Abstract
Studies examining the role of PD-1 family members in allergic asthma have yielded conflicting results. Using a mouse model of allergic asthma, we find that blockade of PD-1/PD-L1 has distinct influences on different CD4+ T cell subsets. PD-1/PD-L1 blockade enhances AHR not by altering the magnitude of the underlying Th2 immune response, but by allowing the development of a concomitant Th17 immune response. Supporting differential CD4+ T cell responsiveness to PD-1-mediated inhibition, naïve PD-1−/− mice displayed elevated Th1 and Th17 levels, but diminished Th2 cytokine levels, ligation of PD-1 limited cytokine production by in vitro-polarized Th1 and Th17 cells, but slightly enhanced cytokine production by in vitro-polarized Th2 cells, and PD-1 ligation enhanced Th2 cytokine production by naïve T cells cultured under non-polarizing conditions. These data demonstrate that different CD4+ T cell subsets respond differentially to PD-1 ligation and may explain some of the variable results observed in control of allergic asthma by the PD-1 family members. As the PD-1/PD-L1 axis limits asthma severity by constraining Th17 cell activity, this suggests that severe allergic asthma may be associated with a defective PD-1/PD-L1 regulatory axis in some individuals.
Keywords: PD-1/PD-L1, Th1/Th2/Th17, Asthma
Introduction
The prevalence of allergic asthma continues to rise in developed nations. As overproduction of Th2 cytokines such as IL-4, IL-5, and IL-13 following allergen exposure are sufficient to explain many of the changes associated with allergic asthma (elevated IgE production, eosinophil dominated inflammation, airway hyperreactivity (AHR)), the Th2 cell is considered central to disease pathology[1]. However, other T cell subsets can markedly influence the course of disease. For example, regulatory T cells have been ascribed a protective role in both mouse models of allergic asthma and human disease[2–4]. In contrast, recent evidence suggests that the production of Th17-associated cytokines my drive the development of more severe disease[5–7]. Thus, factors that subtly alter the balance of T cell effector function serve as important regulators of susceptibility to allergen-induced AHR.
As professional antigen presenting cells dendritic cells (DCs) within the lung integrate a multitude of environmental signals that drive the development of pathogenic or protective T cell responses and as such, play an important role in regulating the development of allergen-induced AHR. One method of conveying these integrated signals to developing T cells is through the expression of the PD-1 family of co-stimulatory molecules. PD-1 (CD279) is expressed primarily by activated CD8+ T cells, CD4++ T cells, and B cells[8]. It binds two unique ligands, PD-L1 (CD274), expressed on both non-hematopoietic and hematopoietic including DCs, and PD-L2 (CD273) whose expression is restricted to activated APCs[9, 10]. However, despite expression on both CD4+ and 8 T cells, the actions of PD-1 in vivo are best described in 8 T cells where high PD-1 drives “exhaustion” of virus-specific 8 T cells observed following chronic infection[11, 12] and restrains 8 memory development after acute infection with lower respiratory tract infections with human metapneumovirus, respiratory syncytial virus, or influenza virus[13]. In contrast to its well-defined role in controlling 8 responses, the importance of PD-1 in regulating different effector CD4+ T cell responses is poorly studied. In CD4+ T cells, PD-1 is best known as a promoter of CD4+CD25+ regulatory T cell activity[14] and the capacity of pulmonary plasmacytoid DCs to induce Tregs and limit asthma development has been linked to their elevated expression of PD-L1[15]. Interestingly, despite the characterization of PD-1 as a driver of Treg development, the role of the PD-1/PD-L axis in regulating allergic asthma is uncertain, with some reports suggesting a protective role for this pathway[15–19], and others reporting a pro-asthmatic role for either PD-L1[17] or PD-L2[20, 21]. Collectively, these data suggest that the ability of PD-1 family members to control CD4+ T cell responses may be more complicated than previously appreciated.
Herein we sought to clarify the role of the PD-1/PD-L1 axis in control of allergic asthma. In contrast to our previous observations of limited impact of PD-1 blockade on allergen-induced AHR in A/J mice, in which allergen uptake is primarily by pulmonary myeloid dendritic cells (mDCs), we found that PD-L1 and PD-1 blockade in C3H mice (a model of mild AHR in which allergen uptake is primarily by plasmacytoid dendritic cells (pDCs)) significantly enhanced the severity of allergen-induced AHR. Surprisingly, the enhanced AHR was associated a shift in the pulmonary inflammatory cell profile towards increased pulmonary neutrophil infiltration and increased production of Th17-associated cytokines while Th2 cytokine responses were unaltered. These findings suggest that the PD-1/PD-L1 axis may variably regulate the activity of different effector CD4+ T cell subsets, and may have differential capacity to inhibit T cell responses in different strains of mice. Consistent with these findings, we observed increased Th1- and Th17-associated cytokine production, concomitant with decreased Th2-associated cytokine production in naïve Pdcd1−/− mice. Additionally, PD-1 ligation (with rPD-L1-Fc) during in vitro Th1/Th17 differentiation or re-stimulation significantly decreased Th1/Th17 cytokine production, while stimulation of PD-1 on Th2 cells via either PD-L1 or PD-L2 enhanced Th2 cytokine production. The ability of PD-1 signaling to enhance Th2 differentiation was even noted in cultures of naïve CD4+ T cells stimulated in the absence of any additional T cell skewing cytokines, wherein PD-1 stimulation was associated with increased expression of the Th2-associated transcription factor GATA3. Collectively, these data suggest that overall regulation of CD4+ T cell responses by PD-1 is more complicated than previously anticipated, and varies between different subsets and strains of mice. It seems likely that differences in cell type and strain responsiveness may have contributed to some of the conflicting data regarding the role of PD-1 family members in allergic asthma.
