adequate baseline risk to ensure a significant benefit of therapy.
When put in context with the updated meta-analysis in women, there was overall about a one third reduction in primary CVD with allocation to statin therapy compared with placebo in women. These findings contrast from prior primary prevention trials and meta-analyses that found that among men, there were significant reductions in coronary events, but among women the reduction was smaller and non-significant.4, 5 When trials that included both primary and secondary prevention populations had been previously analyzed, such as PROSPER19 and HPS20, or only secondary prevention, results were similar in women and men consistent with the present meta-analysis in primary prevention women. This argues against a sex-difference in statin therapy in the primary prevention setting; rather, it suggests that the prior lack of significance may have been due to the inadequate number of events among women in these studies. Compared with prior non-significant sex-specific meta-analyses in primary prevention,4–5 the present meta-analysis findings of a significant reduction in CVD is likely related to the larger number of events by including the recent JUPITER and MEGA trials and using a combined CVD endpoint that included stroke.
Importantly, the hazard ratios in JUPITER showed reduction in all cause death, which, while non-significant in either men or women analyzed separately, was statistically significant when they were analyzed together, as previously reported (P=0.02).8 There was no evidence for increase in cancer death among women or men in JUPITER. This contrasts with a significant increase in cancer death in the Treating to New Targets study among women with stable coronary disease.18 The JUPITER and updated meta-analyses results are, however, consistent with results from meta-analyses that showed no increase in all-cause death in either sex.4, 5 In addition, the present study is the first to find a significant reduction among women for arterial revascularization or unstable angina. This may be important as women present more with angina than myocardial infarction, while the opposite is generally seen in men.
A finding that deserves further investigation is the somewhat higher risk of physician-reported incident diabetes in the statin arm compared with placebo that was observed in women as compared with men, although the test for heterogeneity by sex was not significant. A recent study found that among women, a diagnosis of diabetes carried 37% higher risk of subsequent CVD death than did a diagnosis of myocardial infarction, while the reverse was found among men, with myocardial infarction having 43% higher risk of CVD death compared with diabetes.21 Clinically, women with impaired fasting glucose or overweight/obesity were at greater risk for developing diabetes, and this subgroup had a 40% relative risk reduction in the primary endpoint in JUPITER. Interestingly, the only statin trial that showed a reduction of diabetes with statin therapy was a trial that enrolled only men, the West of Scotland Coronary Prevention Study (WOSCOPS), in which pravastatin resulted in lower incident diabetes compared with placebo.22 However, other statin trials that included both men and women, such as the Heart Protection Study, found a similarly small (0.6%) non-significant increase in incident diabetes with simvastatin20 that was also seen with atorvastatin (0.4%) in ASCOT-LLA.15 The present finding of a potential sex difference for incident diabetes underscores the importance of analyzing and reporting trial efficacy and safety data in women and men separately and combined, as has recently been recommended.23
This study has potential limitations. Median duration of follow-up in JUPITER was 1.9 years (maximum 5 years) due to early termination of the trial for benefit, and long-term safety data for rosuvastatin in a primary prevention setting are limited. While there was a substantial proportion of women younger than 65 years or with low Framingham risk scores, the event rates were low in these subgroups and the question remains as to whether they would significantly benefit from statin therapy. Limitations to the meta-analysis included that the number of both CVD and total deaths could not be exactly determined, since one trial did not report sex-specific event rates, and the degree of LDL cholesterol lowering differed among the trials.
In conclusion, statin treatment of apparently healthy women with elevated high-sensitivity C-reactive protein and non-elevated LDL cholesterol resulted in similar and significant proportional reduction in CVD compared with men. Absolute event rates were lower in women, with more of a benefit for revascularization/unstable angina in women and more of a benefit for stroke in men. Subgroup analysis suggested that women with a family history of premature coronary disease may benefit more from rosuvastatin therapy that those without. Women and men with Framingham risk scores <5% had low event rates, although there was no suggestion of heterogeneity by categories of Framingham risk in either women or men. Taken together with the results of the updated meta-analysis in women, statin therapy resulted in about one third relative reduction in primary CVD in women, a benefit similar to that seen in previous meta-analyses of men.
