Abstract
To evaluated the effect and mechanism of leflunomide (LEF) in murine model of collage-induced arthritis (CIA) on modulating the balance of Th17/Treg cells. DBA mice were divided into: control, CIA, CIA + LEF and CIA + Ibuprofen group. After 14 days immunization with bovine type II collagen (CII), except control group, mice were orally administered saline (CIA group), LEF or ibuprofen daily for 14 days. The severity of arthritis joint was assessed by using arthritis score. The numbers of CD3 + CD4 + interleukin 17 (IL-17) + Th17 cells and CD4 + CD25 + Foxp3+ Tregs were determined by flow cytometry, the expression of ROR-γt, Foxp3 and IL-21 mRNA in spleen were quantified by RT-PCR and IL-17, IL-21 and transforming growth factor β (TGF-β) level in serum was measured by ELISA.
In comparison with CIA group, CIA + LEF and CIA + Ibuprofen group reduced the disease severity obviously (p < 0.05). Leflunomide reduced Th17 cells, the expression of ROR-γt and IL-21 mRNA (p < 0.05) and the levels of IL-17 and IL-21 in serum. In addition, Ibuprofen but not LEF has effect on Treg cells number, Foxp3 mRNA expression and TGF-β secretion.
These results indicate that LEF effect on the balance od Th17/Treg through suppressing Th17 cells, but not stimulating Treg cells. Leflunomide may act as a potential immunomodulator for the treatment of rheumatoid arthritis (RA).
Keywords: rheumatoid arthritis, Th17, Leflunomide, Treg cells, ROR-γt, IL-17
Introduction
Rheumatoid arthritis (RA) is a chronic antigen-driven autoimmune disease that may lead to the destruction of joint architecture and a variety of systemic manifestations. Although the pathogenesis and the underlying mechanisms of RA remain rudimentary, it has been suggested that abnormalities of T cell subsets, such as Th1 and Th2, play an important role in the development of RA [1]. Recently, some research focuses on the balance between proinflammatory Th17 and Treg cells [2, 3]. Th17 cells and Treg cells are characterized by the expression of specific transcription factors, orphan nuclear receptor ROR-γt for Th17 cells [4] and the forkhead box p3 (Foxp3) for Treg [5]. Emerging evidence suggests that Th17 is a key effector cell in the pathogenesis of RA [6]. Its cytokine, interleukin 17 (IL-17), is a very important upstream inflammation factor. It increases the activation of synoviocytes and expression of other cytokines, contributing to cartilage and bone destruction [7]. The expression of IL-17 has been associated with the disease severity of RA. Interleukin 21 also can be produced by Th17 cells and also can be involved in Th17 polarization since in the absence of IL-6, IL-23 in combination with TGF-β, it could function as an alternative signal for the induction of Th17 cells [8]. Tregs are crucial for the suppression of potentially harmful excessive immune responses, they were identified as important mediators of peripheral immune tolerance. Defects in Tregs have been proven to lead to failed tolerance in autoimmune diseases including RA [9]. CD4 + CD25 + - Foxp3+ Tregs specifically express Foxp3, and alterations in Foxp3 expression may affect the functional stability of Tregs. In mice, Tregs have reduced Foxp3 expression with increased susceptibility to autoimmune disease [10]. Tregs modulate many aspects of the immune response, including cytokine production. Tregs secrete the cytokine transforming growth factor β (TGF-β), which contributes to maintenance of tolerance during lymphocyte maturation, thereby preventing the development of autoimmunity. The relationship between Th17 and Tregs cells during the pathogenesis of RA is complex, and the balance of those cells has a great significance for maintaining the normal immunology and defending autoimmune response in RA [11, 12]. So, modulation of the balance between pro-inflammatory and anti-inflammatory T cells is an important method for the treatment of autoimmune arthritis.
Leflunomide (LEF) is a DMARD drug and a frequently used medication, some researchers have demonstrated that it had an anti-proliferation and good curative effect on RA [13]. However, how it takes effect remains unknown. We carry on the research based on CIA aimed to preliminarily learn about the therapeutic mechanism of LEF.