Results
PD-1 blockade enhances Th17, but not Th2 responses in a mouse model of allergic asthma, in select mouse strains
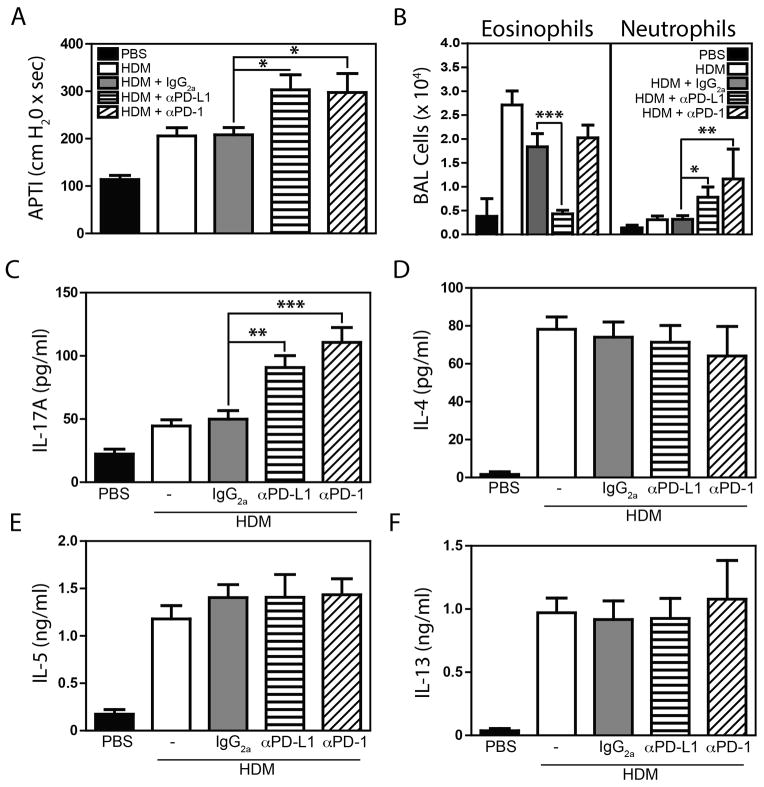
After intratracheal allergen exposure, A/J mice develop allergen-induced airway hyperresponsiveness (AHR) associated with allergen uptake by pulmonary mDCs, and a mixed Th2/Th17 response[7, 22]. In A/J mice, PD-L2 blockade enhanced DC-derived IL-12 production and diminishing AHR, while PD-1 blockade had no impact on AHR severity[20]. In contrast to A/J mice, C3H mice develop mild AHR associated with allergen uptake by pulmonary pDCs and Th2 differentiation[7, 22]. As pDCs exert an anti-asthmatic influence through PD-L1 expression[15], we hypothesized the PD-1/PD-L1 axis may play a greater role in C3H mice. To determine the roles of the PD-1 family members in regulating asthma development in a more asthma-resistant strain, we treated C3H/HeJ mice with house dust mite extract (HDM) in the presence of an isotype control mAb (IgG2a), or blocking mAbs to PD-L2 (clone TY25)[23], PD-L1 (clone MIH-6)[9], or PD-1 (clone RMP1-14)[24]. Consistent with our previous results[20], blockade of PD-L2 in C3H mice decreased AHR, and increased circulating IL-12 levels, (Supplementary Figure 1). However, in contrast to the results reported in A/J animals, blockade of either PD-1 or PD-L1 in HDM-exposed C3H animals resulted in a significant increase in the severity of AHR (Fig 1A). Consistent with our previous results, these findings suggest that PD-L2 acts in a PD-1 independent manner[20]. However, these data also demonstrate a protective role for the PD-1/PD-L1 axis selectively in an asthma-resistant strain.
Figure 1.
Experimental asthma was induced, and PD-1 or PD-L1 was blocked as described in Materials and Methods. At sacrifice, AHR (A) and BAL cellularity (B) were assessed. IL-17A (C), IL-4 (D), IL-5 (E) and IL-13 (F) production by lung cells re-stimulated with HDM (30 μg/ml). Mean + SEM shown. n = 14 – 20 mice pooled from 4 independent experiments. *, ** and *** indicate p < 0.05, p < 0.01, and p < 0.001 between groups (ANOVA).
To dissect the mechanisms responsible for increased AHR observed following PD-1 and PD-L1 blockade, we assessed cellular recruitment to the airways. HDM treatment induced significant pulmonary eosinophilia that was not impacted by isotype or anti-PD-1 treatment (Fig 1B). Surprisingly, anti-PD-L1 completely abrogated eosinophil recruitment (Fig 1B). The reason for the decreased eosinophilia in anti-PD-L1 treated mice is unclear, however, flow cytometric analysis revealed that both bone marrow derived, and pulmonary eosinophils recruited to the lung after HDM-exposure were PD-L1 positive (Supplementary Figure 2) suggesting direct interactions between anti-PD-L1 and eosinophils may contribute. Only a small increase in the number of neutrophils was observed in HDM, or HDM + isotype animals, but treatment of mice with either anti-PD-1 or anti-PD-L1 significantly enhanced pulmonary neutrophilia (Fig 1B). As neutrophil recruitment is associated with Th17 responses, we assessed cytokine production in HDM-stimulated lung cell cultures. Consistent with an enhanced Th17 response, mice receiving anti-PD-1 or anti-PD-L1 produced significantly greater levels of IL-17A than those receiving HDM or HDM + isotype (Fig 1C). In contrast, production of the Th2 cytokines IL-4, IL-5, IL-13 (Fig 1D – 1F), and IL-10, associated with a regulatory T cell response (Supplementary Figure 3) were not significantly altered in cells from anti-PD-1 or -PD-L1 treated mice. IFNγ was not detectable in any HDM-restimulated cultures.
