Abstract
Objective
To analyze peripheral blood interleukin 6 (IL-6) methylation status and its clinical significance in patients with systemic lupus erythematosus (SLE).
Material and methods
Blood samples from 41 adult patients with SLE, and 20 healthy controls were collected. The methylation status of IL-6 was determined by methylation specific polymerase chain reaction (MSP). The IL-6 expression was detected by real-time PCR. Correlations between IL-6 methylation status and clinical features or laboratory findings in patients with SLE were investigated.
Results
The methylation status and expression of IL-6 in peripheral blood could reflect the level in peripheral blood mononucleated cells (PBMCs) of SLE. Significantly positive correlation was found between IL-6 hypomethylation and renal disorder. Interleukin 6 hypomethylation was found negatively correlated with serum C3.
Conclusions
The detection of IL-6 methylation status in peripheral blood could reflect the status in PBMC with SLE. Interleukin 6 may play a role in renal disorder with SLE patients. Interleukin 6 could be considered as a new biomarker for predicting SLE flare.
Keywords: systemic lupus erythematosus, hypomethylation, interleukin-6, peripheral blood
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease, characterized by a multitude of autoantibody production, complement activation and immune- complex deposition, which causes tissue and organ damage. The etiology and pathogenetic mechanisms of SLE have not been clearly elucidated. Cytokines have been shown to be involved in the pathogenesis of SLE [1].
Interleukin 6 (IL-6) is a cytokine that regulates the immune defense response. Interleukin 6 plays a central role in the transition from the acute to the chronic phase of the inflammatory process [2]. Elevated levels of IL-6 have been documented in a variety of autoimmune diseases, such as dermatomyositis [3], rheumatoid arthritis [4], inflammatory bowel disease [5], glomerular nephritis [6], and so forth. Methylation status change is one of the mechanisms of IL-6 deregulation. The hypomethylation levels of the CpG islands in the IL-6 promoters in lupus patients were significantly correlated to the IL-6 expression [7]. But the correlation between IL-6 methylation status and parameters of SLE patients is unknown.
In this study, we compared the expression of IL-6 between peripheral blood, PBMCs in SLE patients and in the control samples, in order to evaluate the application value of peripheral blood IL-6 expression in SLE. Furthermore, clinical and experimental significance (as the references reported [8, 9]) of IL-6 expression and methylation status of peripheral blood was evaluated.
Materials and methods
Patients and controls
Forty-one SLE patients and 20 healthy control subjects were recruited from Guangzhou First People's Hospital from March to May 2013. In the SLE group, there were 37 females and 4 males; the mean of age was 34.1 ±16.6 years. In the control group, there were 14 females and 6 males; the mean of age was 32.6 ±14.1 years. All the control samples were collected from the physical examination center. The patients were diagnosed based on the Criteria of American College of Rheumatology (ACR) for SLE [10]. Approvals were obtained from the Ethics Committee of Jinan University, and informed consent was obtained from all study participants.
Isolation of genomic DNA from peripheral blood and PBMCs and bisulfite DNA
Peripheral blood mononucleated cells (PBMCs) were isolated from peripheral blood obtained from SLE patients and healthy control subjects, by equilibrium centrifugation using Lymphoprep (Nycomed, Oslo, Norway) according to the manufacturer's instructions. Genomic DNA from PBMCs or blood was prepared using the Universal Genomic DNA Extraction Kit Ver.3.0 (Takara, Dalian, China) also according to the manufacturer's instructions. The quality and integrity of DNA from tissues and cells were checked by electrophoresis on 1% agarose gel and quantified spectrophotometrically. Genomic DNA (0.5 mg) extracted from the cells, tumor and the normal tissue specimens was subjected to bisulfite treatment using an Epitect Bisulfite Kit (Qiagen, Hilden, Germany) and stored at –20°C until further use.