Material and methods
Animals and induction of collage-induced arthritis
Male DBA/1 mice (6∼8 weeks, 18-22 g) were obtained from the Laboratory Animal Center of the Xinjiang Medical University, Urumqi, China. All mice were fed on a standard diet, and were housed under standard laboratory conditions. All experiments were approved by the Animal Welfare and Ethics Branch of the Biomedical Ethics Committee of the Xinjaing Medical University.
DBA mice were randomly divided into four groups of 8 mice each: control, collage-induced arthritis (CIA), CIA + LEF; and CIA + ibuprofen. Bovine type II collagen (Sigma) was dissolved in Macetic and then emulsified in complete Freund's adjuvant (CFA, Sigma) at a ratio of 1: 1. Mice were immunized with a subcutaneous injection at the base of the tail on day 1, and received a booster injection on day 7. There has been no injection in control mice. After 14 days of first immunization, CIA + LEF and CIA + ibuprofen groups were treated with daily intragastric administration of 10 mg/kg LEF (Suzhou xin kai long march pharmaceutical co., LTD) or 18 mg/kg ibuprofen (Shanxi taiyuan pharmaceutical co., LTD). The CIA and control groups were administered with equal volumes of distilled water. After administration, the severity of arthritis in each paw in all mice, including the control group, was evaluated on alternate days. The scores were as follows: 0 – normal; 1, slight swelling and erythema; 2 – pronounced edema; 3 – joint rigidity. Each paw was graded, resulting in a maximal clinical score of 12 for each animal. The incidence of CIA (%) in mice was defined according to the arthritis scores, as a total arthritis score ≥ 2 in each mouse.
Flow cytometry analysis
Cells were isolated according to an established method with slight modifications. Briefly, the spleen of the mice was harvested 28 days after the first immunization, pushed through a sieve, single cell suspension was prepared. Then, cells were isolated by density centrifugation on Histopaque 1077 (Sigma, St. Louis, MO, USA). Mononuclear cells were collected, washed with PBS containing 0.5% BSA, pre-incubated for 15 minutes with unlabeled isotype control Abs (IgG1 or IgG2b) (eBioscience, San Diego, CA, USA), and then surface-stained with anti-mouse CD3-FITC/CD4-PerCP or CD4-PerCP/CD25-PE-cy7. After the surface staining, cells were fixed, permeabilized and stained with anti-mouse IL-17-PE or Foxp3-PE (eBioscience, San Diego, CA, USA). Then, cells were incubated on ice for 30 minutes followed by washing with PBS. Subsequently, the cells (1 × 106) were analyzed using the ASR α flow cytometry system (Becton Dickinson, Franklin Lakes, NJ).
Fluorescent quantitative RT-PCR
Total RNA was extracted from splenocytes, using Trizol (Invitrogen) according to the manufacturer's instructions, and 5 µg RNA was reverse-transcribed into cDNA using cDNA synthesis kit (Invitrogen). Polymerase chain reaction was performed using ROR-γt primers (5’-TGTTTTATGGGGTTTGGGTATG-3’ and 5’-CTGTGTGGATGTGTGTCTCTGATTA- 3’), Foxp3 specific primers (5’-TCGCCTACTTCAGAAACCACC-3’ and 5’-CGAAACTCAAATTCATCTAC GG-3’), IL-21 specific primers (5’-CTATTTTCCCTGGAGTGGTATCA- 3’ and 5’-TGCTATCT TAGGGCGGTACAC-3’) and GAPDH specific primers (5’-GGTTGTCTCCTGCGACTTCA- 3’) to generate 122 bp, 130 bp, 167 bp and 183 bp product over 40 cycles of 95°C for 2 min, 62°C for 1 min and 72°C for 2 min. The fold change in expression of each gene was calculated using the ΔΔCt method, with the housekeeping gene GADPH mRNA as an internal control.
Cytokine quantification
The mice were anesthetized at day 28. Peripheral blood was collected, stored at 4°C, overnight, centrifuged at 3000 g for 10 min and the serum was collected. Cytokines were measured in triplicate using IL-17A, IL-21 and TGF-β ELISA kits (R&D Systems or BD Pharmingen) according to the manufacturer's instructions with standard cytokine preparations being used as the internal controls. The amount of cytokines was quantified using an xMark spectrophotometer (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Results were expressed as mean ± SD. Statistical analyses were performed by one-way ANOVA, followed by LSD test using SPSS statistical software (v.14.0). P < 0.05 was considered statistically significant.