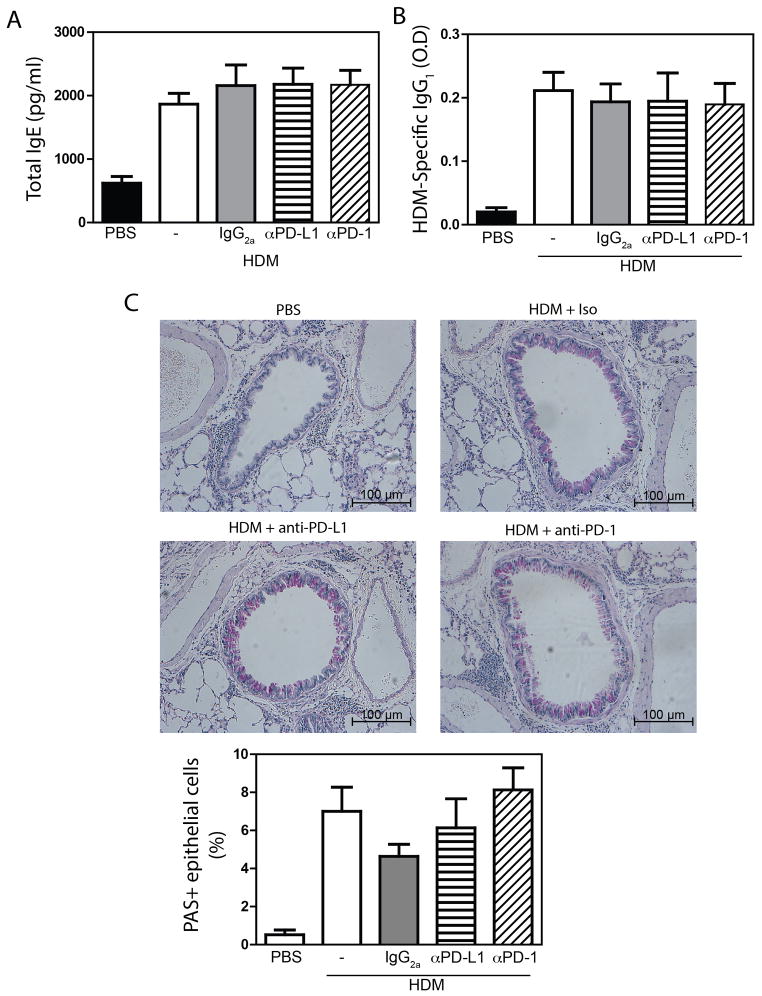
To further characterize the allergic response in mice treated with anti-PD-1/PD-L1, we also assessed serum immunoglobulin levels, and mucus production. HDM exposure induced a marked upregulation in both total IgE (Fig 2A) (a surrogate for antigen specific IgE[25]) and HDM-specific IgG1 (Fig 2B). However, levels of both IgE and IgG1 remained unaffected by anti-PD-1 or anti-PD-L1 (Fig 2A, B). Similarly, while HDM + isotype treated mice displayed a marked increase in the frequency of periodic acid-Schiff positive mucus cells, this was not impacted by PD-1 or PD-L1 blockade (Fig 2C). Thus, in a model of allergen-induced AHR, the PD-1:PD-L1 axis has a limited role in inhibiting Th2 immune responses, but plays an important role in constraining Th17 responses. The differential effect of PD-1:PD-L1 blockade on Th2 and Th17 responses suggest that different effector CD4+ T cell populations may be differentially impacted by PD-1.
Figure 2.
Experimental asthma was induced, and PD-1 or PD-L1 was blocked as described in Materials and Methods. (A) Total IgE, or (B) HDM-specific IgG1. n = 14 – 20 mice pooled from 4 independent experiments. (C) PAS staining to identify mucus secreting cells. PAS+ cell counts from n = 7–10 total mice per group. Mean + SEM shown.
Increased Th1 and Th17, and reduced Th2 cytokine production in Pdcd1−/− mice
To clarify whether PD-1 differentially regulates the activity of CD4+ T cell subsets, we assessed T cell cytokine production using cells from PD-1 deficient mice. To this end, we isolated cells from the spleens of naïve C57Bl/6 and Pdcd1−/− animals. To gauge the relative levels of Th1, Th2 and Th17 activity, cells were stimulated with a variety of polyclonal stimuli (ConA, PMA + Ionomycin, or αCD3 + αCD28), and overall cytokine production was assessed by ELISA. Consistent with the reported inhibitory role for PD-1, spleen cells from Pdcd1−/− mice produced more IFNγ and IL-17A following stimulation when compared to cells from wildtype (WT) mice (Fig 3A, 3B). In contrast, cells from Pdcd1−/− mice produced significantly less IL-4, IL-5 and IL-13 (Fig 3C–E). Similar results were observed when T cells were isolated from the lungs (Supplementary Figure 4). Thus, regardless of the method of T cell stimulation, or the source of cells, PD-1 limits Th1- and Th17-associated cytokine production, while potentially stimulating Th2-associated cytokine production.
Figure 3.
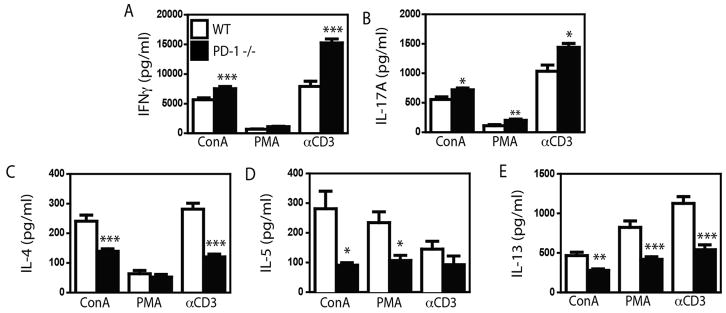
Cytokine levels in the supernatants of spleen cells isolated from naïve WT C57Bl/6 (open bars) and PD-1 −/− (closed bars) mice that were stimulated with ConA, PMA + Ionomycin (PMA), or αCD3 + αCD28. Tissue culture supernatant was collected and assayed for cytokines indicated by ELISA. Mean + SEM shown. n = 5 WT and 4 PD-1 −/− mice. Representative data from 1 of 3 experiments. *, ** and *** indicate p < 0.05, p < 0.01, and p < 0.001 between strains (Student’s t test).
To determine if PD-1 signaling impacted the frequency of Th1, Th2 or Th17 cells in vivo, the frequency of cytokine-secreting CD4+ T cells was determined in WT and Pdcd1−/− mice by flow cytometry (gating strategy depicted in Supplementary Figure 5). Consistent with earlier data, a significantly greater frequency of pulmonary and splenic CD4+IFNγ+ and CD4+IL-17A+ cells were observed in Pdcd1−/− mice, while the frequency of CD4+IL-13+ cells did not differ in Pdcd1−/− and WT mice (Fig 4A). The increased frequency of CD4+IFNγ+ and CD4+IL-17A+ cells observed in Pdcd1−/− mice also correlated with higher levels of circulating IFNγ and IL-17A (Fig 4B) as determined using an in vivo cytokine capture assay (IVCCA)[26]. Paradoxically, while no change in the frequency of Th2 cells was observed, a small but statistically significant decrease in circulating IL-4 and IL-13 levels was noted (Fig 4B). Collectively, these data suggest that PD-1 selectively restricts Th1 and Th17 responses, but supports basal Th2 responses in naïve hosts.
Figure 4.
