Abstract
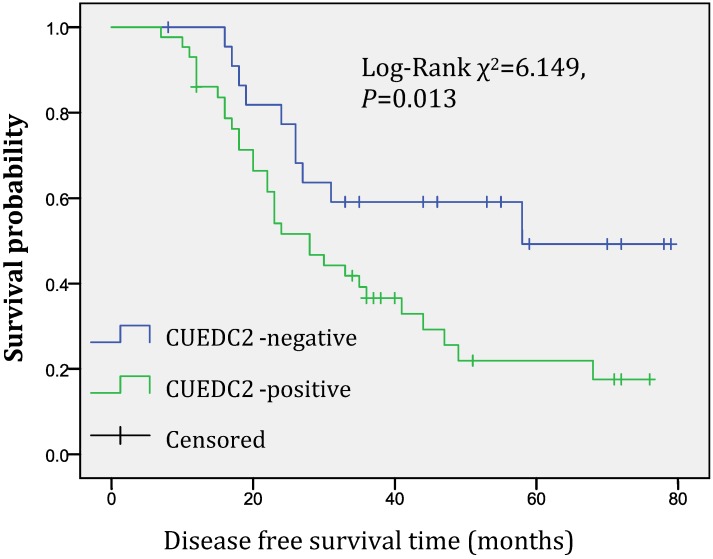
CUEDC2, a newly reported protein, plays critical roles in many biological processes, such as cell cycle, inflammation and tumorigenesis, however, its expression in ovarian serious carcinoma is still poorly understood. In this study, we performed an immunohistochemical study on 101 cases of ovarian serous carcinoma tissues to investigate whether CUEDC2 is a useful biomarker to evaluate the progression of ovarian serous carcinomas. The data showed that the overexpression of CUEDC2 was observed in 59.4% of ovarian serous carcinoma tissue samples and correlated with histopathological grade, patient age at diagnosis, FIGO stage and recurrence. To assess the clinical relevance of CUEDC2, we analyzed the survival follow-up information, the results showed that CUEDC2-positive expression was associated with a shorter disease-free survival time, the median disease-free survival time of CUEDC2-positive patients was 36.0 months compared with 53.9 months of CUEDC2-negative ones (Log-rank χ2=6.149, P=0.013). Collectively, our results suggested that CUEDC2 may be a promising biomarker to evaluate the progression of serous ovarian carcinoma and to predict likely relapse of ovarian serous carcinoma.
Keywords: ovarian serous carcinoma, CUEDC2, recurrence, prognosis
INTRODUCTION
Ovarian carcinoma is the most lethal gynecologic malignancy and leading the mortality with 140,000 deaths annually worldwide 1-2. Ovarian serous carcinoma is the most common subtype of epithelial ovarian cancer which accounts for about 45% of all ovarian cancers 3. The high mortality rate for ovarian serous carcinoma largely remains undetectable in early stage and lack of reliable biomarkers to evaluate its prognostic progression 4-7. Although several prognostic biomarkers have been evaluated, there are still insufficient methods to identify high-risk patients 8-10. Therefore, a challenge for ovarian serous carcinoma research remains identification of tumor biomarkers to aid in predicting early recurrence and metastasis.
CUEDC2, a CUE domain-containing protein, is a small and moderately conserved ubiquitin-binding domain of about 40 amino acids. CUEDC2 has been found in a variety of eukaryotic proteins and plays critical roles in many biological processes, such as cell cycle, inflammation and tumor genesis. It interacts with inhibitor of IkB kinase α (IKKα) and IKKβ, and regulates IKK phosphorylation and activation 11-14. Its overexpression causes earlier activation of anaphase-promoting complex/cyclostome (APC/C), leading to chromosome missegregation and aneuploidy which might contribute to tumor development 15-16.
In this study, we performed an immunohistochemical study on 101 cases of ovarian serious carcinoma tissues. The aims of this study were (1) to evaluate the association between CUEDC2 expression and clinicalpathological factors, such as histopathological grade, patient age at diagnosis, FIGO stage, recurrence and lymph node metastasis, and (2) to investigate the association between CUEDC2 expression in ovarian serous carcinoma and the prognosis of patients who underwent curative laparotomy.