Results
Leflunomide ameliorates the inflammatory reaction in collage-induced arthritis mice
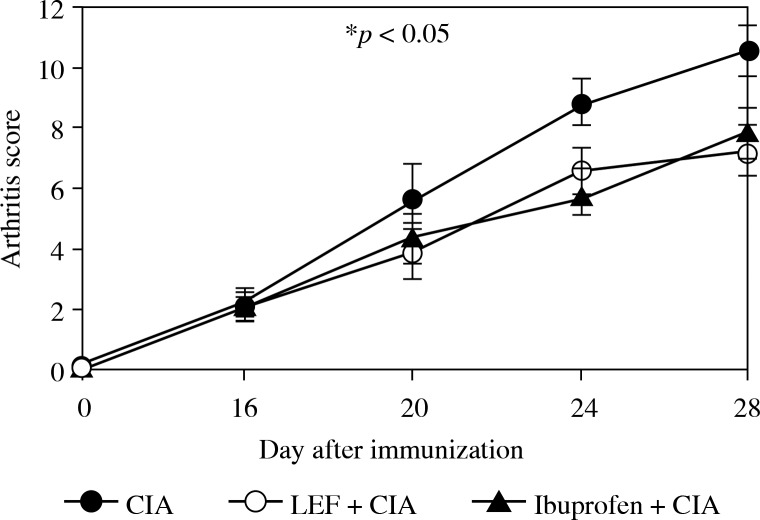
After the first injection of bovine CII on day 14, the mice were treated with daily intragastric administration of LEF or Ibuprofen for 14 days, and the severity of arthritis and signs of CIA were evaluated on alternate days. During the experiment, the arthritis score of the control group were “0” (data not shown) and almost no change. The administration of LEF resulted in amelioration of the paw swelling and poly arthritis score, as compared to the CIA group (Fig. 1). Treatment with LEF or Ibuprofen significantly reduced the incidence of CIA (70% and 60%) compared to that of untreated CIA mice (100%) (p< 0.05). These results suggest that LEF can prevent the development of CIA.
Fig. 1.
Effects of LEF on progression of established CIA in DBA/1J mice. DBA/1 mice were treated with daily intragastric administration of 10 mg/ kg LEF or 18 mg/kg ibuprofen from 14 to 28 days after immunization. Results are expressed as mean ± SD (n = 8) of 3 independent experiments. *p < 0.05 compared to the CIA model
Leflunomide reduces CD4+IL-17+ cells and downregulates ROR-γt and interleukin 21 mRNA
To verify whether LEF can influence the balance of T cell populations in CIA, we first studied the phenotype of CD4 + IL-17+ Th17 cells in splenocytes by flow cytometry. Table 1 presents representative data of flow cytometry analysis of Th17. The number of Th17 in splenocytes of the control, CIA + LEF, CIA + Ibuprofen and CIA groups, was 0.58 ±0.12%, 0.77 ±0.14%, 1.12 ±0.11% and 1.38 ±0.21%, respectively. The number of Th17 in splenocytes was significantly higher in the CIA group compared to the control group (p < 0.05). Both LEF and Ibuprofen lead to decreased numbers of CD4 + IL-17+ Th17 cells in splenocytes, but only the LEF group showed a significant difference compared to the CIA group (p < 0.05).
Table 1.