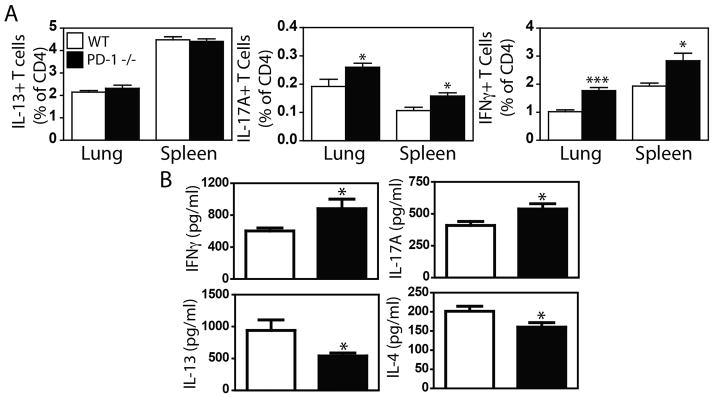
(A) Spleen and lung cells isolated from naïve WT C57Bl/6 (open bars) and PD-1 −/− (closed bars). Cells were stimulated with PMA/Ionomycin overnight, and cultured in the presence of brefeldin A and monensin for 4 hours. (A) Cells were stained with mAbs to CD4+ plus IFNγ, IL-13, or IL-17A for analysis of the frequency of cytokine positive CD4+ cells. (B) Levels of IFNγ, IL-17A, IL-4 and IL-13 secretion were assessed by in vivo cytokine capture assay. Mean + SEM shown. n = 5 WT and 4 PD-1 −/− mice. Representative data from 1 of 3 experiments. * and *** indicate p < 0.05, and p < 0.001 between strains (Student’s t test).
PD-1 stimulation limits cytokine production of established Th1 and Th17 cells, while enhancing Th2 cytokine production
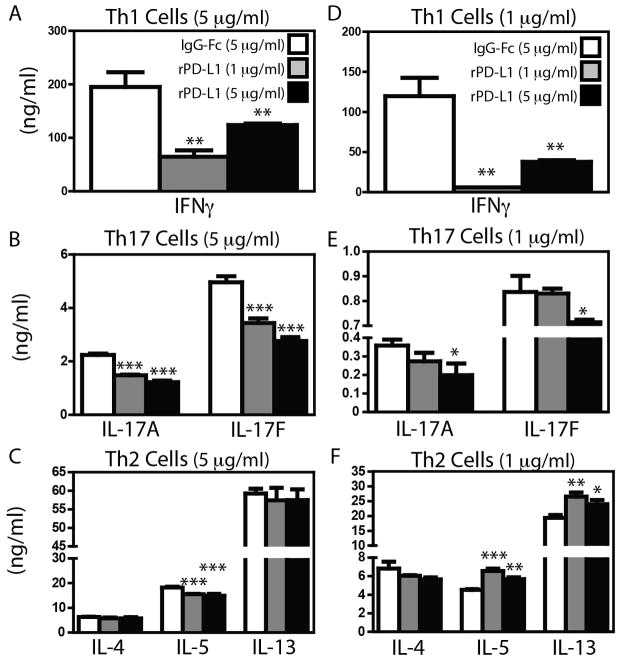
Because PD-1 signaling supports Treg differentiation and activity[14], it is possible that alterations in effector cytokine production observed in Pdcd1−/− and anti-PD-1/PD-L1 treated C3H/HeJ mice may be secondary to altered Treg activity. Therefore, to limit the influence of PD-1 to a single effector T cell subset, we moved to an in vitro system. We isolated naïve (CD4+CD62L+CD25neg) T cells from the lymph nodes of naive C3H/HeJ mice, and cultured them under Th1-, Th2-, or Th17-polarizing conditions. As expected, Th1-polarized T cells expressed abundant message for Tbx21 and Ifng, Th2-polarized cells expressed Gata3, Il4, and Il13, and Th17-polarized cells expressed Rorc, Il17a, and Il17f (Table 1). We then re-stimulated identical numbers of in vitro Th1, Th2 or Th17-polarized T cells on plates coated with αCD3 (5 μg/ml) plus either IgG-Fc or PD-L1-Ig. Compared to Th1 or Th17 cells re-stimulated in the presence of IgG-Fc, Th1 and Th17 cells re-stimulated in the presence of PD-L1-Ig showed marked inhibition of either IFNγ (~66% inhibition - Fig 5A) or IL-17A and IL-17F (~45% inhibition - Fig 5B) production. Interestingly, the Th1 cells restimulated in the presence of 1 μg/ml of rPD-L1 demonstrated more substantial inhibition (66%) than those restimulated in the presence of 5 μg/ml of rPD-L1 (40%) (Fig 5A). In contrast, the magnitude of IL-17A and IL-17F inhibition was dependent upon the amount of PD-L1-Ig present as 5 μg/ml PD-L1-Ig resulted in greater inhibition than 1 μg/ml PD-L1-Ig (Fig 5B). Consistent with our previous observations that Th2 cells are poorly regulated by the PD-1/PD-L1 axis in Pdcd1−/− and anti-PD-1/PD-L1-treated C3H/HeJ mice, PD-1 ligation on Th2 cells had no effect on IL-4 or IL-13 production, and only marginally inhibited IL-5 production (~10%) (Fig 5C). Restimulation of skewed Th2 cells on plates coated with αCD3 (5 μg/ml) and PD-L2-Ig similarly enhanced cytokine production (Supplementary Figure 6). Furthermore, alteration of cytokine production in cultures of skewed Th1, Th2 or Th17 cells was not a result of increased cell death or induction of Treg cells (assessed by flow cytometry – gating strategy depicted in Supplementary Figure 7A), as neither the frequency of dead cells (Supplementary Figure 7B) nor the frequency of Foxp3+ T cells (Supplementary Figure 7C) were consistently altered in cultures restimulated in the presence of PD-L1-Ig or PD-L2-Ig. These suggests that stimulation of PD-1 on Th2 cells with either PD-1 ligand is sufficient to increase their cytokine production.
Table 1.
Gene expression by Th1, Th2 and Th17 skewed T cells
| Gene | Th1 | Th2 | Th17 |
|---|---|---|---|
|
| |||
| Rorc | 5,785 ± 725 | 3,667 ± 549 | 121,156 ± 10,373 |
| IL-17A | 0 | 11 ± 11 | 34,675 ± 2,081 |
| IL-17F | 84 ± 11 | 33 ± 13 | 175,785 ± 24,214 |
| GATA3 | 6,506 ± 877 | 88,314 ± 10,545 | 20,792 ± 485 |
| IL-13 | 24 ± 9 | 3,450 ± 278 | 21 ± 21 |
| IL-4 | 126 ± 11 | 3,853 ± 154 | 59 ± 38 |
| Tbet | 12,429 ± 929 | 91 ± 25 | 163 ± 28 |
| IFNγ | 56,152 ± 1,282 | 365 ± 60 | 225 ± 183 |
Figure 5.