MATERIALS AND METHODS
Patients selection and categorization
For this retrospective cohort study, tumor tissues were collected from 101 patients with primary serous ovarian carcinoma who underwent surgery during January 2007 to December 2010 at the Chinese People's Liberation Army General Hospital. Patients were excluded if 1) undergoing neoadjuvant chemotherapy before the surgical operation, 2) tumor tissue paraffin blocks were unavailable or inadequate, or 3) with other synchronous or metastatic tumors to the ovary. Tissue specimens were formalin fixed and paraffin embedded (FFPE).
Histological classification was performed according to a two-tier system for grading ovarian serous carcinoma. This system based primarily on the assessment of nuclear atypia with the mitotic rate used as a secondary feature. The low-grade ovarian serous carcinoma (LGSC) was characterized by the presence of mild to moderate nuclear atypia. As a secondary feature, they tended to show up to 12 mitoses per 10 high power fields (HPFs), whereas the high-grade category (HGSC) had marked nuclear atypia and as a secondary feature more than 12 mitoses per 10 HPF 17-18. Stage was divided into early stage (FIGO stage I or Ⅱ) and advanced stage (FIGO stage Ⅲ or Ⅳ).
Immunohistochemistry
Sections from FFPE tissues were used for immunohistochemical staining according to a standard method. Briefly, each 4-μm tissue section was deparaffinized with xylene and rehydrated. After rehydration through a graded ethanol series, the sections were autoclaved in 10 mM citrate buffer (pH 6.0) at 120°C for 2.5 min for antigen retrieval, then cooled to 30°C and washed with phosphate buffered saline (PBS, pH 7.3). After endogenous peroxidase had been quenched with aqueous 3% H2O2 for 10 minutes and washed with PBS, the sections were incubated at 4°C overnight with CUEDC2 antibody (donated by Professor Xuemin Zhang, State Key Laboratory of Proteomics, Institute of Basic Medical Science, Chinese National Center of Biomedical Analysis) at a 1:200 dilution in antibody diluent solution and then washed with PBS. Next, the sections were incubated with secondary antibody (Fuzhou Maxim Biotechnology Development Co., China) for 30 min at room temperature. Color development was performed with Polink-2 HRP DAB Detection kit. Nuclei were lightly counterstained with hematoxylin. Two pathologists independently assessed the immunostained slides. Any difference in immunohistochemical scores was resolved by a consensus. Quality assessment was performed on each batch of slides by including negative control (the primary antibody was replaced by PBS) to preclude nonspecific signal and breast carcinoma tissues, known to express high levels of CUEDC2 protein, as positive control.
Evaluation and interpretation of IHC
All of staining was assessed by pathologists blinded to the origination of the samples and subject outcome. The widely accepted German semi-quantitative scoring system in considering the staining intensity and area extent was used. Each specimen was assigned a score according to the intensity of cytoplasmic staining (no staining = 0; weak staining = 1, moderate staining = 2, strong staining = 3) and the extent of stained cells (0% = 0, 1-24% = 1, 25-49% = 2, 50-74% = 3, 75-100% = 4). The final immunoreactive score was determined by multiplying the intensity score with the extent of score of stained cells, ranging from 0 (the minimum score) to 12 (the maximum score). We defined 0 score as negative, 1-6 score as weak positive expression and ≥8 score as strong positive expression, respectively.
Statistical analysis
The correlation between CUEDC2 expression and patient age, histopathological grade, clinical stage and lymph node metastasis was analyzed by chi-square test. We defined disease-free survival (DFS) as the time from the date of diagnosis to the first recurrence (local or distant), and overall survival (OS) as the time from the date of diagnosis to the patient's death from ovarian serous carcinoma. Patients who were alive at the last follow-up were censored at the last follow-up date, and patients who died from causes other than ovarian serous carcinoma were censored at the time of death. Survival curves were constructed with the Kaplan-Meier product-limit method and compared by the log-rank test. To evaluate the independent prognostic factors associated with survival, multivariate Cox proportional hazards regression analysis was used. We performed the statistical analyses using SPSS 17.0 software. All statistical tests were two-sided and P values < 0.05 were considered to be statistically significant.
Ethical Review
The study was approved by the PLA General Hospital Ethics Board. Informed consents were obtained from all patients or their relatives.