Leflunomide influences the balance of TH17/Treg cells during collagen-induced arthritis. Leflunomide decreases the numbers of IL-4+IL17+ T cells rather than regulates Tregs during collagen-induced arthritis (CIA). Splenocytes were obtained on day 28 and analyzed by flow cytometry. Quantification of the number of IL-4+IL-17+ Th17 and CD4+CD25+Foxp3+ Tregs cells. The results shown represent the mean ± SD of 3 independent experiments. *p < 0.05 compared to the CIA model
| Group | CD4 + IL-17 + T (%) | CD4 + CD25 + T (%) | CD4 + CD25 + Foxp3 + T (%) |
|---|---|---|---|
| control | 0.58 ±0.12 | 9.43 ±1.22 | 1.98 ±0.08 |
| LEF | 0.77 ±0.14 | 6.45 ±1.30 | 1.21 ±0.10 |
| ibuprofen | 1.12 ±0.11 | 7.29 ±1.16* | 1.48 ±0.08* |
| CIA | 1.38 ±0.21 | 5.45 ± 1.06 | 0.90 ±0.12 |
LEF – leflunomide, CIA – collage-induced arthritis
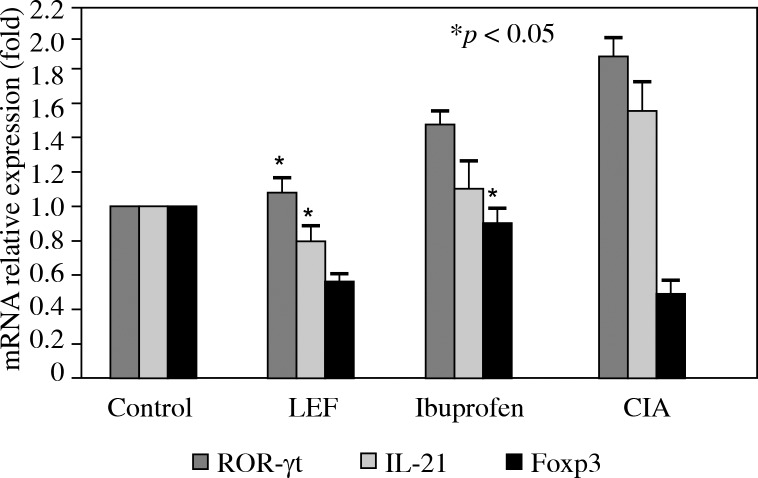
And then, the mRNA expression levels of the transcription factor ROR-γt and IL-21 in splenocytes from each experimental group were quantified using RT-PCR. Compared to control animals, ROR-γt and IL-21 were upregulated in splenocytes of the CIA group (Fig. 2, p < 0.05). Leflunomide and Ibuprofen treatment both lead to downregulation of ROR-γt and IL-21 mRNA in splenocytes, but only the LEF group showed a significant difference compared to the CIA group (Fig. 2, p < 0.05).
Fig. 2.
The expression of ROR-γt, IL-21 and Foxp3 mRNA in mouse spleen. Splenocytes were collected on day 28 and RT-PCR were performed to detect the ROR-γt, IL-21 and Foxp3 mRNA expression. Relative expression levels of mRNA were measured by quantitative real-time RT-PCR using the ΔΔ Ct method with the GAPDH mRNA as an internal control. The results shown represent the mean ± SD of 3 independent experiments. *p < 0.05 compared to the CIA model
Ibuprofen but not leflunomide increases and upregulates Foxp3 mRNA
The phenotype of CD4 + CD25 + Foxp3+ Treg cells in splenocytes were detected by flow cytometry. The data showed that the number of Treg in splenocytes of the control, CIA + LEF, CIA + ibuprofen and CIA groups, was 1.98 ±0.08%, 1.21 ±0.10%, 1.48 ±0.08% and 0.90 ±0.12%, respectively. Compared to the control group, the number of Treg in splenocytes was significantly lower in the CIA group (p < 0.05) and both LEF and Ibuprofen lead to increased numbers of CD4 + CD25 + Foxp3+ Treg cells in splenocytes, however, only the Ibuprofen group showed a significant difference compared to the CIA group (p < 0.05). Consistent with FCM data, the mRNA expression levels of the transcription factor Foxp3 in splenocytes from each experimental group had the same change. Foxp3 was downregulated in splenocytes of the CIA group (Fig. 2, p < 0.05) compared to the control group. Leflunomide and ibuprofen treatment both lead to an upregulation of Foxp3+ mRNA in splenocytes, but only the Ibuprofen group showed a significant difference compared to the CIA group (Fig. 2, p < 0.05).