Methylation-specific PCR
Methylation specific polymerase chain reaction (MSP) were conducted as described previously (Reed K). 2.5 U of Taq mix (Takara), 0.5 ml of 1 mM forward and reverse primers (forward methylated primer: 5’-GGAGGGGTAGGAGGGTAGAGTTATAC-3’, reverse methylated primer: 5’-AAAATAATCCCGATACAATACCGTT-3’, forward unmethylated primer: 5’-GGGGTAGGAGGGTAGAGTTATATGA-3’, reverse unmethylated primer: 5’-TAAAATAATCCCAATACAATACCATT-3’), was used in a 25 ml of total reaction volume. Here, 50 ng of bisulfite- treated DNA was used as the template of the PCR. The PCR cycles were as follows: at 95°C for 5 min, followed by 35 cycles at 95°C for 0.5 min, at 60°C for 2 min and at 72°C for 1 min, followed by a final extension at 72°C for 10 min. Polymerase chain reaction products were separated on 1% agarose gels and analyzed by ethidium bromide staining.
RNA extraction and reverse transcription
Total RNA was extracted with TRIZOL (Invitrogen) from resected cells. One microgram of total RNA was reverse-transcribed in a total volume of 20 μl with IMProm- IITM reverse transcriptase kit (Promega) by using oligo-dT primers, according to the manufacturer's instructions.
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was performed using the ABI Prism 7500 real-time PCR system. The sequences of primers and probe used for specific IL-6 PCR amplification were as follows: 5’-CAAATTCGGTACATCCTC-3’ (primer forward), 5’- CTGGCTTGTTCCTCACTA- 3’ (primer reverse). Thermal cycling conditions included activation at 95°C (10 min) followed by 40 cycles, each of denaturation at 95°C (15 sec) and annealing/ elongation (1 min) at 60°C. Each sample was analyzed in triplicate with β-actin (Applied Biosystems) as housekeeping gene, and the mean values of IL-10 were calculated. The cycle threshold (Ct) was used to calculate relative amounts of IL-6. Data were normalized for β-actin levels by the 2−ΔΔCt method.
Measurement of blood parameters
Blood parameters including complement C3 (C3), complement C4 (C4), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), antinuclear antibody (ANA), immunoglobulin (Ig) G, IgM, IgA were measured by standard methods. Measurement was done in triplicate. Clinical data were obtained from medical records on admission.
Statistical analysis
Data are presented as mean ± SD from at least three separate experiments. Multiple group comparisons were performed using ANOVA with a post hoc test for subsequent individual group comparisons. The difference in IL-6 promoter methylation status between SLE patients and the control group was examined by an independent sample t-test. The relationships between IL-6 methylation status, expression and clinicopathological parameters were examined by the χ2-test. Results were considered significant when the p value (two sided) of ≤ 0.05 was obtained. All the statistical analyses were performed using SPSS13.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
The expression of IL-6 in peripheral blood could reflect the level in PBMCs of SLE
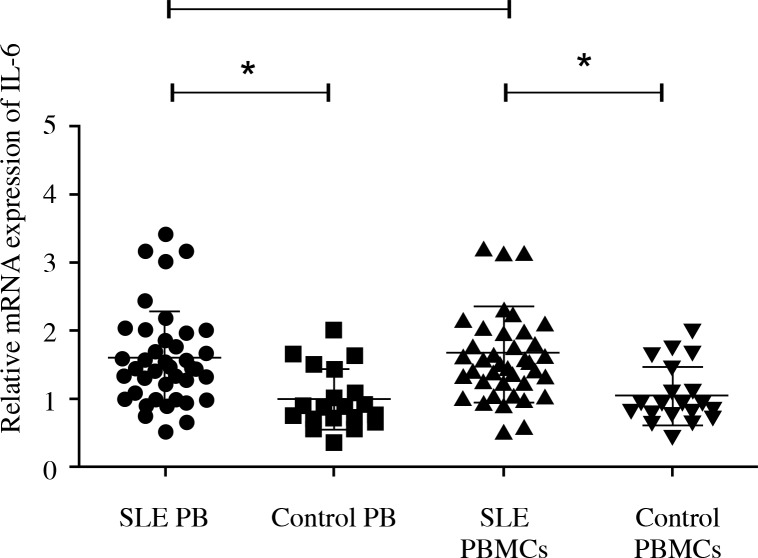
A total of 41 SLE samples and 20 controls, positive for β-actin reaction, were submitted to qRT-PCR. The peripheral blood IL-6 expression of SLE was higher than the control group (p < 0.01). The IL-6 in PBMCs of SLE was upregulated compared to the control group (p < 0.01). There is no significant difference of the IL-6 expression between PBMCs and peripheral blood in SLE (Fig. 1). So we considered that the expression of IL-6 in peripheral blood could reflect the level in PBMCs of SLE.