RESULTS
Clinicopathological characterization
The mean age of 101 cases at the time of diagnosis was 51.6 years old (range 19-74 y). Forty six cases (45.5%) demonstrated lymph node metastasis after lymph node sampling or dissection. Seventy three cases were categorized into HGSC and 28 cases were LGSC. Twenty four out of 101 cases were in early stage (10 cases for stage I and 14 cases for Ⅱ) and 77 cases in advanced stage (62 cases for Ⅲ and 15 cases for Ⅳ).
A total of 74 cases had followed up records and were therefore included in data analysis. The mean DFS time of 74 cases was 34.5 months. Eight cases had failure of the treatment due to persistent local lesion or recurred within 6 months and 41cases recurred (40.6%) in 7 to 68 months after the operation with a median DFS of 25.4 months. The mean OS time of 74 cases was 41.7 months. Twenty two cases (21.8%) had died of this disease in 3 to 69 months after the operation.
CUEDC2 expression in ovarian serous carcinoma
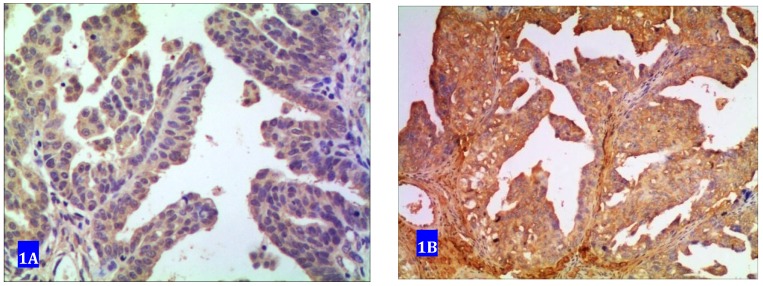
Forty one samples showed no expression of CUEDC2. Sixty samples exhibited positive immunostaining among which 33 samples were determined as weak positive with cytoplasm in light yellow color and 27 were strong positive with yellow or dark yellow as shown in Fig 1.
Fig 1.
CUEDC2 expression in ovarian serous carcinoma. A weakly immunostained with cytoplasm in light yellow color. B strongly immunostained with cytoplasm in yellow or brown color.
Expression of CUEDC2 was associated with age at diagnosis, histopathological grade, FIGO stage and recurrence
The expression of CUEDC2 in different histopathological grade subgroups were highly correlated. Almost seventy percent (69.9%) samples were positive in HGSC subgroup compared with 32.1% of the LGSC subgroup (P=0.001). More samples with advanced FIGO stage (66.2%) exhibited positive expression than early FIGO stage (37.5%) (P=0.012). Recurrence, age at diagnosis ≥50 y were observed more frequently in CUEDC2-positive subgroup compared with negative subgroup (75.6% versus 48.0%, P=0.022; and 67.8% versus 47.6%, P=0.042; respectively). In addition, the positive expression rate of the cases with lymph node metastasis (65.2%) was higher than cases without lymph node metastasis (54.5%), but no statistically significant difference (P=0.277) as shown in table 1.
Table 1.
Correlation of clinicopathological variables with CUEDC2 expression in patients with serous ovarian cancer
