Abstract
Perfluorinated compounds (PFCs) have been recognized as an important class of environmental contaminants commonly detected in blood samples of both wildlife and humans. These compounds have been in use for more than 60 years as surface treatment chemicals, polymerization aids, and surfactants. They possess a strong carbon-fluorine bond, which leads to their environmental persistence. There is evidence from both epidemiology and laboratory studies that PFCs may be immunotoxic, affecting both cell-mediated and humoral immunity. Reported effects of PFCs include decreased spleen and thymus weights and cellularity, reduced antibody production, reduced survival after influenza infection, and altered cytokine production. Immunosuppression is a critical effect associated with exposure to PFCs, as it has been reported to reduce antibody responses to vaccination in children. Mounting evidence suggests that immunotoxicity in experimental animals can occur at serum concentrations below, within, or just above the reported range for highly exposed humans and wildlife. Considering bioaccumulation and exposure to multiple PFCs, the risk of immunotoxicity for humans and wildlife cannot be discounted. This review will discuss current and recently published work exploring the immunomodulatory effects of PFCs in experimental animals and humans.
Keywords: perfluorinated compounds, immunosuppression, PPAR-α receptor, cytokine, vaccination
1. Uses, environmental and serum concentrations of PFCs
Perfluorinated compounds (PFCs), such as perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), have been produced since the 1950s and are used in many industrial and manufacturing applications, including production of nonstick cookware, waterproof and breathable textiles, and protective coatings for paper, food packing materials, and carpets. PFCs comprise a heterogeneous class of chemicals consisting of an alkyl chain (4–14 carbons), which is partially or fully fluorinated, and have different functional groups attached. Among the PFCs are the perfluoroalkyl carboxylic acids (i.e. PFOA, PFDA), perfluoroalkyl sulfonic acids (i.e. PFOS, PFBS), perfluoroalkyl sulfonamides (i.e. PFOSA), and other polyfluorinated compounds, such as fluorotelomer alcohols (i.e. fluorotelomer). PFCs are commonly detected in wildlife and human blood samples (Suja et al., 2009; Rayne and Forest, 2009). Food intake appears to be the major factor contributing to background PFC levels in human sera, while exposure to contaminated water and soils results in elevated levels in both human and wildlife populations (Vestergren and Cousins, 2009).
These compounds are present in surface, ground, marine, and drinking waters at concentrations that vary from pg/l to μg/l and in soils at levels up to approximately 50 ng/g of dry matter. Some wastewaters contain PFCs at mg/l to low g/l levels (Rayne and Forest, 2009; Zareitalabad et al., 2013). Although western manufacturers began a voluntary phase out of PFOS and PFOA production in 2002, both PFOS and PFOA persist in the environment because of extremely stable carbon-fluorine bonds and circulate through environmental media including air, soil, plants and food stocks (Davis et al., 2007, Domingo, 2012).
The PFOA elimination half-life in serum markedly differs among species and between sexes, being 1 to 9 days in rats, 18 days in mice, 20 to 40 days in dogs, 20 to 32 days in monkeys, and 3.8 years in humans (Calafat et al., 2007). Blood samples of occupationally exposed individuals and the general human population in various countries were found to contain PFOS and PFOA at measurable levels. In the United States, the mean serum concentration in the general population was reported as 20.7 ng/ml for PFOS and as 3.7 ng/ml for PFOA (Calafat et al., 2007), with children often showing higher serum concentrations than adults (Kato et al., 2009). One explanation for the apparent greater mean concentrations of PFCs in children than in adults is increased exposure among children resulting from increased contact with carpeted floors and upholstered furniture coupled with hand-to-mouth activity (Kato et al., 2009). PFOA serum concentrations reported in occupationally exposed humans were between 428 and 12000 ng/ml (Steenland et al., 2010), and between 145 and 3490 ng/ml for PFOS (Olsen et al., 2007).
2. Toxicology findings
In the context of toxicology, PFOA and PFOS have attracted most attention because they represent a high proportion of environmental PFCs. Adverse effects reported in experimental animals exposed to PFOA or PFOS include hepatomegaly and hepatic peroxisome proliferation; liver, testicular, and pancreatic tumors; reproductive and developmental deficits, including reduced fetal weights, skeletal and cardiac malformations; neurotoxicity; and immunotoxicity. Data from experimental animals demonstrate that the perfluorinated alkyl acids induce peroxisomal proliferation, hepatomegaly, altered steroidogenesis, and body weight loss associated with a wasting syndrome (Kennedy, 2004; Pastoor et al., 1987; Liu et al., 1996b; Olsen et al., 1999; Biegel et al., 2001; Lau et al., 2007). Ammonium perfluorooctanoate, the ammonium salt of PFOA, produces increased aromatase activity and plasma estradiol levels, and induces Leydig cell adenomas (Liu et al., 1996a, 1996b).
The peroxisome proliferator-activated receptors (PPAR) belong to the nuclear hormone receptor superfamily, and there are three primary subtypes: PPAR α, β/δ, and γ. These receptors regulate important physiological processes that impact lipid homeostasis, inflammation, adipogenesis, reproduction, wound healing, and carcinogenesis (Chinetti et al., 2000). PFC-associated toxicity in laboratory rodents has been related to ligand activation of PPARα (Peraza et al., 2006). It is, however, important to stress that there are important species differences in receptor-specificity, receptor activity and related ligand binding/activation, which are all critical factors that mechanistically influence susceptibility to PFC toxicity. For example, PPARα-humanized mice do not exhibit the liver toxicities found in wild-type mice (Cheung et al., 2004). The hepatocarcinogenic effect of PPARα ligands in rodents is one of the best examples of such a species-specific effect: epidemiological studies of humans taking fibrates (a potent class of PPARα agonists) to control serum lipid levels found no evidence of increased cancer, even though all rodents exposed to the same compounds develop liver tumors (Gonzales et al., 1998). Therefore, the role of species differences in signal transduction in extrapolating animal data to human risk assessment needs to be carefully considered.