Fig. 1.
The expression of IL-6 in SLE compared to controls. Forty-one SLE samples and 20 controls, positive for β-actin reaction, were detected by qRT-PCR. The peripheral blood IL-6 expression was higher than the control group. Interleukin 6 in PBMCs of SLE was upregulated compared to the control group. There is no significant difference of the IL-6 expression between PBMCs and peripheral blood in SLE. SLE PB – peripheral blood with SLE; control PB – peripheral blood with control; SLE PBMCs – PBMCs with SLE; control PBMCs – PBMCs with control; *p = 0.05
Interleukin 6 was hypomethylated in peripheral blood of SLE, the same as in PBMCs
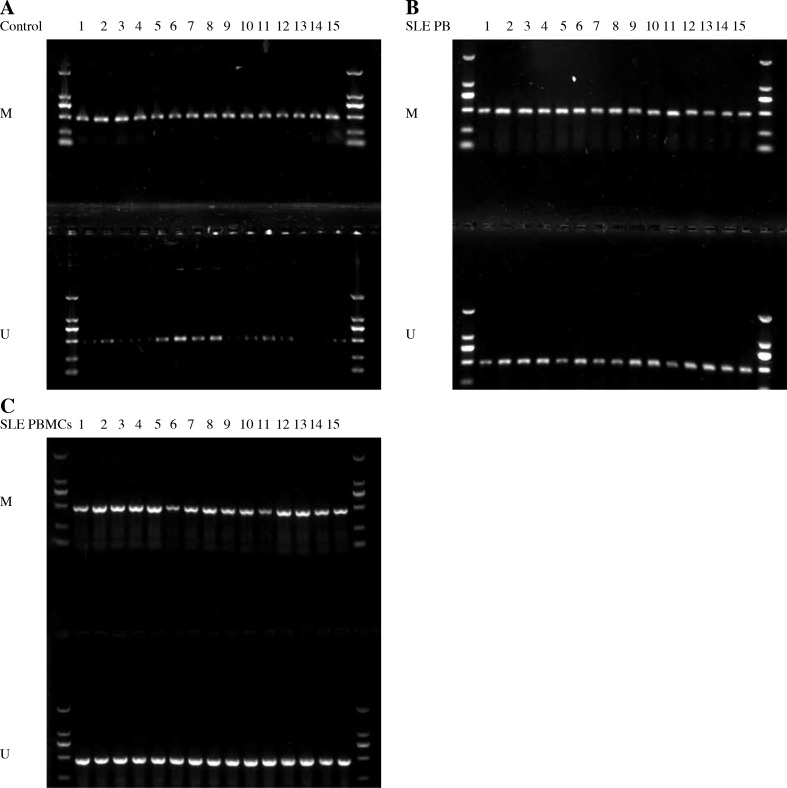
We designed and validated MSP for IL-6 methylation within the region which included 13 CpGs. The methylation status of the IL-6 promoter was determined by MSP. Interleukin 6 was significantly hypomethylated in SLE patients (32/41) (Fig. 2B). In contrast, IL-6 was hypomethylated in 6 of 20 healthy controls (p < 0.01) (Fig. 2A). Then, we performed MSP to detect the methylation status of IL-6 promoter in PBMCs. Interleukin 6 was hypomethylated in 33 of 41 SLE patients’ PBMCs (p < 0.01) (Fig. 2C). Therefore, the methylation status of IL-6 in peripheral blood could reflect the methylation status in PBMCs of SLE.