The use of statins in patients with manifest cardiovascular disease (CVD) is established, with similar benefit in women and men, but statin use for primary prevention of CVD is controversial particularly for women. We analyzed sex-specific outcomes in JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) and conducted an updated meta-analysis of statin use for women in primary prevention (20,147 women, >276 CVD events, mean age 63–69 years). JUPITER was a multi-center randomized trial designed to assess the benefits and risks of statin therapy in apparently healthy individuals selected on the basis of elevated high-sensitivity C-reactive protein, a marker of higher cardiovascular risk, without a concomitant elevation in LDL cholesterol. JUPITER participants were 6,801 women ≥60 years and 11,001 men ≥50 years with high-sensitivity C-reactive protein ≥2mg/L and LDL cholesterol <130mg/dL randomized to rosuvastatin 20 mg/day versus placebo. Absolute CVD rates for rosuvastatin and placebo in JUPITER were lower for women than men, but there was similar and significant relative risk reduction in both women (by 46%) and men (by 42%). In women, there was significant reduction in revascularization/unstable angina and non-significant reductions in other components of the primary endpoint. Furthermore, in this updated meta-analysis of statin therapy for primary prevention in women, statin allocation yielded a significant relative risk reduction in CVD by one third, similar to prior results seen in men and in secondary prevention women.
Acknowledgments
Funding Sources
JUPITER was funded by AstraZeneca. The sponsor collected data and monitored study sites but played no role in the conduct of the analyses or drafting of the manuscript.
Disclosures
Dr Mora received research grant support from NHLBI (K08 HL094375), AstraZeneca, and Merck. Dr Glynn received grant support from AstraZeneca and Bristol-Myers Squibb. Dr Hsia is employed by AstraZeneca. Dr Genest received research grants from Merck, AstraZeneca, and Resverlogix, and speaker’s fees from Merck, AstraZeneca, and GlaxoSmithKline. Dr Ridker received research support from Astra-Zeneca, Novartis, Roche, and Sanofi-Aventis, and non-financial research support from Amgen. Dr Ridker is co-inventor on patents held by Brigham and Women’s Hospital related to the use of inflammatory biomarkers in CVD that have been licensed to Siemens and AstraZeneca, and has served as a research consultant to Schering-Plough, Sanofi/Aventis, AstraZeneca, Isis, Siemens, Merck, Novartis, and Vascular Biogenics.
Footnotes
Clinical Trial Registration URL http://www.clinicaltrials.gov. Unique Identifier: NCT00239681
References
- 1.Grundy SM. Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? Yes. BMJ. 2007;334:982. doi: 10.1136/bmj.39202.399942.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kendrick M. Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? No. BMJ. 2007;334:983. doi: 10.1136/bmj.39202.397488.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson J, Wright JM. Are lipid-lowering guidelines evidence-based? Lancet. 2007;369:168–169. doi: 10.1016/S0140-6736(07)60084-1. [DOI] [PubMed] [Google Scholar]
- 4.Walsh JM, Pignone M. Drug treatment of hyperlipidemia in women. JAMA. 2004;291:2243–2252. doi: 10.1001/jama.291.18.2243. [DOI] [PubMed] [Google Scholar]
- 5.Petretta M, Costanzo P, Perrone-Filardi P, Chiariello M. Impact of gender in primary prevention of coronary heart disease with statin therapy: A meta-analysis. Int J Cardiol. 2010;138:25–31. doi: 10.1016/j.ijcard.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:2307–2313. doi: 10.1001/archinte.166.21.2307. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno K, Nakaya N, Ohashi Y, Tajima N, Kushiro T, Teramoto T, Uchiyama S, Nakamura H. Usefulness of pravastatin in primary prevention of cardiovascular events in women: analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA study) Circulation. 2008;117:494–502. doi: 10.1161/CIRCULATIONAHA.106.671826. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108:2292–2297. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 10.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 14.Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 15.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 17.Clearfield M, Downs JR, Weis S, Whitney EJ, Kruyer W, Shapiro DR, Stein EA, Langendorfer A, Beere PA, Gotto AM. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): efficacy and tolerability of long-term treatment with lovastatin in women. J Womens Health Gend Based Med. 2001;10:971–981. doi: 10.1089/152460901317193549. [DOI] [PubMed] [Google Scholar]
- 18.Wenger NK, Lewis SJ, Welty FK, Herrington DM, Bittner V. Beneficial effects of aggressive low-density lipoprotein cholesterol lowering in women with stable coronary heart disease in the Treating to New Targets (TNT) study. Heart. 2008;94:434–439. doi: 10.1136/hrt.2007.122325. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 20.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 21.Hu G, Jousilahti P, Qiao Q, Peltonen M, Katoh S, Tuomilehto J. The gender-specific impact of diabetes and myocardial infarction at baseline and during follow-up on mortality from all causes and coronary heart disease. J Am Coll Cardiol. 2005;45:1413–1418. doi: 10.1016/j.jacc.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 23.Blauwet LA, Hayes SN, McManus D, Redberg RF, Walsh MN. Low rate of sex-specific result reporting in cardiovascular trials. Mayo Clin Proc. 2007;82:166–170. doi: 10.4065/82.2.166. [DOI] [PubMed] [Google Scholar]