In spite of a number of experimental and epidemiological studies, the health effects of PFCs in humans still remain controversial (Butenhoff et al., 2004; Emmett et al., 2006; Steenland et al., 2010). Epidemiological studies on large cohorts of occupationally exposed workers, mainly from manufacturing sites in the US (3M and DuPont) in the period 1947–2002, found no association between PFOA exposure and significant adverse health effects (Ubel, 1989; Gilliland Mandel, 1993; Alexander, 2001; Alexander and Olsen, 2007; Leonard et al., 2008). The slight excess of prostate cancer reported by Gilliland and Mandel (1993) and of bladder cancer found in the study by Alexander (2001) was not confirmed in two occupational cohorts or a general population study (Leonard et al., 2008; Eriksen et al., 2009, Lundin et al., 2009). In a comprehensive review, Steenland et al. (2010) evaluated the epidemiologic literature on PFOA and its association with several health outcomes, including immunomodulation. The authors concluded that the most consistent health findings were modest increases in cholesterol, which may increase the risk of cardiovascular diseases, and elevated serum uric acid. The later finding was associated with an increased risk of chronic kidney disease in the study cohort, although the relationship between elevated uric acid and kidney disease is not absolute (Cain et al., 2010). The mechanism by which PFCs are related to changes in serum cholesterol in humans is unknown, but the finding of positive associations between perfluorinated compounds and altered cholesterol levels contradicts what would be expected from animal studies. In animals, PFOA is a strong PPARα activator in the liver and this proliferation has been shown to alter lipid metabolism with decreases, not increases, in serum lipids (Kennedy et al., 2004). Peroxisome proliferation is much less apparent in humans (Palmer et al., 1998) and may clarify the discrepancy between human and animal studies on the associations between PFCs and cholesterol levels.
In humans, the possible interference of PFCs with metabolism of fatty acids should not be underestimated and deserves further investigations on the mechanisms of action and as a possible risk factor for metabolic disorders and/or cardiovascular diseases (Costa et al., 2009). Certain chronic diseases with an inflammatory component, including diabetes, heart disease, and stroke, have been reported to be elevated in studies of exposed populations; however, the evidence is too meager and inconsistent for solid conclusions (Steenland et al., 2010).
3. Evidences of immunotoxicity in rodents
In a review of the U.S. Environmental Protection Agency (US EPA) risk assessment of PFOA and its salts by the US EPA Science Advisory Board, immunotoxicity was mentioned as an endpoint of concern for PFCs (http://yosemite.epa.gov/sab/sabproduct.nsf/ded77e69fc8ced288525711e006fe16b/$file/kropp-ewg.pdf). Early studies with mice indicated that ingestion of feed containing 0.02% PFOA by weight (approximately 30 mg/kg bw/day) suppressed antigen-specific IgM antibody production, caused thymic and splenic atrophy, and altered T-cell populations (Yang et al., 2000, 2001, 2002a, 2002b). It is important to note that at this dose, overt toxicity, as indicated by body weight loss, was observed, and, therefore, the observed immunosuppressive effects could be secondary to severe toxicity rather than a specific effect of PFOA on the immune system (Loveless et al., 2008; Fang et al., 2008). However, DeWitt et al. (2008) demonstrated that suppression of the antigen-specific IgM antibody response occurs at doses that produce no signs of acute toxicity or effects on spleen weight. Furthermore, this group (DeWitt et al. (2009a) determined that adrenalectomy did not protect mice from the immunosuppressive effects of PFOA at doses that reduced body weight gain. Although the HPA axis may contribute to immunotoxicity at high doses in intact animals, suppression of humoral immunity at lower doses (e.g., 3.75 and 7.5 mg PFOA/kg PFOA) is independent of elevated corticosterone and therefore represents a true immunotoxic effect (DeWitt et al., 2009a). DeWitt et al. (2008) calculated a benchmark dose for immunosuppression, based on 3.75 mg PFOA/kg as a lowest observed adverse effect level (LOAEL), an exposure that resulted in 7.4×104 ng PFOA/ml of serum measured one day post-exposure. This concentration is approximately 150 times lower than that reported in highly exposed individuals living near a PFOA production facility, 6 times greater than concentrations observed in highly exposed humans (12 μg/ml) and 20,000 times greater of the mean serum concentration measured in the general population (3.7 ng/ml).
Recent studies have shown that PFOS affects antibody production in mice at levels found in the general human population (Peden-Adams et al., 2008). Peden-Adams et al. (2008) reported that serum concentrations of PFOS associated with the LOEL (lowest observed effect level) for suppression of humoral immunity were 91.5 ng/ml for males and 666 ng/ml for females. Additionally, PFOS exposure (5 or 25 μg/kg PFOS/kg/d) suppressed immunity to influenza A infection in mice without altering body or lymphoid organ weights, resulting in significant increases in emaciation and mortality (Guruge et al., 2009). The pesticide sulfluramid, which is rapidly metabolized to PFOS, also has been demonstrated to target T-dependent, IgM antibody production in mice at exposure levels 10-fold lower than overtly toxic doses (Peden-Adams et al., 2007), further confirming the immune system as a sensitive target of PFC toxicity. Interestingly, the immune system of nonmammalian species has also been shown to be affected at current environmental levels of PFCs (Peden-Adams et al., 2009)). In contrast, Qazi et al. (2010) reported that 28 days of dietary exposure to PFOS did not affect IgM TDAR in B6C3F1 male mice at serum concentrations 8- to 85-fold greater than occupationally exposed humans. Similarly, Lefebvre et al. (2008) indicated that when Sprague-Dawley rats were given PFOS in their diet for 28 days, IgG TDAR was not statistically suppressed, although they did not measure IgM TDAR. Regardless of the presence of some conflicting data, the weight of evidence clearly supports the conclusion that antibody production is an endpoint sensitive to modulation by PFCs.
4. Evidences of immunotoxicity in humans
Data on the immunotoxicity of PFCs in humans are limited; however, they seem to suggest that exposure to PFCs may be associated with immunosuppressive effects. Emmett et al. (2006) reported a slight increase in absolute monocyte counts of residents who lived in a water district contaminated with PFOA, while there was no significant relationship between serum PFOA and the percentage of monocytes in differential white cell counts. The C8 Health Project, which collected data from residents living in the vicinity of a PFOA plant, found that immunoglobulins (Ig) A and E (female only) and C reactive protein (CRP) levels decreased with increasing blood levels of PFOA and antinuclear antibodies increased with increasing PFOA serum concentration (Fletcher et al., 2009). Brieger et al. (2011) explored the impact of PFOS and PFOA on selected functions of human leukocytes in vitro, and reported that PFOA and PFOS were associated with reduced NK cell activity and reduced the release of the pro-inflammatory cytokine TNF-α following lipopolysaccharide (LPS)-stimulation.