Fig. 2.
The methylation status of the IL-6 promoter was determined by MSP. A) Interleukin 6 was hypomethylated in 6 of 20 healthy controls. B) Interleukin 6 was hypomethylated in 32 SLE patients. C) Interleukin 6 was hypomethylated in 33 SLE patients’ PBMCs. M – methylated primer; U – unmethylated primer
Associations of peripheral blood IL-6 methylation status with clinical parameters of SLE patients
Considering the clinical characteristics, a significantly positive correlation was found between IL-6 hypomethylation and renal disorder (p = 0.010, Table 1). In addition, no correlation was found between IL-6 hypomethylation and sex, age, cardiac disorder, pulmonary inflammation, nervous system disorder, butterfly erythema, and arthritis.
Table 1.
Associations of serum IL-6 methylation status with clinical parameters of SLE patients
| Group | Hypomethylation | methylation | χ2 | p |
|---|---|---|---|---|
| Gender | 0.024 | 0.878 | ||
| male (4) | 3 | 1 | ||
| female (37) | 29 | 8 | ||
| Age | 1.324 | 0.250 | ||
| > 34(25) | 21 | 4 | ||
| ≤ 34(16) | 11 | 5 | ||
| Cardiac disorder | 3.720 | 0.054 | ||
| with (10) | 10 | 0 | ||
| without (31) | 22 | 9 | ||
| Pulmonary inflammation | 1.759 | 0.185 | ||
| with (17) | 15 | 2 | ||
| without (24) | 17 | 7 | ||
| Renal disorder | 6.549 | 0.010* | ||
| with (20) | 19 | 1 | ||
| without (21) | 13 | 8 | ||
| Nervous system disorder | 0.115 | 0.735 | ||
| with (6) | 5 | 1 | ||
| without (35) | 27 | 8 | ||
| Arthritis | 0.212 | 0.645 | ||
| with (21) | 17 | 4 | ||
| without (20) | 15 | 5 | ||
| Butterfly erythema | 3.703 | 0.054 | ||
| with (16) | 10 | 6 | ||
| without (25) | 22 | 3 |
p = 0.05
Associations of peripheral blood IL-6 methylation status with laboratory parameters of SLE patients
Interleukin 6 hypomethylation was found negatively correlated with serum C3 (p = 0.020) and C4 p = 0.007, Table 2). No correlation was found between IL-6 hypomethylation and IgG, IgM, IgA, double-stranded DNA (ds-DNA), anti-nuclear ribonuclear protein antibody (anti-nRNP), anti- ribosomal P protein antibody (anti-rRNP), anti-Sjögren syndrome type A antibody (anti-SSA), anti-Sjögren syndrome type B antibody (anti-SSB), anti-Sm, anti-nucleosome antibody (AauA) or anti-histone antibody (Table 2).
Table 2.