Naïve C3H/HeJ CD4+CD62L+CD25neg T cells were skewed towards Th1, Th2 and Th17 cells as described in Materials and Methods. After skewing without PD-L1, cells were rested for 24 hours, and 5 × 104 were stimulated with αCD3 at 5 μg/ml (A – C) or 1 μg/ml (D – F) in the presence of IgG-Fc (5 μg/ml) (open bars), or rPD-L1-Fc at 1 μg/ml (grey bars), or 5 μg/ml (black bars). After 72 hours, tissue culture supernatant was collected for analysis of cytokines by ELISA. Mean + SEM shown. n = 4 individual cultures per condition. Representative data from 1 of 2 experiments. *, ** and *** indicate p < 0.05, p < 0.01, and p < 0.001 (ANOVA).
PD-1 recruits phosphatases to the TCR complex, resulting in dephosphorylation of signaling molecules downstream of TCR activation[27]. Thus, the inability of PD-1 to limit cytokine production by established Th2 cells may be due to excessively strong activation signals overwhelming the capacity of PD-1-activated phosphatases to limit T cell activation. Thus, we assessed the capacity of PD-1 to inhibit Th1, Th2 and Th17 cells stimulated with a lower dose of αCD3 (1 μg/ml). Stimulation with 1 μg/ml αCD3, resulted in a 50 – 85% reduction in overall cytokine production (Fig 5D–F) compared to cultures re-stimulated with 5 μg/ml αCD3 (Fig 5A–C). However, even with reduced TCR stimulation, re-stimulation of Th1 or Th17 cells in the presence rPD-L1 significantly decreased IFNγ (Fig 5D), or IL-17A and IL-17F production (Fig 5E). In contrast, Th2 cells stimulated with 1 μg/ml of αCD3 in the presence of rPD-L1 produced significantly more (~30%) IL-5 and IL-13 (Fig 5F). Again, restimulation of skewed Th2 cells in the presence of PD-L2-Ig also enhanced cytokine production (Supplementary Figure 8), and neither PD-L1-Ig or PD-L2-Ig consistently altered cell viability (Supplementary Figure 9A) or the frequency of Foxp3+ cells (Supplementary Figure 9B). Thus, while PD-1 signaling significantly inhibits the activity of Th17 cells, PD-1 can provide a co-stimulatory signal for Th2 cells under sub-optimal stimulation conditions.
PD-1 ligation during T cell differentiation inhibits Th1 and Th17 activity, but enhances Th2 cell activity
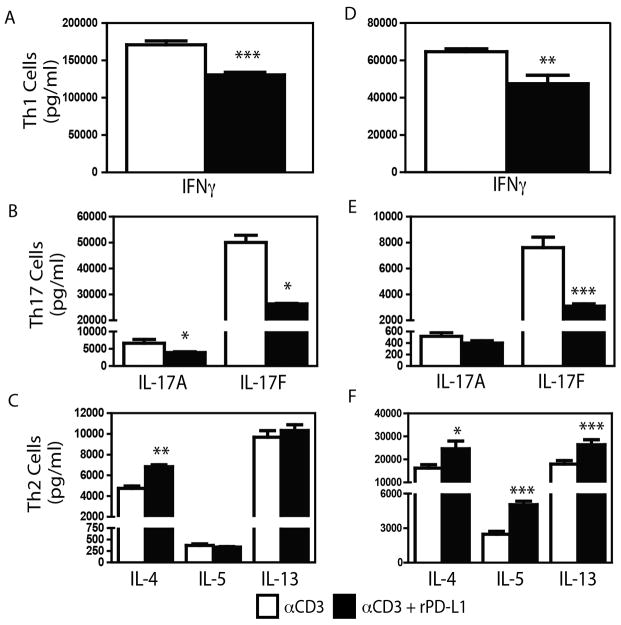
To determine whether PD-1 ligation during effector differentiation had an impact on cytokine producing capacity, naïve T cells were cultured under Th1-, Th2- or Th17-polarizing conditions in the presence of IgG-Fc or rPD-L1. As with Th1- and Th17-polarized cells restimulated in the presence of rPD-L1, the presence of PD-L1-Ig during polarization, decreased cytokine production by both Th1 (IFNγ-Fig 6A) and Th17 cells (IL-17A, IL-17F - Fig 6B). In contrast, the presence of rPD-L1 during Th2 polarization did not significantly impact IL-5 and IL-13 production, but enhanced production of IL-4 by ~ 40% (Fig 6C). However, as ligation of PD-1 limits T cell proliferation[28], it is conceivable that the observed differences in cytokine production were due to differences in the number of T cells present in the culture, rather than an overall decreased capacity for cytokine production. To determine if overall cytokine producing capacity was altered by polarization in the presence of PD-L1, T cells polarized in the presence of IgG-Fc or rPD-L1-Ig were rested overnight, and equivalent numbers of T cells were re-stimulated for 24 hours with αCD3/αCD28 (in the absence of either IgG-Fc or PD-L1-Ig). Th1 and Th17 cells differentiated in the presence of rPD-L1 produced significantly lower IFNγ or IL-17A and IL-17F (Fig 6D, E) respectively, while rPD-L1-polarized Th2 cells produced significantly greater levels of IL-4, IL-5 and IL-13 (Fig 6F). Th2 cells polarized in the presence of PD-L2-Ig displayed elevated IL-4 production, and comparable IL-5 and IL-13 production in the skewing cultures, but significantly increased levels of cytokines were produced upon restimulation (Supplementary Figure 10). Skewing in the presence of PD-L1-Ig or PD-L2-Ig did not alter cell death, or induce the differentiation of Tregs (Supplementary Figure 11). Thus, PD-1 ligation during T cell differentiation selectively restricts Th1 and Th17 cell cytokine production, even following restimulation in the absence of further PD-1 ligation. In contrast, PD-1 enhances Th2-derived cytokine production.
Figure 6.
Naïve C3H/HeJ CD4+CD62L+CD25neg T cells were skewed towards Th1, Th2 and Th17 cells as described in Materials and Methods. A–C: T cells were skewed in the presence of plate bound IgG-Fc (5 μg/ml, white bars) or PD-L1-Ig (5 μg/ml, black bars). Cytokine production was measured by ELISA. D–F: After skewing as in A–C, cells were rested, and 5 × 104 cells were stimulated with plate bound αCD3 (5 μg/ml) and soluble αCD28 (5 μg/ml). Mean + SEM shown. n = 4 individual cultures per condition. One representative experiment of two performed shown. *, ** and *** indicate p < 0.05, p < 0.01, and p < 0.001 (Student’s t test).