Age, gender, genetic background, and social factors typically influence the response to immunotoxic compounds, resulting in a great deal of variability in biological outcomes. Thus, exposure-related alterations in immune functions that are well tolerated by “normal” individuals could have more serious consequences in those individuals who are chronically ill, or malnourished, in the very young, whose immune systems have yet to complete development, or in the elderly, whose immune systems are in decline (Germolec, 2004; Holsapple et al., 2004; Dieter, 2008). Two recently published studies indicate that PFCs may lower vaccine protection in children (Grandjean et al., 2012; Granum et al., 2013). The antibody response to immunization is considered a feasible parameter to evaluate in population studies; it is clinically relevant and reflects immune system functionality (van Loveren et al., 1999; Luster et al., 2005; WHO, 2012). In laboratory rodents, the antibody response to T cell-dependent antigens is considered one of the most sensitive and predictive measures of immune function currently in use, as it requires T cells, B cells, and antigen-presenting cells to function properly to elicit an antibody response (Luster et al. 1992) and correlates well with resistance to infection (Luster et al., 1993). Therefore, reduced vaccine responses in children may reflect impacts on a sensitive immunotoxicological endpoint in a sensitive subpopulation.
In a prospective study of a birth cohort from the National Hospital in the Faroe Islands involving a total of 587 children, Grandjean et al. (2012) found that five to seven year old children with high serum concentrations of PFCs did not respond as well to diphtheria and tetanus immunizations as children with lower PFC levels: a 2 fold increase in serum PFC concentrations at 5 years of age was reported to reduce responses to tetanus and diphtheria booster immunizations by half. The fishing community of the Faroe Islands was chosen due to the frequent intake of marine food, which is associated with increased exposure to PFCs (Weihe et al., 2008). The mean maternal serum concentrations of PFOS and PFOA were 27.3 ng/ml and 3.2 ng/ml, respectively, while in five year old children serum concentrations were 16.7 ng PFOS/ml and 4.06 ng PFOA/ml. Childhood immunizations began with vaccination at age 3 months against diphtheria and tetanus, along with pertussis, polio, and Haemophilus influenzae type B. Repeat inoculations were given at 5 and 12 months of age, with a booster vaccination against diphtheria and tetanus at age 5 years. To evaluate the long-term antibody response to the vaccinations, the birth cohort underwent prospective follow-up until age 7 years. Examinations took place at age 5 years prebooster (587 children), approximately 4 weeks after the booster, and at age 7 years (464 children). Prenatal exposure to five selected PFCs was assessed by analyses of serum obtained from the mother at the last antenatal examination at week 32 of gestation; postnatal exposure was assessed by analysis of serum from the child at age 5 (prebooster). Multiple regression analyses with covariate adjustment showed that prenatal exposures to both PFOS and PFOA were negatively associated with antidiphtheria and antitetanus antibody concentrations. The authors concluded that elevated PFC concentrations, particularly of PFOS and PFOA, were responsible for antibody responses to vaccination below those associated with effective protection for tetanus and diphtheria (0.1 IU/ml). This study was the first to link PFC exposures in children to deficits in immune system functions.
In a second prospective study, involving the birth-cohort BraMat, Granum et al. (2013) found an inverse association between the level of anti-rubella antibodies in children at age 3 years and maternal plasma concentrations of PFCs. The antibody levels specific for the four vaccines included in the Norwegian Childhood Vaccination Program, included measles, rubella, tetanus and Haemophilus influenzae type B were assessed. Clinical health outcomes were collected using a specific questionnaire administered to the participants. The mean maternal serum concentrations were 1.1 ng/ml for PFOA and 5.5 ng/ml for PFOS. In multivariate models, increased concentrations of PFCs in maternal blood were significantly associated with reduced anti-rubella antibodies in children at age 3 years, while no significant associations were found between the concentrations of PFCs and antibody titers to the other vaccines. In addition, these investigators found a positive association between the maternal concentration of PFOA and PFNA (perfluorononanoate) and the number of episodes of common cold for the children in this cohort, and between maternal PFOA and PFHxS (perfluorohexane sulfonate) concentration and the number of episodes of gastroenteritis. This study indicates that pre-natal exposure to PFCs is associated with immunosuppression in early childhood with effects on both response to pediatric vaccine and immune-related clinical outcomes.
In a study involving 1400 pregnant women and their offspring, characterized by mean maternal serum concentrations of 5.6 ng/ml for PFOA and 35.3 ng/ml for PFOS during the first trimester, Fei et al., (2010) did not find an association between PFC exposure and increased risk of hospitalization for infectious diseases in children followed for up to 11 years, using data from the Danish National Birth Cohort. The outcome of interest was any hospitalization due to infections in early childhood. The number of hospitalizations was counted for each child during the follow-up period, which began on the date of birth and ended on the date of death, emigration, or December 31, 2008, which ever occurred first. Out of 1400 children, 363 (25.9%) were hospitalized at least once during the follow-up period due to infectious diseases. If considered all together, children who were prenatally exposed to higher levels of PFOA had lower risks of hospitalization due to infections, but the incidence rate ratio (IRR) was statistically significant only for the second quartile of exposure [IRR1/40.71, 95% confidence interval (CI) 0.53–0.94] compared with the first. No significant association was observed for PFOS. However, if children were stratified by sex, girls had a higher risk of hospitalization for infections associated with higher maternal PFOS or PFOA levels, with the IRRs ranging from 1.14 to 1.61 for PFOS and 1.20 to 1.74 for PFOA in the higher three quartiles compared with the lowest. In contrast, the IRRs were all below 1.0 among boys. This sex difference is of interest, and it is consistent with in vitro data we recently published (Corsini et al., 2012): in terms of cytokine production, leukocytes obtained from female donors appear to be more sensitive to the immunosuppressive effects of PFCs compared to leukocytes from male donors. Interestingly, sex also significantly modified the positive association between PFOA and cholesterol, with a stronger association among women as demonstrated in a middle-aged Danish population (Eriksen et al., 2013).
While the majority of published human data are indicative of immunosuppressive effects of PFCs, especially in children, the role of PFCs in allergic diseases is still not well-known. T-helper (Th)-1 and Th2 cells play specific roles in immunity. Th1 cells promote cell-mediated immunity against intracellular pathogens, and the prototypical cytokines secreted are IFNγ and IL-2. Th2 cells are involved in humoral immunity and secrete IL-4 to promote production of immunoglobulins. If cytokine profiles are shifted toward one subset over another, the risk of immune dysfunction increases. For example, in separate studies, Dong et al. (2011) and Zheng et al. (2011) evaluated the effect of short-term or subchronic PFOS exposure on the balance between Th1 and Th2 cytokines in C57BL/6 male mice. Results indicated that the cytokine balance favored Th2 responses in experimental animals (Zheng et al. 2011; Dong et al. 2011).
In a study by Wang et al. (2011) involving 244 children and investigating correlations among cord blood IgE and serum total IgE at 2 years of age and cord blood PFC levels, prenatal PFOA and PFOS exposures positively correlated with cord blood IgE levels in boys. In contrast, in a study by Okada et al. (2012), cord blood IgE levels decreased significantly with high maternal PFOA levels among female infants, and no relationship was found between maternal PFOS and PFOA levels and infant allergies and infectious diseases as assessed from self-administered questionnaires at 18 months of age. In agreement, the study by Granum et al. (2013) showed no significant associations between prenatal exposure to PFCs and allergy. Further studies are necessary to definitively exclude or include a role of PFCs in allergy.