Associations of serum IL-6 methylation status with laboratory parameters of SLE patients
| Group | Hypomethylation | Methylation | χ2 | p |
|---|---|---|---|---|
| C3 (g/l) | 5.423 | 0.020* | ||
| < 0.9 (27) | 24 | 3 | ||
| ≥ 0.9 (14) | 8 | 6 | ||
| C4 (g/l) | 7.278 | 0.007* | ||
| < 0.1 (25) | 23 | 2 | ||
| ≥ 0.1 (16) | 9 | 7 | ||
| IgM (g/l) | 0.290 | 0.591 | ||
| < 0.4 (7) | 6 | 1 | ||
| ≥ 0.4 (34) | 26 | 8 | ||
| IgA (g/l) | 1.247 | 0.264 | ||
| < 4 (37) | 28 | 9 | ||
| ≥ 4 (4) | 4 | 0 | ||
| IgG (g/l) | 0.245 | 0.621 | ||
| < 16 (38) | 30 | 8 | ||
| ≥ 16 (3) | 2 | 1 | ||
| dsDNA | 0.518 | 0.472 | ||
| +(8) | 7 | 1 | ||
| −(33) | 25 | 8 | ||
| anti-SSA | 1.916 | 0.166 | ||
| +(19) | 13 | 6 | ||
| −(22) | 19 | 3 | ||
| anti-SSB | 0.024 | 0.877 | ||
| +(4) | 3 | 1 | ||
| −(37) | 29 | 8 | ||
| anti-Sm | 2.035 | 0.154 | ||
| +(4) | 2 | 2 | ||
| −(37) | 30 | 7 | ||
| anti-nRNP | 0.864 | 0.353 | ||
| +(13) | 9 | 4 | ||
| −(28) | 23 | 5 | ||
| anti-rRNP | 0.531 | 0.466 | ||
| +(6) | 4 | 2 | ||
| −(35) | 28 | 7 | ||
| AauA | 0.054 | 0.816 | ||
| +(8) | 6 | 2 | ||
| −(33) | 26 | 7 | ||
| anti-histone | 0.157 | 0.692 | ||
| +(16) | 13 | 3 | ||
| −(25) | 19 | 6 |
p = 0.05
Discussion
Although the precise etiology and pathogenetic mechanisms of SLE are not entirely known, several factors have been proposed in the initiation and progression of SLE. Two important factors that are suggested to be involved are apoptosis imbalance and dysregulation of several cytokines. It is well known that overproduction of several cytokines such as IL-18 [8] and downregulation of several cytokines such as IL-21 [11] play a significant role in the pathogenesis of SLE. Accumulating studies have indicated that DNA methylation abnormalities play a pivotal role in the pathogenesis of SLE [12]. Data demonstrated that patients with SLE had a significantly lower level of DNA methylation than the controls. The expression of both DNMT1 and MBD2 mRNA was significantly increased in SLE patients compared with the controls. It also showed a positive correlation between DNMT1 and MBD2 mRNA levels in patients with SLE [13].
Interleukin 6, a cytokine featuring redundancy and pleiotropic activity, contributes to host defense against acute environmental stress, while dysregulated persistent IL-6 production has been demonstrated to play a pathological role in various autoimmune and chronic inflammatory diseases. High concentrations of IL-6 are found in the synovial fluid and sera of patients with RA [14]. Mi et al. verified that IL-6 was hypomethylated in T cells from SLE. High levels of IL-6 mRNA and protein expressions were detected in freshly isolated lymphocytes and hypomethylation of IL-6 promoter was detected in T cells from SLE patients [7]. We planned to investigate the difference of IL-6 hypomethylation status between peripheral blood and PBMCs in this study. And, we revealed that IL-6 was overexpressed and hypomethylated in peripheral blood from SLE patients. This result indicated that the expression and methylation status of IL-6 in peripheral blood could accurately reflect the information in PBMC from SLE patients. Then, we analyzed the association of peripheral blood IL-6 methylation status with clinical parameters of SLE patients. It has shown a significantly positive correlation between IL-6 hypomethylation and renal disorder. However, IL-6 hypomethylation was not related to sex, age, cardiac disorder, pulmonary inflammation, nervous system disorder, butterfly erythema or arthritis of SLE patients. The data obtained suggest that IL-6 may participate in renal disorder with SLE. At last, associations of peripheral blood IL-6 methylation status with laboratory parameters of SLE patients were statistically analyzed. Interleukin 6 hypomethylation correlated negatively with C3 and C4, but was not correlated with IgG, IgM, IgA, ds-DNA, anti- nRNP, anti-rRNP, anti-SSA, anti-SSB, anti-Sm, AauA or anti-histone antibody. The decrease in C3 and C4 indicates SLE flare [15]. In this study, it was explained that the IL-6 hypomethylation could predict SLE flare.
In conclusion, the detection of IL-6 methylation status in peripheral blood could reflect the status in PBMC with SLE. Interleukin 6 may play a role in renal disorder with SLE patients. Interleukin 6 could be considered as a new biomarker for predicting SLE flare.