PD-1 signaling during T cell differentiation promotes a Th2-skewing environment
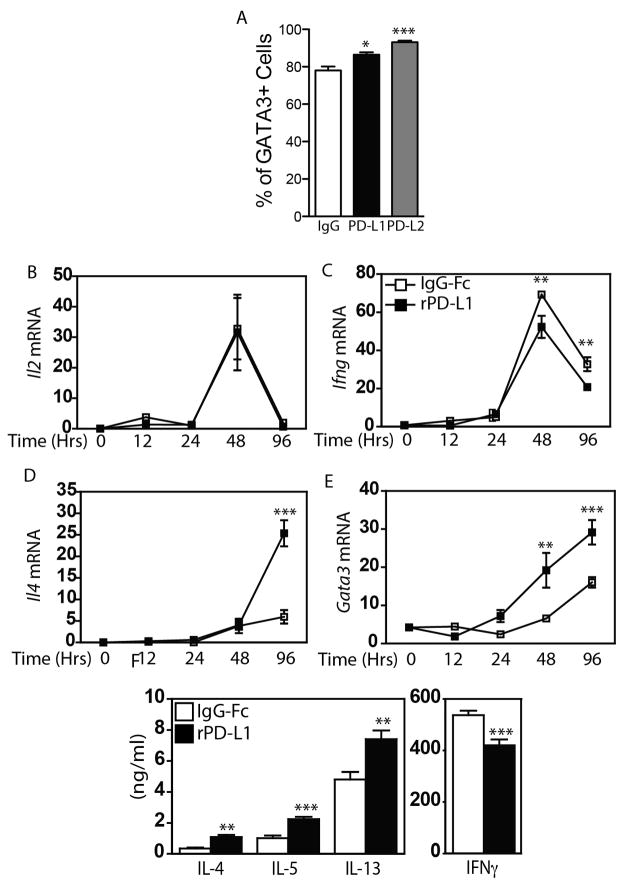
As the presence of rPD-L1-Ig and rPD-L2-Ig during skewing enhanced the production of Th2 cytokines, and GATA3 is a master regulator of Th2 identity, we also assessed the frequency of GATA3+ cells in cells cultured under Th2 polarizing conditions in the presence and absence of PD-1 stimulation. As shown in Figure 7A, polarizing T cells in the presence of PD-L1-Ig and PD-L2-Ig enhanced the frequency of GATA3+ cells, suggesting that PD-1 signaling can support Th2 differentiation. To determine if PD-1 signaling may be sufficient to drive Th2 differentiation directly, naïve T cells were isolated from C3H/HeJ mice, stimulated with αCD3 and αCD28 in the presence of IgG-Fc, or PD-L1-Ig, and the production of cytokines was assessed over time by RT-PCR. Under these conditions, a spike in IL-2 mRNA was observed at 48 hours, but, surprisingly, the magnitude of this induction was not impacted by PD-L1 (Fig 7B). In contrast, while expression of Ifng followed similar kinetics as IL-2, synthesis of Ifng mRNA was significantly reduced by rPD-L1-Ig (Fig 7C). Significant induction of message for IL-17A or IL-17F was not seen under these conditions. Consistent with our previous observations of enhanced Th2 cytokine production in the presence of PD-L1, there was a significant (~5 fold) increase in the levels of Il4 mRNA in cultures stimulated in the presence of PD-L1 (Fig 7D). Similar patterns were seen with expression of Il5 and Il13 (data not shown). As GATA3 expression is an important regulator of Th2 cell differentiation, we also assessed the levels of Gata3 mRNA. Consistent with the rise in expression of mRNA for multiple Th2 cytokines, we also observed a significant enhancement of Gata3 mRNA in PD-L1 stimulated cultures (Fig 7E). Finally, changes in mRNA expression resulted in significantly increased production of IL-4, IL-5 and IL-13, and significantly decreased production of IFNγ (Fig 7E). Collectively, these data show that PD-L1 can provide a co-stimulatory signal that selectively enhances Th2 cell differentiation.
Figure 7.
Naïve C3H/HeJ CD4+CD62L+CD25neg T cells were skewed towards Th2 cells as described in Materials and Methods, and the frequency of viable CD3+ CD4+ GATA3+ T cells was assessed by flow cytometry (A). Naïve C3H/HeJ CD4+CD62L+CD25neg T cells were stimulated with αCD3 and αCD28 in the presence of IgG-Fc (open squares) or PD-L1-Fc (closed squares). mRNA levels of (B) IFNγ, (C) IL-2, (D) IL-4 and (E) GATA3 were assessed at the indicated time points by RT-PCR. (F) Secretion of Th2 (IL-4, IL-5, IL-13) and Th1 (IFNγ) cytokines were measured at times of maximal transcription (Th2 - 96 hours; IFNγ 48 hours). Mean + SEM shown. One representative experiment of two performed shown. Each experiment included three to five replicate wells per condition ** and *** indicate p < 0.01, and p < 0.001 (ANOVA (A), and Student’s t test (B – F)).
Discussion
Our observation of reduced Th1/Th17 cytokine production following PD-1 ligation is consistent with previous reports of PD-1-mediated inhibition of cytokine production in non-polarized CD4+ T cells[13, 29–35]. While inhibition of Th2 cytokine production by PD-1 has been reported (in non-polarized T cells)[30, 34, 36–38], we find that PD-1 consistently enhances Th2 cytokine production. Our findings are consistent with the observation of reduced Th2 cytokine production in PD-L1−/− mice following Leishmania mexicana infection[39], and in the placenta in a model of allogeneic fetomaternal tolerance[31]. Similarly, PD-1 blockade decreases Th2 cytokines in superantigen stimulated PBMCs from cancer patients[29], suggesting that PD-1 can stimulate Th2 responses in human CD4+ cells. While the reasons for the discrepancies regarding an inhibitory/stimulatory role for PD-1 on Th2 cytokine production are unclear, our findings suggest that PD-1 enhances Th2 responses in conditions of sub-optimal TCR stimulation. Thus, in conditions where PD-1 plays an inhibitory role, overall TCR signaling may be very robust, while in conditions where PD-1 plays a stimulatory role, TCR signaling may be comparatively weaker. However, as our study is the first to examine the effect of PD-1 on purified Th2 cells, it is also plausible that inhibition of Th2 cytokine production reported elsewhere[30, 34, 36–38], reflects reduced availability of T cell growth factors (e.g. IL-2) resulting from inhibition of non-Th2 cells, rather than direct effects on Th2 cells.