5. Mechanisms of action
Studies suggest that some of the biological effects of the PFCs are mediated through PPARs and because PFOA and PFOS both activate murine PPARα, the role of PPARα in PFOA and PFOS immunotoxicity has been investigated. However, the specific role of PPARs in PFC-induced immunotoxicity is still a matter of debate (DeWitt et al., 2009b). Some data suggest that PPARα mediates many processes connected with the immune system in an indirect fashion, by modulating lipid levels and leading to hepatotoxicity and stress effects (Qazi et al., 2009). Yang and co-workers (2002a) compared the immunomodulating effects of PFOA in wild-type and PPARα null mice and reported that reductions in spleen weight and cellularity, in thymus weight and cellularity, and in mitogen-induced lymphocyte proliferation caused by PFOA in wild-type mice were not observed in PPARα null mice, indicating that PPARα plays a major role in the immunomodulation caused by PFOA. In contrast, DeWitt et al. (in preparation) found that suppression of antigen-specific IgM antibody responses occurred in PPARα null mice was equivalent to that of wild-type mice exposed to the same dose of PFOA. The difference between the Yang et al. (2002a) and the DeWitt et al. (in preparation) studies was based on the strain of mouse; Yang et al. (2002a) evaluated immune responses in null animals on an Sv129 background and used a C57BL/6 animal as the corresponding wild-type whereas DeWitt et al. (in preparation) used C57BL/6 animals for both the null and wild-type. Therefore, it appears as if the immunotoxicity of PFOA may depend on PPARα null mice in some strains of mice and may not in others. The immunotoxicity of PFOS appears to be partially dependent upon PPARα activation (Qazi et al., 2009): reductions in thymus weight and in total number of thymocytes were only partially attenuated in PPARα-null animals. In vitro studies have demonstrated that PFOA is a more potent agonist of murine PPARα than PFOS (Takacs and Abbott, 2007), which may explain the differences in the immune responses in vivo. Effects independent of PPARα activation may have greater potential impact on humans exposed to PFCs as human hepatic PPARα expression is only one-tenth that of rodents (Kennedy et al., 2004). Furthermore, the affinity of PFOA and PFOS for human or murine PPARs is quite different. In vitro studies conducted on Cos-1 cells transfected with mouse or human PPAR α, β/δ, or γ reporter plasmids by Takacs et al. (2007) demonstrated that PFOA (0.5–100 μM) significantly increased mouse and human PPARα and mouse PPARβ/δ activity. Whereas PFOS (1–250 μM) significantly increased activation of mouse PPARα and PPAR β/δ isoforms, no significant activation of mouse or human PPAR γ was observed with PFOA or PFOS. The mouse PPARα appears to be more sensitive to PFCs than the human PPARα, with PFOA having more activity than PFOS with both the mouse and human PPAR isoforms, further supporting the hypothesis of a different role for PPARα in PFOA and PFOS toxicity.
Modulation of cell-signaling responses is likely to be involved in PFC-induced suppression of antibody production, as alterations in the numbers of relevant leukocyte populations have not been demonstrated (Peden-Adams et al. 2010; Fair et al. 2011). After 28 days of oral exposure to PFOS and an in vivo antigen challenge, IL-6 production in B cells stimulated with anti-CD40 or soluble CD154 in vitro was suppressed. In the same study, nuclear translocation of NF-κB (p65) and AP-1 (c-Jun) in splenic nuclear extracts was not altered, suggesting a possible role for altered CD40-CD154 signaling in the B cell (Peden-Adams et al. 2010).
PFOA and PFOS also inhibited T-cell-independent IgM antibody responses (TIAR) in mice: suppression of TIAR was observed in C57BL/6 female mice given 1.88 mg/kg of PFOA in drinking water for 15 days (DeWitt, unpublished data), and Peden-Adams et al. (2008) reported that exposure to PFOS also suppressed TIAR in B6C3F1 mice. The suppression of both TDAR and TIAR suggest that B cells, macrophages, or both may be specific targets of PFCs. Although these studies begin to elucidate possible pathways altered in PFOS-induced antibody suppression, additional studies are needed to clarify the mechanism associated with this effect.
Considering the ability of PFCs to directly affect immune cells, using human immune cells exposed to several PFCs in vitro, we demonstrated that PFCs directly affected immune cell activation and reduced cytokine production (both pro- and anti-inflammatory) (Corsini et al., 2011; Corsini et al., 2012). As plasma levels of PFOA and PFOS ranging between 3.7–12000 ng/ml have been found in the human population, the concentrations (0.1–10 μg/ml) used in our in vitro studies are likely to be relevant for the human situation (Calafat et al., 2006; Olsen et al., 2007; Rayne and Forest, 2009; Steenland et al., 2010). We also demonstrated a role for PPARα in PFOA-induced inhibition of cytokine secretion in human cells exposed in vitro. In contrast, we showed that the inhibitory effect of PFCs on in vitro cytokine production by human leukocytes can also occur independently of PPARα, and involves the inhibition of NF-κB activation. All of the PFCs studied, including PFOA and PFOS, decreased LPS-induced phosphorylation of p65 and NF-κB driven transcription. However, with the exception of PFOA, none of the PFCs tested was able to activate PPARα in transiently transfected THP-1 cells, excluding a role for this receptor in the immunomodulation observed. Similar to our previous findings with PFOS, PFBS and PFDA prevented LPS-induced I-κB degradation (Corsini et al 2011). All of the PFCs tested inhibited p65 phosphorylation and NF-κB driven transcription; and while it appears that PFOS, PFBS and PFDA act upstream by inhibiting I-κB degradation, PFOA, PFOSA and fluorotelomer do not interfere with LPS-induced I-κB degradation, suggesting a downstream effect. The phosphorylation of p65 is regulated by both cell- and stimulus-dependent activating kinases. Ser276 phosphorylation has a crucial role in the interaction with and the engagement of the cofactor CBP/p300, being therefore important in order to establish gene activation (Saccani et al., 2002; Vermeulen et al., 2002, 2003). The phosphorylation of p65, which is required for optimal NF-κB dependent gene transcription (Schmitz et al., 2001), appears to be an important target of PFC-induced immunotoxicity. Figure 1 depicts the complexity associated with PFC-induced immunomodulation. The toxicity and immunomodulation induced by exposure to PFCs may occur via multiple pathways, and the relative contributions of these pathways and the role of PPARα likely differ among PFCs and animal species and/or strains.