Leflunomide reduces interleukin 17A, interleukin 21 levels in serum
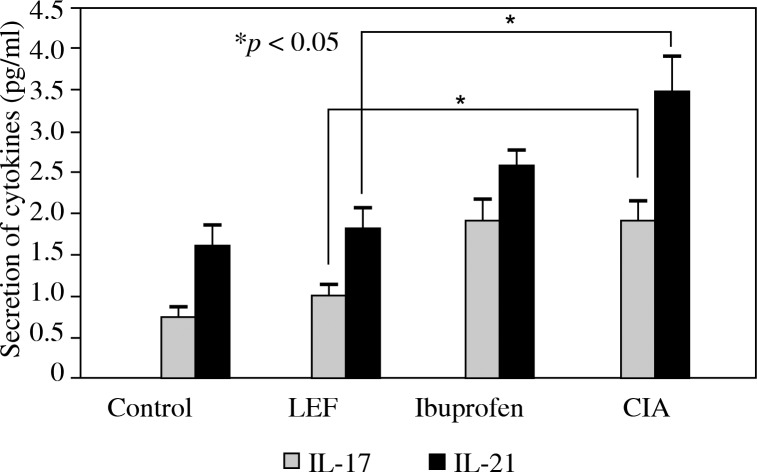
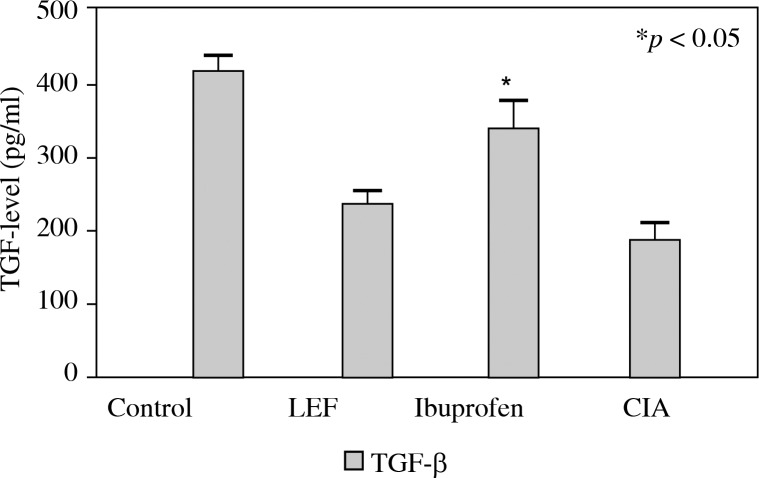
In order to evaluate the ability of LEF to modulate the balance of cytokine production during CIA in mice, the effects of LEF on pro-inflammatory cytokines IL-17 and IL-21 and the anti-inflammatory cytokines TGF-β were determined. Compared to the control group, the levels of IL-17 and IL-21 in the serum increased (Fig. 3, p < 0.05) and TGF-β decreased in the CIA group (Fig. 4, p < 0.05). The levels of pro-inflammatory in the serum treated with LEF were significantly lower than in the CIA group. However, the serum level of TGF-β was not significantly different in LEF groups, compared to the CIA group.
Fig. 3.
The concentration of IL-17 and IL-21 in serum. The serum of the CIA mouse was collected and the concentrations of IL-17 and IL-21 were measured by ELISA. The results which are shown represent the mean ± SD of 3 independent experiments. *p < 0.05 compared to controls
Fig. 4.
The concentration of TGF-β in serum. The serum of the CIA mouse was collected and the TGF-β levels were measured by ELISA. The results which are shown represent the mean ± SD of 3 independent experiments. *p < 0.05 compared to the control
Discussion
In the present study, we investigated for the first time the effect of LEF on the balance of Th17 and Treg cells. Furthermore, we also looked at its action on specific transcription factors, ROR-γt and Foxp3, and cytokine secretion such as IL-17, IL-21 and TGF-β. The main goal of current clinical and pharmacological treatment for RA was to relieve signs and symptoms such as inflammation, swelling and pain. Such treatment may include analgesics, anti-inflammatory drugs, corticosteroids and drugs known as disease-modifying antirheumatic drugs (DMARDs). Disease-modifying antirheumatic drugs can be divided into traditional DMARDs and biological agents such as TNF antagonists. Clinically, patients with RA primarily utilize traditional DMARDs initially, as these drugs have fewer side effects and are not associated with lymphocyte proliferative disorders [13]. Leflunomide, a traditional DMARD, was approved by the FDA used in RA in 1998, and have been used worldwide. As an isoxazole derivative and prodrug, LEF gets converted into active metabolite in the intestine, plasma and liver. It was shown that LEF monotherapy was better than placebo and almost equal to sulfasalazine and methotrexate. Moreover, numerous clinical research studies demonstrated that LEF was the most efficacious drug to decrease the severity of signs and symptoms of anxiety in RA patients [14] and effective in improving all clinical outcomes and delaying radiographic progression. In the present study, we demonstrated that LEF can ameliorate the severity of arthritis and inhibit the progression of CIA in mice. And this effect may relate to Th17/Treg cell balance.