The reasons for the differential effects of PD-1 ligation in Th2 and Th1/Th17 cells also remain unclear. However, we speculate that this is due to intrinsic differences that govern metabolic activity of Th2 cells and Th1/Th17 cells. Different mTOR complexes are utilized in different CD4+ T cell subsets; while Th1 and Th17 differentiation is supported by mTOR complex 1 (mTORC1), Th2 differentiation requires mTORC2[40, 41]. Activation of mTORC1 in T cells follows the classical mTOR activation pathway and is dependent upon the PI3K/AKT pathway[42]. While the mechanisms of mTORC2 activation in Th2 cells is not entirely clear, it may be independent of the CD28/PI3K/Akt pathway[42]. As PD-1 inhibits the activation of the PI3K/Akt pathway in CD4+ T cells[14], it is likely that PD-1 directly limits mTORC1, while mTORC2 may be insensitive to inhibition via PD-1. This is an area of ongoing investigation in our laboratory.
We observe that PD-1 ligation enhances both GATA3 expression, and production of IL-4. As GATA3 expression is both downstream of IL-4 signaling[43] and can promote IL-4 expression[44, 45], it is unclear whether PD-1 influences IL-4 production, which indirectly enhances Gata3 expression, or vice versa. However, it is interesting to note that when PD-1 is ligated on naïve T cells under non-polarizing conditions, increased Gata3 expression is observed at 24 hours, a time point that precedes a significant induction of Il4 mRNA (96 hours). Thus, PD-1 may influence Th2 differentiation by directly enhancing GATA3 expression. This finding also argues that the ability of PD-1 to influence Th2 differentiation may be most evident in conditions in which there are no other powerful GATA3-inducing factors present (i.e. in the absence of IL-4). Such results may help reconcile our potentially confounding observations of increased Th2 cytokine production in naive Pdcd1−/− mice and the failure of anti-PD-1 treatment of C3H/HeJ to substantially alter Th2 cytokine production in a model of allergic asthma.
The role of PD-1 family members in allergic asthma is complex. Some reports suggest a protective role for the PD-1 family[15–17, 46] and others describe a pro-asthmatic role for either PD-L1 (resulting from decreased iNKT cell-derived IFNγ production[17]) or PD-L2 (resulting from a PD-1 independent reduction in DC-derived IL-12 production[20, 21]). Moreover, in contrast to our observation that PD-1/PD-L1 blockade enhances asthma severity in C3H/HeJ mice described herein, we previously found that PD-1/PD-L1 blockade has no impact asthma severity in A/J animals[20], suggesting that the capacity of PD-1 ligation to regulate CD4+ T cell cytokine production may vary by strain. In support of this, stimulation of C3H/HeJ, or BALB/c-derived (data not shown) Th1 or Th17 cells in the presence of rPD-L1 significantly inhibits Th1 and Th17 cytokine production, while similar stimulation of A/J-derived Th1 or Th17 cells fails to alter cytokine production (data not shown), suggesting that PD-1 responsiveness may be limited in A/J mice. As we observe increased pDC activity in C3H mice[20], and pDCs have been shown to limit the development of AHR through a PD-1 dependent mechanism[15], it is plausible that the selective effect of PD-1/PD-L1 blockade in C3H mice may be indicative of a major regulatory role for pDCs. However, given the wide expression pattern of PD-L1 on both hematopoietic and non-hematopoietic cells[9, 10] it seems likely that the global PD-1 unresponsiveness present in A/J mice plays a much larger role in the development of Th17 cells and severe asthma than a decreased frequency of activated pulmonary pDCs. It is also possible that natural variation in PD-1-responsiveness amongst common laboratory mouse strains may contribute to some of the confusion regarding the role of PD-1/PD-L1/PD-L2 in control of allergic asthma.
While asthma is widely regarded as the result of a Th2-dominated immune response, there is increasing evidence in both humans[5] and animal models[7] that a mixed Th2/Th17 response drives the development of more severe AHR. However, while IL-17A enhances IL-13 driven AHR, IL-17A does not exacerbate all aspects of asthma, inasmuch as IL-17A neutralization has no impact on IgE synthesis or mucus cell metaplasia in HDM-sensitized A/J mice[7]. The failure of PD-1 to stimulate Th2 responses in this model may be related to the Th2-dominant immune response, because PD-1 does not stimulate Th2 cytokine production under conditions of high TCR stimulation (Fig 4C). Rather, as only the Th17 arm of the immune system is constrained by PD-1/PD-L1 in our model of allergic asthma, these data suggest that the Th17 cell, rather than the Th2 cell is the target of PD-1/PD-L1 blockade. Thus, our observation that in vivo PD-1/PD-L1 blockade enhances AHR, pulmonary neutrophilia and IL-17A production, without impact on Th2 cytokine synthesis, IgE levels or mucus cell metaplasia, is wholly consistent with a pathogenic role for IL-17A.
From a clinical perspective, these data suggest that the PD-1/PD-L1 axis does exert an inhibitory influence in asthma, but is only involved in regulating the severity of disease, no disease initiation. Our observation that the PD-1/PD-L1 axis constrains the development of severe allergic asthma by targeting Th17 cell activity suggests that in certain individuals, more severe allergic asthma may be associated with a defective PD-1/PD-L1 regulatory axis. Thus these findings highlight the complexity of immune regulation by PD-1 and suggest that the variability seen in studies examining the role of PD-1 family members in control of allergic asthma may be due the differential capacity to regulate specific effector CD4+ T cell subsets. As Th17 cells appear to be strongly inhibited by the PD-1/PD-L1 axis, and excessive Th17 responses are associated with the development of more severe allergic asthma, stimulating the PD-1/PD-L1 regulatory axis may represent a novel therapeutic approach for the treatment of severe allergic asthma in certain individuals.
Materials and Methods
Mice
Male C3H/HeJ, C57Bl/6J (Jackson Laboratories), and Pdcd1−/− mice on a C57Bl/6 background (a gift of Tasuku Honjo)[47] were housed in an SPF facility at Cincinnati Children’s Hospital Medical Center. All experiments were approved by the CCHMC IACUC.