Figure 1.
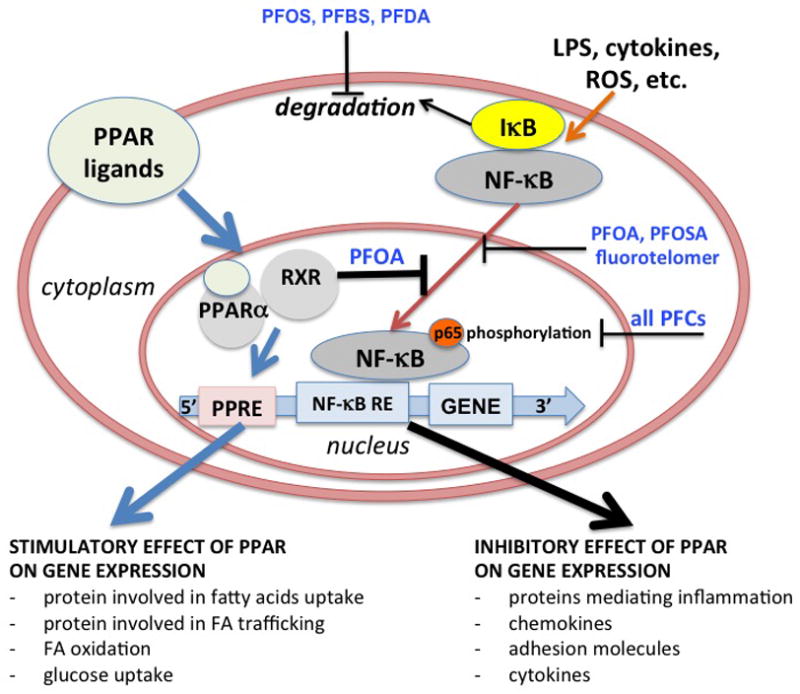
complexity associated with PFC-induced immunomodulation. The toxicity and immunomodulation induced by exposure to PFCs may occur via multiple pathways, and the relative contributions of these pathways and the role of PPARα likely differ among PFCs and animal species. In human leukocytes, PFCs directly inhibit LPS-induced cytokine production. Both inflammatory cytokines as well as adaptive cytokines were affected by PFCs, with PFOA being the least effective. A role of PPARα in PFOA-induced inhibition of cytokine secretion in human cells exposed in vitro was demonstrated. In contrast, the inhibitory effect of PFCs on in vitro cytokine production by human leukocytes occurred independently of PPARα, and involves the inhibition of NF-kB activation. In particular, PFOS, PFBS and PFDA act upstream by inhibiting I-kB degradation, PFOA, PFOSA and fluorotelomer do not interfere with LPS-induced I-kB degradation, suggesting a downstream effect. All PFCs tested inhibited the phosphorylation of p65 at Ser536, which is required for optimal NF-kB dependent gene transcription and can explain the defective cytokine production following PFCs treatment (Corsini et al., 2012). PFBS, perfluorobutane sulfonic acid; PFDA, perfluorodecanoic acid; Fluorotelomer, 1-Decanol, 3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-(8:2 Telomer), RE, responsive elements.
6. Risk of immunotoxicity
Regulatory interest in PFCs recently has increased because of their ubiquitous presence in the environment, biological and environmental persistence, and reported experimental animal toxicities. In Europe, the European Food Safety Authority established an acceptable daily intake (ADI) for mammals of 0.0015 mg/kg bw/day for PFOA and 0.00015 mg/kg bw/day for PFOS (EFSA, 2012).
Regarding immunotoxicity, the weight of evidence supports the conclusion that antibody production is an endpoint sensitive to modulation by PFCs both in animals and humans. In mice, the LOAL for suppression of antibody production for PFOA was 3.75 mg/kg/d (corresponding to a serum concentration of 7.4×104 ng/ml) when given to female C57BL/6 mice by drinking water for 15 days (DeWitt et al. 2008) and 0.00166 mg/kg/day (corresponding to a serum concentration of 91.5 ng/ml) in male B6C3F1 mice given PFOS by oral gavage for 28 days (Peden-Adams et al. 2008). The margin of exposure (MOE), which is the ratio of the NOAEL (no observed adverse effect level) to expected human exposure, calculated from these studies in relation to the general human population serum PFC concentrations is approximately 15,000 for PFOA and 0.5 to 10.8 for PFOS. For occupational exposures, the calculated MOEs are 12 for highly exposed PFOA workers and approximately 0.005 for workers highly exposed to PFOS. Although MOEs are not values of risks themselves, when an MOE is less than 100, it is suggestive of potential health risks to exposed populations (Faustman and Omenn 2001). The serum levels associated with PFOA-induced immune effects are higher than the concentrations reported for humans and wildlife, whereas for PFOS, some human and wildlife species have serum concentrations that exceed the threshold for PFOS-induced immune effects, suggesting a potential risk for altered disease resistance.
Very recently, Grandjean et al. (2013) used the results obtained from the Faroese birth cohort study to establish a benchmark dose using linear and logarithmic curves. Calculations relied on serum-PFC measurements at age 5 years, and serum concentrations of specific antibodies two years later as clinically relevant measures of immune functions. The associations that appeared the strongest were selected for the benchmark dose calculations. Benchmark dose levels were about 1.3 ng/ml serum for PFOS and 0.3 ng/ml for PFOA at a benchmark dose response of 5%, and 0.8 and 5 ng/ml at a benchmark dose response of 10%. These results are below the range of average serum concentrations reported for general population. Using animal data, calculation of benchmark dose levels resulted in serum concentrations of 23 μg/ml and 35 μg/ml for PFOA and PFOS, respectively (Butenhoff et al., 2004). Based on animal data, US EPA has issued provisional health advisories of 400 ng/l for PFOA and 200 ng/l for PFOS in drinking water (US EPA, 2009). A lower guidance limit of 40 ng/l has been determined for PFOA by the state of New Jersey (Post et al., 2009). The authors concluded that if results are converted to approximate exposure limits for drinking water, current limits appear to be several hundred folds too high, and should be reconsidered.
7. Conclusions
The body of evidence concerning the ability of PFCs to modulate the immune system has grown in the last decade, and several studies have reported serum concentrations in rodents at immune effect levels within the range of human and wildlife exposures, suggesting risk of immunosuppression in humans, especially in children following pre-natal exposure, and in wildlife (DeWitt et al., 2012). Dysregulation(s) of immune system homeostasis can lead to adverse changes in immune functions, increasing the susceptibility to infections and cancer, as well as favoring the development of autoimmune diseases.