| Variable | n | CUEDC2 expression | χ2 | P Value | |
|---|---|---|---|---|---|
| -ive (n=41) | +ive (n=60) | ||||
| Age | |||||
| <50 | 42 | 22 | 20 | 4.142 | 0.042 |
| ≥50 | 59 | 19 | 40 | ||
| Grading | |||||
| LGSC | 28 | 19 | 9 | 11.940 | 0.001 |
| HGSC | 73 | 22 | 51 | ||
| FIGO stage | |||||
| Ⅰ-Ⅱ | 24 | 15 | 9 | 6.264 | 0.012 |
| Ⅲ-Ⅳ | 77 | 26 | 51 | ||
| LN* metastasis | |||||
| Yes | 46 | 16 | 30 | 1.183 | 0.277 |
| No | 55 | 25 | 30 | ||
| Recurrence** | |||||
| Yes | 41 | 10 | 31 | 5.214 | 0.022 |
| No | 25 | 13 | 12 | ||
* LN: lymph node; ** detailed follow up information of 66 cases was available
Univariate and multivariate analysis of CUEDC2 expression for survival time of patients with ovarian serous carcinomas
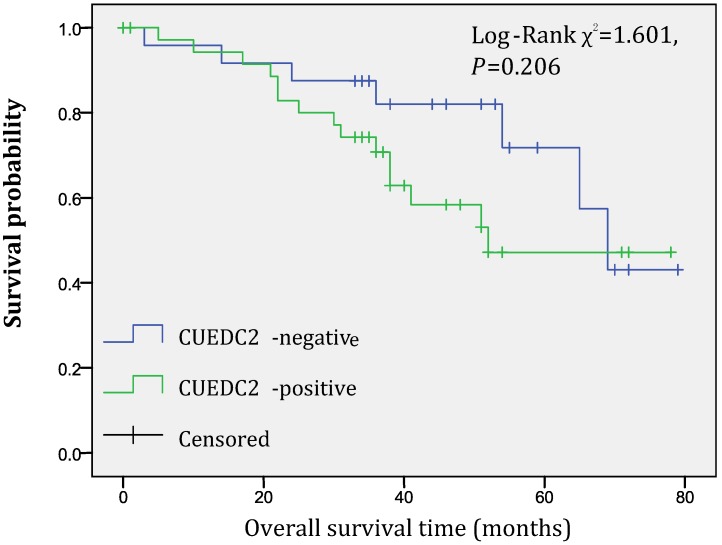
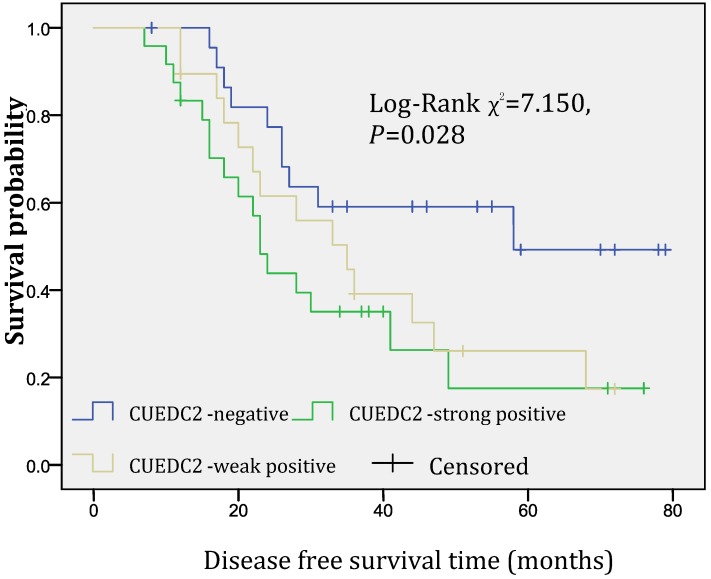
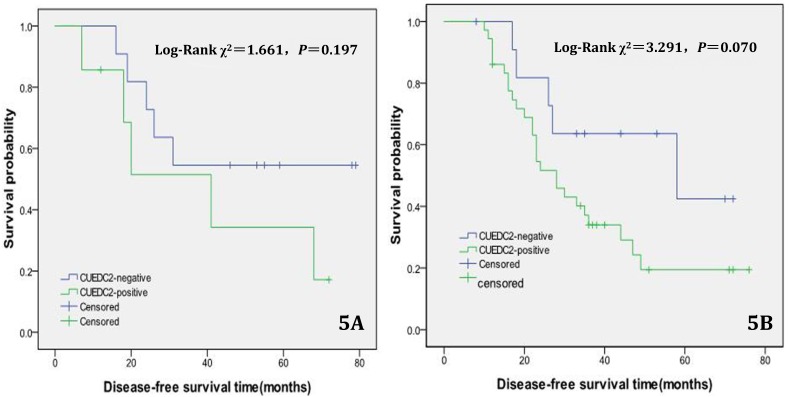
In univariate survival analysis, CUEDC2 expression, FIGO stage, and age at diagnosis reached significance for DFS time; meanwhile, histopathological grade, FIGO stage, and age at diagnosis reached significance for OS time (Table 2). Although CUEDC2-positive expression was not related to OS time (Fig 2, P=0.206), it is associated with a shorter DFS time (Fig 3, P=0.013). Moreover, a further analysis was carried out, the results showed that weak CUEDC2 expression was associated with a poor prognosis compared with negative CUEDC2 expression (P=0.010), whereas there was no difference in survival between the “strong” and “weak” expression groups (Fig 4). In addition, we analyzed the relationship of CUEDC2 expression and the prognosis in LGSC and HGSC group respectively. The results showed that the mean DFS time of CUEDC2-positive cases was 34.0 months versus 44.2 months of CUEDC2-negative cases in LGSC subgroup; correspondingly, the data was 29.4 months versus 38.4 months in the HGSC subgroup. Survival curve showed that the CUEDC2-positive cases had shorter DFS time tendency compared to CUEDC2-negative cases, although there were no statistically significant difference (P=0.197 and P=0.070, Fig 5). Multivariate survival analysis based on the Cox proportional hazards regression model was performed on all parameters. The result of this analysis was shown in table 3. It can be seen that the FIGO stage was independent predictor of a shorter DFS time for patients undergoing curative resection (P=0.000) and CUEDC2 was a potential predictor.