The authors declare no conflict of interest.
This study was supported by the Guangdong Technology Foundation (2012B031800274).
References
- 1.Pan HF, Ye DQ, Li XP. Type 17 T-helper cells might be a promising therapeutic target for systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2008;4:352–353. doi: 10.1038/ncprheum0815. [DOI] [PubMed] [Google Scholar]
- 2.Iwanami K, Matsumoto I, Tanaka-Watanabe Y, et al. Crucial role of the interleukin-6/interleukin-17 cytokine axis in the induction of arthritis by glucose-6-phosphate isomerase. Arthritis Rheum. 2008;58:754–763. doi: 10.1002/art.23222. [DOI] [PubMed] [Google Scholar]
- 3.Yang M, Cen X, Xie Q, et al. Serum interleukin-6 expression level and its clinical significance in patients with dermatomyositis. Clin Dev Immunol. 2013;2013:717808. doi: 10.1155/2013/717808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida Y, Tanaka T. Interleukin 6 and rheumatoid arthritis. Biomed Res Int. 2014;2014:698313. doi: 10.1155/2014/698313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hur SJ, Kang SH, Jung HS, et al. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr Res. 2012;32:801–816. doi: 10.1016/j.nutres.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Gigante A, Gasperini ML, Afeltra A, et al. Cytokines expression in SLE nephritis. Eur Rev Med Pharmacol Sci. 2011;15:15–24. [PubMed] [Google Scholar]
- 7.Mi XB, Zeng FQ. Hypomethylation of interleukin-4 and -6 promoters in T cells from systemic lupus erythematosus patients. Acta Pharmacol Sin. 2008;29:105–112. doi: 10.1111/j.1745-7254.2008.00739.x. [DOI] [PubMed] [Google Scholar]
- 8.Hatef MR, Sahebari M, Rezaieyazdi Z, et al. Stronger correlation between interleukin 18 and soluble Fas in lupus nephritis compared with mild lupus. ISRN Rheumatol. 2013;2013:850851. doi: 10.1155/2013/850851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang WH, Pan HF, Zhao XF, et al. Anti-alpha-actinin antibodies in relation to new-onset systemic lupus erythematosus and lupus nephritis. Mol Biol Rep. 2010;37:1341–1345. doi: 10.1007/s11033-009-9513-7. [DOI] [PubMed] [Google Scholar]
- 10.Bertsias GK, Pamfil C, Fanouriakis A, Boumpas DT. Diagnostic criteria for systemic lupus erythematosus: has the time come? Nat Rev Rheumatol. 2013;9:687–694. doi: 10.1038/nrrheum.2013.103. [DOI] [PubMed] [Google Scholar]
- 11.Pan HF, Wu GC, Fan YG, et al. Decreased serum level of IL-21 in new-onset systemic lupus erythematosus patients. Rheumatol Int. 2013;33:2337–2342. doi: 10.1007/s00296-013-2724-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhao S, Long H, Lu Q. Epigenetic perspectives in systemic lupus erythematosus: pathogenesis, biomarkers, and therapeutic potentials. Clin Rev Allergy Immunol. 2010;39:3–9. doi: 10.1007/s12016-009-8165-7. [DOI] [PubMed] [Google Scholar]
- 13.Liu CC, Ou TT, Wu CC. Global DNA methylation, DNMT1, and MBD2 in patients with systemic lupus erythematosus. Lupus. 2010;20:131–136. doi: 10.1177/0961203310381517. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Platt A, Upmanyu R, et al. IL-6 pathway- driven investigation of response to IL-6 receptor inhibition in rheumatoid arthritis. BMJ Open. 2013;3:e003199. doi: 10.1136/bmjopen-2013-003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda-Hernández D, Cruz-Reyes C, Angeles U. Prognostic factors for treatment response in patients with lupus nephritis. Reumatol Clin. 2013;10:164–169. doi: 10.1016/j.reuma.2013.08.001. [DOI] [PubMed] [Google Scholar]