Our understanding of the mechanisms underlying the immune effects of PFCs is incomplete, as is our knowledge of how chemical structure and PPAR activation impact immune system toxicity. Because humans are exposed to both endogenous and exogenous PPAR ligands, the total ligand contribution on receptor occupancy and activities and subsequent influence on physiological processes deserves more attention.
While the phase-out of PFOA and PFOS resulted in a decrease in blood concentrations of these compounds in humans in the last decade (Kato et al, 2011), the concentration of other PFCs in humans has, however, increased in the same time period. Further studies are warranted, particularly studies that include biomarkers of immune system function and different PFCs in current and/or increasing use.
Footnotes
Disclaimers:
This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
This report has been reviewed by the Environmental Protection Agency’s Office of Research and Development, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Conflict of Interest
For the past 3 years since the beginning of this work, all Authors declare of not having any financial, personal, or association with any individuals or organizations that could have inappropriately influenced the submitted work.
References
- Alexander BH. Mortality Study of Workers Employed at the 3M Cottage Grove Facility. St. Paul, MN: 3M Company; Washington DC: U.S. EP A Public Docket; 2001. AR-226-1030a018. [Google Scholar]
- Alexander BH, Olsen GW. Bladder cancer in perfluorooctanesulfonyl fluoride manufacturing workers. Ann Epidemiol. 2007;17:471–78. doi: 10.1016/j.annepidem.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Biegel LB, Hurtt ME, Frame SR, O’Connor JC, Cook JC. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci. 2001;60:44–55. doi: 10.1093/toxsci/60.1.44. [DOI] [PubMed] [Google Scholar]
- Brieger A, Bienefeld N, Hasan R, Goerlich R, Haase H. Impact of perfluorooctanesulfonate and perfluorooctanoic acid on human peripheral leukocytes. Toxicol In Vitro. 2011;25:960–8. doi: 10.1016/j.tiv.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Gaylor DW, Moore JA, Olsen GW, Rodricks J, Mandel JH, Zobel LR. Characterization of risk for general population exposure to perfluorooctanoate. Regul Toxicol Pharmacol. 2004;39:363–380. doi: 10.1016/j.yrtph.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Cain L, Shankar A, Ducatman AM, Steenland K. The relationship between serum uric acid and chronic kidney disease among Appalachian adults. Nephrol Dial Transplant. 2010;25(11):3593–3599. doi: 10.1093/ndt/gfq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Silva MJ, Kuklenyik Z, Needham LL. Human exposure assessment to environmental chemicals using biomonitoring. Int J Androl. 2006;29:166–71. doi: 10.1111/j.1365-2605.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the US population: data from the National Health and Nutrition examination survay. Environ Sci Technol. 2007;41:2237–42. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- Cheung C, Akiyama TE, Ward JM, Nicol CJ, Feigenbaum L, Vinson C, Gonzalez FJ. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor-a. Cancer Res. 2004;64:3849–54. doi: 10.1158/0008-5472.CAN-04-0322. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Corsini E, Avogadro A, Galbiati V, dell’Agli M, Marinovich M, Galli CL, Germolec DR. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs) Toxicol Appl Pharmacol. 2011;250:108–16. doi: 10.1016/j.taap.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Corsini E, Sangiovanni E, Avogadro A, Galbiati V, Viviani B, Marinovich M, Galli CL, Dell’Agli M, Germolec DR. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs) Toxicol Appl Pharmacol. 2012;258:248–55. doi: 10.1016/j.taap.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Costa G, Sartori S, Consonni D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med. 2009;51:364–72. doi: 10.1097/JOM.0b013e3181965d80. [DOI] [PubMed] [Google Scholar]
- Davis KL, Aucoin MD, Larsen BS, Kaiser MA, Hartten AS. Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere. 2007;67:2011–2019. doi: 10.1016/j.chemosphere.2006.11.049. [DOI] [PubMed] [Google Scholar]
- Dewitt JC, Copeland CB, Strynar MJ, Luebke RW. Perfluorooctanoic acid-induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ Health Perspect. 2008;116:644–650. doi: 10.1289/ehp.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, Cunard R, Anderson SE, Meade BJ, Peden-Adams MM, Lubcke RW, Luster MI. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol. 2009;39:76–94. doi: 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Copeland CB, Luebke RW. Suppression of humoral immunity by perfluorooctanoic acid is independent of elevation serum corticosterone concentration in mice. Toxicol Sci. 2009a;109:106–12. doi: 10.1093/toxsci/kfp040. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, Cunard R, Anderson SE, Meade BJ, Peden-Adams MM, Luebke RW, Luster MI. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol. 2009b;39:76–94. doi: 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicologic Pathology. 2012;40:300–11. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- Dietert RR. Developmental immunotoxicology (DIT): windows of vulnerability, immune dysfunction and safety assessment. J Immunotoxicol. 2008;5:401–12. doi: 10.1080/15476910802483324. [DOI] [PubMed] [Google Scholar]
- Domingo JL. Health risks of dietary exposure to perfluorinated compounds. Environ Int. 2012;40:187–195. doi: 10.1016/j.envint.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Dong GH, Liu MM, Wang D, Zheng L, Liang ZF, Jin YH. Sub-chronic effect of perfluorooctanesulfonate (PFOS) on the balance of type 1 and type 2 cytokine in adult C57BL6 mice. Arch Toxicol. 2011;85:1235–44. doi: 10.1007/s00204-011-0661-x. [DOI] [PubMed] [Google Scholar]
- EFSA. Perfluorinated substances in food: occurrence and dietary exposure. The EFSA Journal. 2012;10:2743. [Google Scholar]
- Emmett EA, Zhang H, Shofer FS, Freeman D, Rodway NV, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum levels and certain health parameters. J Occup Environ Med. 2006;48:771–9. doi: 10.1097/01.jom.0000233380.13087.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen KT, Sørensen M, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, Raaschou-Nielsen O. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J Natl Cancer Inst. 2009 Apr 15;101(8):605–9. doi: 10.1093/jnci/djp041. [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, Sørensen M. Association between Plasma PFOA and PFOS Levels and Total Cholesterol in a Middle-Aged Danish Population. PLoS ONE. 2013;8:e56969. doi: 10.1371/journal.pone.0056969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair PA, Driscoll E, Mollenhauer MA, Bradshaw SG, Yun SH, Kannan K, Bossart GD, Keil DE, Peden-Adams MM. Effects of environmentally-relevant levels of perfluorooctane sulfonate on clinical parameters and immunological functions in B6C3F1 mice. J Immunotoxicol. 2011;8:17–29. doi: 10.3109/1547691X.2010.527868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Feng Y, Shi Z, Dai J. Alterations of cytokines and MAPK signaling pathways are related to the immunotoxic effect of perfluorononanoic acid. Toxicol Sci. 2009;108:367–76. doi: 10.1093/toxsci/kfp019. [DOI] [PubMed] [Google Scholar]
- Faustman EM, Omenn GS. Risk assessment. In: Klaassen CD, editor. Casarett and Doull’s Toxicology: The Basic Science of Poisons. New York: McGraw-Hill; 2001. pp. 83–104. [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to PFOA and PFOS and risk of hospitalization for infections diseases in early childhood. Environ Res. 2012;110:773–7. doi: 10.1016/j.envres.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Fletcher T, Steenland K, Savitz D. Status Report: PFOA and immune biomarkers in adults exposed to PFOA in drinking water in the mid Ohio valley. 2009 Available at: http://www.c8sciencepanel.org/study_results.html.
- Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D. Perfluorinated compounds-exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212:239–70. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Germolec DE. Sensitivity and predictivity in immunotoxicity testing: Immune endpoints and disease resistance. Toxicol Lett. 2004;149:109–14. doi: 10.1016/j.toxlet.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Mandel JS. Mortality among employees of a perfluorooctanoic acid production plant. J Occup Med. 1993;35:950–954. doi: 10.1097/00043764-199309000-00020. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Peters JM, Cattley RC. Mechanism of action of the non-genotoxic peroxisome proliferators: role of the peroxisome proliferator activator receptor alpha. J Natl Cancer Inst. 1998;90:1702–1709. doi: 10.1093/jnci/90.22.1702. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Wreford Andersen E, Budtz-Jorgensen E, Nielsen F, Molbak K, Wheihe P, Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–97. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, Stolevik SB, Thomsen C, Aagerge IS, van Loveren H, Lovik M, Nygaard UC. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicology. doi: 10.3109/1547691X.2012.755580. early online 2013. [DOI] [PubMed] [Google Scholar]
- Guruge KS, Hikono H, Shimada N, Murakami K, Hasegawa J, Yeung LW, Yamanaka N, Yamashita N. Effect of perfluorooctane sulfonate (PFOS) on influenza A virus-induced mortality in female B6C3F1 mice. J Toxicol Sci. 2009;34:687–91. doi: 10.2131/jts.34.687. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Becher G, Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds--comparing exposure estimates with biomarkers of exposure. Environ Int. 2011;37:687–93. doi: 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Paustenbach DJ, Chatnley G, West LJ, Luster MI, Dietert RR, Burns-Naas LA. Symposium summary: children’s health risk-what’s so special about the developing immune system? Toxicol Appl Pharmacol. 2004;199:61–70. doi: 10.1016/j.taap.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Kato K, Calafat AM, Wong LY, Wanigatunga AA, Caudill SP, Needham LL. Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001–2002. Environ Sci Technol. 2009;43:2641–7. doi: 10.1021/es803156p. [DOI] [PubMed] [Google Scholar]
- Kennedy GL, Jr, Butenhoff JL, Olsen, O’Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34:351–84. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–94. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lefebvre DE, Curran I, Armstrong C, Coady L, Parenteau M, Liston V, Barker M, Aziz S, Rutherford K, Bellon-Gagnon P, Shenton J, Mehta R, Bondy G. Immunomodulatory effects of dietary potassium perfluorooctane sulfonate (PFOS) exposure in adult Sprague- Dawley rats. J Toxicol Environ Health A. 2008;71:1516–25. doi: 10.1080/15287390802391943. [DOI] [PubMed] [Google Scholar]
- Leonard RC, Kreckmann KH, Sakr CJ, Symons JM. Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol. 2008;18:15–22. doi: 10.1016/j.annepidem.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Liu RC, Hahn C, Hurtt ME. The direct effect of hepatic peroxisome proliferators on rat Leydig cell function in vitro. Fundam Appl Toxicol. 1996a;30:102–8. doi: 10.1006/faat.1996.0047. [DOI] [PubMed] [Google Scholar]
- Liu RC, Hurtt ME, Cook JC, Biegel LB. Effect of the peroxisome proliferator, ammonium perfluorooctanoate (C8), on hepatic aromatase activity in adult male Crl:CDBR (CD) rats. Fundam Appl Toxicol. 1996b;30:220–8. doi: 10.1006/faat.1996.0059. [DOI] [PubMed] [Google Scholar]
- Loveless SE, Hoban D, Sykes G, Frame SR, Everds NE. Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate (APFO) Toxicol Sci. 2008;105:86–96. doi: 10.1093/toxsci/kfn113. [DOI] [PubMed] [Google Scholar]
- Lundin JI, Alexander BH, Olsen GW, Church TR. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology. 2009 Nov;20(6):921–8. doi: 10.1097/EDE.0b013e3181b5f395. [DOI] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, White KL, Jr, Gennings C, Munson AE, Rosenthal GJ. Risk assessment in immunotoxicology: I. Sensitivity and predictability of immune tests. Fund Appl Toxicol. 1992;18:200–10. doi: 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- Luster MI, Johnson VJ, Yucesoy B, Simeonova PP. Biomarkers to assess potential developmental immunotoxicity in children. Toxicol Appl Pharmacol. 2005;206:229–36. doi: 10.1016/j.taap.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, Rosenthal GJ, Germolec DR, Corsini E, Blaylock BL, Pollock P, Kouchi Y, Craig W, White KL, Munson AE, Comment CE. Risk assessment in immunotoxicology. II Relationships between immune and host resistance tests. Fundam Appl Toxicol. 1993;21:71–82. doi: 10.1006/faat.1993.1074. [DOI] [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DG, Jr, Sjödin A, Turner WE, Weihe P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45:1121–6. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada E, Sasaki S, Saijo Y, Washino N, Miyashita C, Kobayashi S, Konishi K, Ito YM, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H, Kishi R. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ Res. 2012 Jan;112:118–25. doi: 10.1016/j.envres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Mandel JH, Zobel LR. Serum perfluorooctane sulfonate and hepatic and lipid clinical chemistry tests in fluorochemical production employees. J Occup Environ Med. 1999;41:799–806. doi: 10.1097/00043764-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CN, Hsu MH, Griffin KJ, Raucy JL, Johnson EF. Peroxisome proliferator activated receptor-alpha expression in human liver. Mol Pharmacol. 1998;53:14–22. [PubMed] [Google Scholar]
- Pastoor TP, Lee KP, Perri MA, Gillies PJ. Biochemical and morphological studies of ammonium perfluorooctanoate-induced hepatomegaly and peroxisome proliferation. Exp Mol Pathol. 1987;47:98–109. doi: 10.1016/0014-4800(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Peden-Adams MM, Keil DE, Romano T, Mollenhauer MAM, Fort DJ, Guiney PD, Houde M, Kannan K, Muir DC, Rice CD, Stuckey J, Segars AL, Scott T, Talent L, Bossart GD, Fair PA, Keller JM. Health effects of perfluorinated compounds—what are the wildlife telling us? Repro Toxicol. 2009;27:414–15. [Google Scholar]