Table 2.
Univariate survival analysis of prognostic factors and CUEDC2 expression in patients with serous ovarian cancer
| Variable | Median DFS time(months) | P value | Median OS time(months) | P value |
|---|---|---|---|---|
| Cuedc2 | ||||
| negative | 53.9 | 0.013 | 62.5 | 0.206 |
| positive | 36.0 | 53.9 | ||
| age | ||||
| <50y | 50.2 | 0.006 | 66.9 | 0.031 |
| ≥50y | 34.1 | 51.6 | ||
| grading | ||||
| Low-grade | 45.6 | 0.329 | 72.6 | 0.010 |
| High-grade | 37.9 | 52.6 | ||
| FIGO stage | ||||
| Ⅰ-Ⅱ | 60.9 | 0.000 | 72.4 | 0.004 |
| Ⅲ-Ⅳ | 33.7 | 51.6 | ||
| Lymph nodes metastasis | ||||
| Yes | 36.6 | 0.210 | 52.8 | 0.309 |
| No | 42.6 | 58.9 |
Fig 2.
Overall survival of CUEDC2-negtive patients versus CUEDC2 -positive patients, there was no statistically significant difference (P=0.206)
Fig 3.
Disease-free survival of CUEDC2-negtive patients versus CUEDC2 -positive patients, CUEDC2-positive expression was associated with a shorter disease-free survival time (P=0.013)
Fig 4.
Disease-free survival of CUEDC2 negative, weak, and strong expression groups, significant difference only was observed among three groups (p=0.028).
Fig 5.
Disease-free survival of CUEDC2-negtive patients versus CUEDC2 -positive patients in LGSC subgroup and HGSC subgroup, respectively. 5A LGSC subgroup, 5B HGSC subgroup.
Table 3.
Independent predictors of survival in patients undergoing primary chemotherapy
| Factor | B | SE | Exp(B) | 95.0% CI Exp(B) | P |
|---|---|---|---|---|---|
| CUEDC2 expression | -0.618 | 0.370 | 0.539 | 0.261-1.113 | 0.095 |
| Age at diagnosis | 0.449 | 0.292 | 0.638 | 0.360-1.132 | 0.125 |
| FIGO stage | 1.364 | 0.388 | 0.256 | 0.120-0.546 | 0.000 |
| grade | -0.004 | 0.316 | 1.004 | 0.541-1.863 | 0.991 |
| LN metastasis | -0.251 | 0.271 | 1.286 | 0.755-2.186 | 0.354 |
DISCUSSION
CUEDC2, a CUE domain-containing protein, plays critical roles in many biological processes, such as cell cycle, inflammation and tumor genesis 19. Chen reported that CUEDC2 is a key regulator of macrophage function and critical for protection against colitis-associated tumor genesis 20. Zhang suggested that the expression level of CUEDC2 had an inverse correlation with imatinib resistance and activity of NF-κB signaling pathway in CML cells, CUEDC2 could regulate imatinib sensitivity in CML cells at least partially through NF-κB signaling pathway 21.