Th1 and Th2 cells were thought to play a critical role in the development of RA. Recently, interest has expanded from the research of Th1/Th2 cells to Th17 cells, which initiate and maintain inflammation, and regulatory T cells (Tregs), which have an anti-inflammatory effect and can maintain immune homeostasis [11]. Th17 cells have recently been identified as a new effector Th cell subset characterized by the production of proinflammatory cytokines, including IL-17, IL-21 and IL-22. Importantly, this unique subset contributes to the pathogenesis of various autoimmune diseases [15]. In active RA patients, the percentage and MFI of peripheral CD4 + IL-17+ Th17 cells were significantly higher comparing to normal controls. The infiltration of Th17 cells into the inflammatory lesion is the link between the abnormal T cell response and bone damage. Clearly, Th17 cell subset is an auspicious target for future therapeutic investigations, and cytokines related to Th17 cell differentiation and function will be of great clinical importance [16]. Tregs are also crucial for RA immune response, they modulate many aspects of the immune response, including antigen presenting cell (APC) function, T-cell proliferation, cytokine production and antibody secretion by B cells [17, 18]. Additionally, Tregs can prevent autoimmune disease by suppressing potentially harmful excessive immune responses [19]. The defects in Tregs function have been proven to lead to failed tolerance in RA. Our study showed that in splenocytes the number of Th17 cells increased and Tregs decreased in CIA mouse and treatment with LEF significantly reduced the number of Th17 cells. In contrast to the results for Th17, we observed no significant alternation in the proportions of CD4 + CD25 + Foxp3+ Tregs in LEF treated CIA mice comparing to CIA groups. In Di Jin research [20], differential changes of CD4 + CD25 + Foxp3+ Treg cells in different peripheral immune tissues or organs were observed after LEF treatment in mice with and without body mass index (BMI). The ratios of Treg cells were enhanced in the thymus, but significantly decreased in the spleens but not in PBLs and LNs. At present, the reasons for the different response of Treg cells in different tissues or in different animal models are unclear, but possibly are related to different animal models, different doses and treatment time courses of LEF. In any case, LEF failed to induce CD4 + CD25+ Foxp3+ Treg cells in RA, indicating that it may not be an ideal choice for the purpose of immune tolerance induction. Those findings suggested that LEF modulate imbalance of numbers of Th17/Treg is mostly contributed by decreasing Th17 cells rather than increasing Tregs cell numbers. Moreover, the anti-inflammatory function of LEF is partly explained by suppressing the Th17 cells.
Soon after the discovery of Th17 cells, retinoid-related orphan receptor (ROR)γt was reported to be selectively expressed in Th17 cells and is required for Th17 cell differentiation. Furthermore, overexpression of ROR-γt has been shown to be sufficient to drive IL-17 production whilst its deficiency was shown to lead to an impairment of Th17 cell differentiation both in vitro and in vivo. Tregs specifically express Foxp3, and alterations in Foxp3 expression may affect the functional stability of Tregs [21]. Tregs have reduced Foxp3 expression and increased susceptibility to autoimmune disease in mice. In RA patients, TGF-β inhibits the Tregs by downregulating the expression of Foxp3, which can be reversed by anti-TNF therapy. Recent studies have shown that the function of ROR-γt can be inhibited by Foxp3, suggesting that the balance between ROR-γt and Foxp3 plays a major role in the commitment of Th cells toward either the Th17 or the Treg lineage. Our results demonstrate that LEF treatment downregulated ROR-γt expression and has no significant effect on Foxp3 expression, which correlated with decreased numbers of CD4 + IL-17+ Th17 but not Tregs in LEF treated mice.