- Peden-Adams MM, EuDaly JG, Dabra S, EuDaly A, Heesemann L, Smythe J, Keil DE. Suppression of humoral immunity following exposure to the perfluorinated insecticide sulfluramid. J Toxicol Environ Health (A) 2007;70:1130–41. doi: 10.1080/15287390701252733. [DOI] [PubMed] [Google Scholar]
- Peden-Adams MM, Keller JM, Eudaly JG, Berger J, Gilkeson GS, Keil DE. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol Sci. 2008;104:144–154. doi: 10.1093/toxsci/kfn059. [DOI] [PubMed] [Google Scholar]
- Peden-Adams MM, Mollenhauer M, Morse M, Wills L, Keil DE. Effects of perfluorooctanesulfonic acid (PFOS) on NF-kB, AP-1, and PPAR-alpha. The Toxicologist-An Official Journal of the Society of Toxicology. 2010;114:330. [Google Scholar]
- Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicol Sci. 2006;90:269–95. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]
- Post GB, Louis JB, Cooper KR, Boros-Russo BJ, Lippincott RL. Occurrence and potential significance of perfluorooctanoid acid (PFOA) detected in New Jersey public drinking water systems. Environ Sci Technol. 2009;43:4547–54. doi: 10.1021/es900301s. [DOI] [PubMed] [Google Scholar]
- Qazi MR, Nelson BD, Depierre JW, Abedi-Valugerdi M. 28-Day dietary exposure of mice to a low total dose (7 mg/kg) of perfluor- ooctanesulfonate (PFOS) alters neither the cellular compositions of the thymus and spleen nor humoral immune responses: does the route of administration play a pivotal role in PFOS-induced immunotoxicity? Toxicology. 2010;267:132–9. doi: 10.1016/j.tox.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Rayne S, Forest K. Perfluoroalkyl sulfonic and carboxylic acids: a critical review of physicochemical properties, levels and patterns in waters and wastewaters, and treatment methods. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2009;44:1145–99. doi: 10.1080/10934520903139811. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Bacher S, Kracht M. I kappa B-independent control of NF-kappa B activity by modulatory phosphorylations. Trends Biochem Sci. 2001;26:186–190. doi: 10.1016/s0968-0004(00)01753-9. [DOI] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA) Environ Health Perspect. 2010;118:1100–8. doi: 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suja F, Pramanik BK, Zain SM. Contamination, bioaccumulation and toxic effects of perfluorinated chemicals (PFCs) in the water environment: a review paper. Water Sci Technol. 2009;60:1533–44. doi: 10.2166/wst.2009.504. [DOI] [PubMed] [Google Scholar]
- Takacs ML, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci. 2007;95:108–117. doi: 10.1093/toxsci/kfl135. [DOI] [PubMed] [Google Scholar]
- Ubel FA, Sorenson SD, Roach DE. Health status of plant workers exposed to fluorochemicals-a preliminary report. Am Ind Hyg Assoc J. 1980;41:584–9. doi: 10.1080/15298668091425310. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol. 2002;64:963–70. doi: 10.1016/s0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–24. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loveren H, Germolec D, Koren HS, Luster MI, Nolan C, Repetto R, Smith E, Vos JG, Vogt RF. Report of the Bilthoven Symposium: advancement of epidemiological studies in assessing the human health effects of immunotoxic agents in the environment and the workplace. Biomarkers. 1999;4:135–57. doi: 10.1080/135475099230949. [DOI] [PubMed] [Google Scholar]
- Vestergren R, Cousins IT. Tracking the pathways of human exposure to perfluorocarboxylates. Environ Sci Technol. 2009;43:5565–75. doi: 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Perfluorooctanoic acid (PFOA) and fluorinated telomers. 2010 www.epa.gov/opptintr/pfoa.
- Yang Q, Xie Y, Depierre JW. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin Exp Immunol. 2000;122:219–66. doi: 10.1046/j.1365-2249.2000.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Xie Y, Eriksson AM, Nelson BD, DePierre JW. Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluoroctanoic acid in mice. Biochem Pharm. 2001;62:1133–40. doi: 10.1016/s0006-2952(01)00752-3. [DOI] [PubMed] [Google Scholar]
- Yang Q, Xie Y, Alexson SEH, Nelson BD, DePierre JW. Involvement of the peroxisome proliferators- activated receptor-α in the immodulation caused by peroxisome proliferators in mice. Biochem Pharm. 2002a;63:1893–1900. doi: 10.1016/s0006-2952(02)00923-1. [DOI] [PubMed] [Google Scholar]
- Yang Q, Abedi-Valugerdi M, Xie Y, Zhao X, Möller G, Nelson BD, DePierre JW. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferators perfluorooctanoic acid. Int Immunopharm. 2002b;2:389–97. doi: 10.1016/s1567-5769(01)00164-3. [DOI] [PubMed] [Google Scholar]
- Wang IJ, Hsieh WS, Chen CY, Fletcher T, Lien GW, Chiang HL, Chiang CF, Wu TN, Chen PC. The effect of prenatal perfluorinated chemicals exposures on pediatric atopy. Environ Res. 2011;111:785–91. doi: 10.1016/j.envres.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Weihe P, Kato K, Calafat AM, Nielsen F, Wanigatunga AA, Needham LL, Grandjean P. Serum concentrations of polyfluoroalkyl compounds in Faroese whale meat consumers. Environ Sci Technol. 2008;42:6219–95. doi: 10.1021/es800695m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Guidance for immunotoxicity risk assessment for chemicals. Geneva: World Health Organization; 2012. IPCS harmonization project document; No 10. [Google Scholar]
- Zareitalabad P, Siemens J, Hamer M, Amelung W. Perfluorooctanoic acid (PFOA) and perfluoroocanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater - a reveiw on concentrations and distributions coefficients. Chemosphere. 2013;91:725–32. doi: 10.1016/j.chemosphere.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Zheng L, Dong GH, Zhang YH, Liang ZF, Jin YH, He QC. Type 1 and Type 2 cytokines imbalance in adult male C57BL/6 mice following a 7-day oral exposure to perfluorooctanesulfonate (PFOS) J Immunotoxicol. 2011;8:30–8. doi: 10.3109/1547691X.2010.537287. [DOI] [PubMed] [Google Scholar]