In the present study, we evaluated the frequency of CUEDC2 in ovarian serous carcinoma by using immunohistochemistry, and assessed whether overexpression of this protein had any clinical or pathological significance. To our knowledge, this is the first study to evaluate CUEDC2 expression in ovarian serous carcinoma. We found 60 out of 101 (59.4%) ovarian serous carcinoma samples were positive, among which 33 samples were weakly immunostained and 27 strongly immunostained. Regarding a potential correlation with clinicopathologic variables, we found significant association between CUEDC2 expression and patient's age at diagnosis, FIGO stage, histopathological grade and recurrence. To assess the clinical relevance of CUEDC2, we analyzed the survival follow-up information, and the result showed CUEDC2-positive expression was associated with a shorter disease-free survival time. Considering the effect of histopathological grade to relapse, we analyzed the relationship of CUEDC2 expression and the prognosis in LGSC and HGSC group, respectively. The results showed that the CUEDC2-positive cases had shorter DFS time tendency compared to CUEDC2-negative cases in both groups; the reason for no statistically significant difference was possibly due to insufficient samples with follow up data.
In previous study, Pan reported that subjects with breast cancer highly expressing CUEDC2 had a dramatically weaker responsiveness to tamoxifen treatment and a greater likelihood of relapse. For the subjects who received tamoxifen, those with tumors that highly expressed CUEDC2 showed significantly poorer DFS and OS than those with low CUEDC2 expression 22. Our result is consistent with their findings. Thus, CUEDC2 might be a novel marker for predicting likely relapse in ovarian serous carcinoma patients. However, multivariate survival analysis showed CUEDC2 was not an independent predictor of poor disease-free survival of patients undergoing curative resection. The reasons for this result may be related with the insufficient follow-up data, 27 samples were lost due to various reasons in the study.
There was strong epidemiological evidence that etiology, pathogenesis, and progression of ovarian cancers were greatly dependent on the activity of estrogens 23. Studies provided some evidence that the chance of a clinical response to therapy was directly related to ER expression of ovarian cancer tissues 24. Studies found that CUEDC2 bound both the progesterone receptor (PR) and ERα, resulted in degradation of these receptors and reduction of ligand activated gene transcription 25-26. CUEDC2 may be a potential biomarker for endocrine therapy of ovarian serous carcinomas. The focus of our next study will be analysis of the relationship among CUEDC2, ER, PR and p53.
In summary, the current study is a pilot assessment. We reported here for the first time that CUEDC2 expression was seen in 59.4% of serous ovarian carcinoma and was correlated with histological grade, FIGO stage and recurrence. It is indicted that CUEDC2 might be a promising biomarker to evaluate the progression of serous ovarian carcinoma and to predict likely relapse of ovarian serous carcinomas.
Acknowledgments
We thank Professor Xuemin Zhang for his generous donation of the antibody to CUEDC2, Mr. Changhe Wang for his assistance with statistical analysis. This work was supported by Science & Technology Innovation Fund of PLA General Hospital (No.13KMM47).
References
- 1.Jemal A, Bray F, Center MM. et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O. et al. Cancer statistics, 2011. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Sung PL, Chang YH, Chao KC. et al. Task Force on Systematic Review and Meta-analysis of Ovarian Cancer. Global distribution pattern of histological subtypes of epithelial ovarian cancer: a database analysis and systematic review. Gynecol Oncol. 2014;133(2):147–54. doi: 10.1016/j.ygyno.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Tognon G, Carnazza M, Ragnoli M. et al. Prognostic factors in early-stage ovarian cancer. E cancer medical science. 2013;7:325. doi: 10.3332/ecancer.2013.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su Z, Graybill WS, Zhu Y. Detection and moni¬toring of ovarian cancer. Clin Chim Acta. 2013;415:341–5. doi: 10.1016/j.cca.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 6.Simon AE, Wardle J, Grimmett C. et al. Ovarian and cervical cancer awareness: development of two validated measurement tools. J Fam Plann Reprod Health Care. 2012;38(3):167–74. doi: 10.1136/jfprhc-2011-100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu XH, Man YN, Wu XZ. Recurrence season impacts the survival of epithelial ovarian cancer patients. Asian Pac J Cancer Prev. 2014;15(4):1627–32. doi: 10.7314/apjcp.2014.15.4.1627. [DOI] [PubMed] [Google Scholar]