The balance of cytokines produced by Th17/Treg also plays an important role in the development of RA [22]. Interleukin 17 is mainly produced by Th17 and can induce the production of proinflammatory cytokines, upregulation of RANK ligand, stimulate the activity of matrix metalloproteinases and bone destruction. Interleukin 17 is detected at higher levels within the joints of patients with RA [23] and in CIA models, furthermore, increased levels of IL-17 correlate with more severe joint damage. Mice deficient in IL-17 are protected from the development of arthritis in CIA. Transforming growth factor β could be secreted by Tregs and induce Treg cell proliferation and/or activation in vivo. It was thought that TGF-β contributes to self-reactive T-cell apoptosis, the maintenance of tolerance and prevention of the development of autoimmunity. The secretion of TGF-β was markedly increased in the serum of CIA rats and this was consistent with down-regulation of Tregs number during CIA. In the murine system, the TGF-β and IL-6 are the critical cytokines controlling the initial commitment of a T helper cell to become a Th17 cell [24]. As the presence of TGF-β alone, Foxp3+ Tregs will contribute to development, there appears to be a reciprocal relationship between Th17 cells and Tregs whereby, in the presence of IL-6, TGF-β will drive the development of Th17 cells rather than promote the generation of Tregs. In this study, the administration of LEF reduced the level of IL-17 in the serum and has a little effect on TGF-β secretion, suggesting that the anti-inflammatory action of LEF is associated with significantly reduced IL-17 release rather than an increased TGF-β level. The decrease of serum IL-17 level was consistent with decreased Th17 cells.
Interleukin 21, an autocrine cytokine that regulates human Th17 cells in RA, potently enhances Th17 proliferation and suppresses Foxp3 expression, leading to the expression of ROR-γt and production of IL-17 [25]. Although exposure to TGF-β and IL-6 initiates the commitment a naive Th cell to the Th17, additional stimuli are required for Th17 cell programme. The acquisition of IL-21 production is believed to play a key amplification role in the generation of Th17 cells [26]. Moreover, IL-21 can substitute for IL-6 in the initial differentiation of Th17 cells, upregulate differentiation of B cells and the activation of NK cells. Research suggested that downregulation of Th17 cells is one of mechanisms of anti-TNF therapy. Interleukin 21 and IL-21 mRNA was elevated in RA and blocking IL-21 action has attenuated the disease in MRL/lpr mice and in CIA. Therefore, IL-21 can serve as a good target for treating autoimmune disease. Our study revealed that the IL-21 in peripheral blood serum and IL-21 mRNA in splenocytes of CIA mice were decreased after treatment with LEF. Moreover, the anti- inflammation effect of LEF was also reflected in the suppression of TNF secretion (data not shown).
We also checked the other cytokines level, including TGF-β, IL-10 and tumor necrosis factor α (TNF-α) et al. but only TGF-β result was shown in the present data, because it related not only to Treg differentiation and function but also Th17 differentiation. Tumor necrosis factor α is a very important cytokine for inflammation and modulation of TNF, it may be another anti-mechanism for LEF.
In summary, the results of this study provide a comprehensive assessment of immunomodulating function by which LEF exerts its protective effect against CIA. Leflunomide, as a traditional drug for therapy of RA, can modulate the imbalance of Th17/Treg cells in spleen of CIA, and such influence may be partially due to decreasing the number of Th17 by downregulation of the transcription factor of ROR-γt and IL-21, these findings are consistent with the change of Th17. Furthermore, we detect the serum cytokine microenvironment of LEF-treated CIA mice. The results show that the administration of LEF decreased the serum levels of IL-17 and IL-21 in mice with CIA. Interestingly, there have no significant changes in the number of splenocyte Tregs, expression of Foxp3 mRNA and secretion of TGF-β in LEF-treated CIA mice. They may suggest that LEF can decrease the production of pro-inflammatory cytokines in serum, downregulate the number of CD4 + IL-17+ Th17 and Th17 related transcription factor to suppress the Th17 rather than have an effect on Tregs, to regulate the imbalance of Th17/Tregs and induce immunosuppression during CIA. The relationship between Th17 and Treg cells during the pathogenesis of RA is complex, and modulation of the balance between Th17/Treg cells is an important method for the treatment of RA. These insights are relevant to explore drug combination of LEF and further develop the therapeutic interventions for RA clinical medication.
The authors declare no conflict of interests.
This work was supported by Grant, XJEDU2010S26, from the Xinjiang University scientific research project, Doctoral Fund of Xinjiang Medical University and SRTP( CX2012026), China.
References
- 1.Yamada H, Nakashima Y, Okazaki K, et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1299–1304. doi: 10.1136/ard.2007.080341. [DOI] [PubMed] [Google Scholar]
- 2.Boissier MC, Assier E, Falgarone G, Bessis N. Shifting the imbalance from Th1/Th2 to Th17/treg: the changing rheumatoid arthritis paradigm. Joint Bone Spine. 2008;75:373–375. doi: 10.1016/j.jbspin.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhu M, Mo H, Li D, et al. Th17/Treg imbalance induced by increased incidence of atherosclerosis in patients with systemic lupus erythematosus (SLE) Clin Rheumatol. 2013;32:1045–1052. doi: 10.1007/s10067-013-2237-z. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 6.Hemdan NY, Birkenmeier G, Wichmann G, et al. Interleukin- 17-producing T helper cells in autoimmunity. Autoimmun Rev. 2010;9:785–792. doi: 10.1016/j.autrev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Paradowska A, Maślińiski W, Grzybowska-Kowalczyk A, Lacki J. The function of interleukin 17 in the pathogenesis of rheumatoid arthritis. Arch Immunol Ther Exp. 2007;55:329–334. doi: 10.1007/s00005-007-0032-8. [DOI] [PubMed] [Google Scholar]
- 8.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Amelsfort JM, Jacobs KM, Bijlsma JW, et al. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 10.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Shao S, Jiao Z, et al. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int. 2012;32:887–893. doi: 10.1007/s00296-010-1710-0. [DOI] [PubMed] [Google Scholar]
- 12.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 13.Smolen JS, Emery P, Kalden JR, et al. The efficacy of leflunomide monotherapy in rheumatoid arthritis: towards the goals of disease modifying antirheumatic drug therapy. J Rheumatol. 2004;71:13–20. [PubMed] [Google Scholar]
- 14.Pinho de Oliveira Ribeiro N, Rafael de Mello Schier A, Ornelas AC, et al. Anxiety, depression and suicidal ideation in patients with rheumatoid arthritis in use of methotrexate, hydroxychloroquine, leflunomide and biological drugs. Compr Psychiatry. 2013;54:1185–1189. doi: 10.1016/j.comppsych.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 17.Venken K, Hellings N, Liblau R, Stinissen P. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med. 2010;16:58–68. doi: 10.1016/j.molmed.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Wright GP, Notley CA, Xue SA, et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci U S A. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdulahad WH, Stegeman CA, van der Geld YM, et al. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener's granulomatosis in remission. Arthritis Rheum. 2007;56:2080–2091. doi: 10.1002/art.22692. [DOI] [PubMed] [Google Scholar]
- 20.Jin D, Duan K, Zhang L, et al. The effects of leflunomide on CD4(+)CD25(+)Foxp3(+) T regulatory cells in mice receiving allogeneic bone marrow transplantation. Inflamm Res. 2012;61:53–60. doi: 10.1007/s00011-011-0388-4. [DOI] [PubMed] [Google Scholar]
- 21.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4 + CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 22.Eastaff-Leung N, Mabarrack N, Barbour A, et al. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 23.Metawi SA, Abbas D, Kamal MM, Ibrahim MK. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clin Rheumatol. 2011;30:1201–1207. doi: 10.1007/s10067-011-1737-y. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 25.Niu X, He D, Zhang X, et al. IL-21 regulates Th17 cells in rheumatoid arthritis. Hum Immunol. 2010;71:334–341. doi: 10.1016/j.humimm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]