- 8.Kang KW, Lee MJ, Song JA. et al. Overexpression of goosecoid homeobox is associated with chemoresistance and poor prognosis in ovarian carcinoma. Oncol Rep. 2014;32(1):189–98. doi: 10.3892/or.2014.3203. [DOI] [PubMed] [Google Scholar]
- 9.Oshima T, Sato S, Kato J. et al. Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers. Mol Cancer. 2013;12(1):60. doi: 10.1186/1476-4598-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Wang H, Mohammad Shahidul Makki. et al. Overexpression of β-catenin and cyclinD1 predicts a poor prognosis in ovarian serous carcinomas. Int J Clin Exp Pathol. 2014;7(1):264–71. [PMC free article] [PubMed] [Google Scholar]
- 11.Donaldson KM, Yin H, Gekakis N. et al. Ubiquitin signals protein trafficking via interaction with a novel ubiquitin binding domain in the membrane fusion regulator, Vps9p. Curr Biol. 2003;13:258–62. doi: 10.1016/s0960-9822(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 12.Ponting CP. Proteins of the endoplasmic-reticulum-associated degradation pathway: domain detection and function prediction. Biochem J. 2000;351:527–35. [PMC free article] [PubMed] [Google Scholar]
- 13.Li HY, Liu H, Wang CH. et al. Deactivation of the kinase IKK by CUEDC2 through recruitment of the phosphatase PP1. Nat Immunol. 2008;9(5):533–41. doi: 10.1038/ni.1600. [DOI] [PubMed] [Google Scholar]
- 14.Thorne JL, Ouboussad L, Lefevre PF. Heterochromatin protein 1 gamma and IκB kinase alpha interdependence during tumor necrosis factor gene transcription elongation in activated macrophages. Nucleic Acids Res. 2012;40(16):7676–89. doi: 10.1093/nar/gks509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang WN, Zhou J, Zhou T. et al. Phosphorylation-triggered CUEDC2 degradation promotes UV-induced G1 arrest through APC/C(Cdh1) regulation. Proc Natl Acad Sci USA. 2013;110(27):11017–22. doi: 10.1073/pnas.1221009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao YF, Li T, Chang Y. et al. Cdk1-phosphorylated CUEDC2 promotes spindle checkpoint inactivation and chromosomal instability. Nat Cell Biol. 2011;13(8):924–33. doi: 10.1038/ncb2287. [DOI] [PubMed] [Google Scholar]
- 17.Malpica A, Deavers MT, Lu K. et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28(4):496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Malpica A, Deavers MT, Tornos C. et al. Interobserver and intraobserver variability of a two-tier system for grading ovarian serous carcinoma. Am J Surg Pathol. 2007;31(8):1168–74. doi: 10.1097/PAS.0b013e31803199b0. [DOI] [PubMed] [Google Scholar]
- 19.Man J, Zhang X. CUEDC2: an emerging key player in inflammation and tumorigenesis. Protein Cell. 2011;2(9):699–703. doi: 10.1007/s13238-011-1089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Wang SX, Mu R. et al. Dysregulation of the MiR-324-5p-CUEDC2 Axis Leads to Macrophage Dysfunction and Is Associated with Colon Cancer. Cell Rep. 2014;7(6):1982–93. doi: 10.1016/j.celrep.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Chang G, Wang J. et al. CUEDC2 sensitizes chronic myeloid leukemic cells to imatinib treatment. Leuk Res. 2013;37(11):1583–91. doi: 10.1016/j.leukres.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Pan X, Zhou T, Tai YH. et al. Elevated expression of CUEDC2 protein confers endocrine resistance in breast cancer. Nat Med. 2011;17:708–14. doi: 10.1038/nm.2369. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo K, Sheridan TB, Mabuchi S. et al. Estrogen receptor expression and increased risk of lymphovascular space invasion in high-grade serous ovarian carcinoma. Gynecol Oncol. 2014;133(3):473–79. doi: 10.1016/j.ygyno.2014.03.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gourley C. Hormone receptors and ovarian cancer survival. Lancet Oncol. 2013;14(9):794–95. doi: 10.1016/S1470-2045(13)70323-1. [DOI] [PubMed] [Google Scholar]
- 25.Musgrove EA. Estrogen receptor degradation: a CUE for endocrine resistance? Breast Cancer Res. 2011;13(4):312. doi: 10.1186/bcr2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang PJ, Zhao J, Li HY. et al. CUE domain containing 2 regulates degradation of progesterone receptor by ubiquitin-proteasome. EMBO J. 2007;26:1831–42. doi: 10.1038/sj.emboj.7601602. [DOI] [PMC free article] [PubMed] [Google Scholar]