Assessment of asthmatic phenotype
Mice were treated with 40 μl PBS, or 100 μg HDM (Greer Laboratories, Lenoir, NC) i.t. on days 0 and 14 and treated with intraperitoneally with 250 μg anti-PD-L1 (MIH6), anti-PD-1 (RMP1-14) or rat IgG2a (GL117) on days 0, 2, 14 and 16. AHR was determined as previously described[20]. BALF was collected in HBSS and cells were places on slides by cytocentrifugation, and stained with Diff-Quik (Dade Behring). Differential cell counts were determined using morphologic criteria under a light microscope by evaluation of ≥ 500 cells/slide. Whole lung cells were restimulated with HDM for 72 hours, and cytokine production was assessed by ELISA using mAbs from eBioscience. To enumerate mucous-producing airway epithelial cells, PAS+ and PAS- epithelial cells were counted from at least 7 medium sized airways, and the percentage of PAS+ cells was calculated for an individual mouse.
Skewing and stimulation of T cell subsets
Naïve CD4+CD25negCD62L+ T cells were isolated using CD4+ T cell isolation kit II (negative selection), CD25 microbead kit (negative selection), and CD62L microbeads (all from Miltenyi Biotec, Auburn, CA). Isolated CD4+CD25negCD62L+ T cells were plated in flasks coated with αCD3 (5 μg/ml) in the presence of αCD28 (5 μg/ml) and Th1-(10 ng/ml IL-12, 1 μg/ml αIL-4), Th2- (10 ng/ml IL-4, μg/ml αIFNγ), or Th17- (20 ng/ml IL-6, 5 ng/ml TGFβ1, 10 ng/ml IL-23, 1 μg/ml αIL-4, 1 μg/ml αIFNγ) skewing cytokines. Cells were cultured for 5 days. Cultures were skewed or stimulated in the presence of either plate bound IgG-Fc (5 μg/ml), or plate bound PD-L1-Ig (R&D Systems, Minneapolis, MN). Cells were rested overnight in the absence of stimulation, and then restimulated on plates coated with various concentrations of αCD3, IgG-Fc and PD-L1-Ig. Cytokine production was assessed by ELISA using eBioscience mAbs.
Quantitative real-time PCR
PCR primer pairs for S14, Pdcd1, Tbx21, Ifng, Gata3, Il4, Il13, Rorc, Il17a and Il17f were obtained from the mouse primer depot[48] and designed to span an intronic region to avoid co-amplification of genomic DNA. Gene expression was analysed by real-time PCR using the iCycler system (BioRad) and SYBR green.
IVCCA
A kit for measuring in vivo IL-4 production by IVCCA was purchased from Becton-Dickinson. For measurement of in vivo IL-13, IFNγ and IL-17A production was done following injection of mice with biotin-labeled eBio1316H (IL-13), R46A2 (IFNγ) or IL-17A (eBio17B7) and using plates coated with eBio13A (IL-13), XMG1.2 (IFNγ) or eBio17CK15A5 (IL-17A) (all from eBioscience).
Assessment of PD-1 and PD-L1 expression on eosinophils
Total bone marrow cells were isolated, and erythrocytes were lysed by RBC lysis buffer (Sigma-Aldrich). After a density gradient of Histopaque 1083 (Sigma-Aldrich), the low-density bone marrow (LDBM) cells were collected and plated at 1 × 106 cells/ml in IMDM (Life Technologies) supplemented with 10% FBS (HyClone), penicillin-streptomycin (Life Technologies), 200 mM l-glutamine (Life Technologies), and 55 μM 2-ME (Sigma-Aldrich). During the first 4 d, the medium contained stem cell factor (PeproTech) at 50 ng/ml each. From day 4 to day 14, the cells were cultured in medium containing 10 ng/ml IL-5 (PeproTech). The medium was changed every 2 d until day 14. Eosinophils were stained with mAb to CC11b (clone M1/70; eBioscience) and CCR3 (clone 83101, RnD Systems). To assess PD-L1 expression on eosinophils in vivo, mice were treated with HDM as above, and eosinophils were identified as CD3negCCR3+ cells following staining with anti-CD3 (clone 145-2C11; eBioscience) and CCR3 (clone 83101, RnD Systems). PD-1 and PD-L1 expression was detected using antibodies to PD-1 (clone RMP1-30; eBioscience) and PD-L1 (clone MIH5; eBioscience)
Statistical Analysis
To determine differences between multiple groups, analysis of variance (ANOVA) was used with post hoc comparisons using Tukey’s method. For comparison between two groups, a Student’s t-test was performed. Significance was assumed at p < 0.05.
Supplementary Material
Acknowledgments
Funded by a Parker B. Francis Fellowship (IPL, SL), an ATS Unrestricted Research Grant (IPL), ALA Biomedical Research Grant (IPL), NHLBI R01 HL67736-09 (MWK), NIAID U19 A1070235 (MWK), NIAID R01 A1083315 (MWK), and NHLBI R01 HL122300 (IPL).
Footnotes
Conflict of Interest Disclosure:
The authors have no potential conflicts of interest to disclose.
References
- 1.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 2.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 10.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 12.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 13.Erickson JJ, Gilchuk P, Hastings AK, Tollefson SJ, Johnson M, Downing MB, Boyd KL, Johnson JE, Kim AS, Joyce S, Williams JV. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Invest. 2012;122:2967–2982. doi: 10.1172/JCI62860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, Smit JJ, Coyle A, Clausen BE, Hoogsteden HC, Lambrecht BN, Hammad H. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol. 2009;183:1074–1082. doi: 10.4049/jimmunol.0900471. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, Tsushima F, Hoshino T, Aizawa H, Akiba H, Pardoll D, Hara N, Yagita H, Azuma M, Nakanishi Y. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol. 2004;172:2530–2541. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- 17.Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, Sharpe AH, Umetsu DT, Dekruyff RH. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3:81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto K, Fukuyama S, Eguchi-Tsuda M, Nakano T, Matsumoto T, Matsumura M, Moriwaki A, Kan-o K, Wada Y, Yagita H, Shin T, Pardoll DM, Patcharee R, Azuma M, Nakanishi Y, Inoue H. B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem Biophys Res Commun. 2008;365:170–175. doi: 10.1016/j.bbrc.2007.10.156. [DOI] [PubMed] [Google Scholar]
- 19.McGee HS, Yagita H, Shao Z, Agrawal DK. PD-1 Antibody Blocks Therapeutic Effects of T-regulatory Cells in Cockroach Antigen-induced Allergic Asthma. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0258OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewkowich IP, Lajoie S, Stoffers SL, Suzuki Y, Richgels PK, Dienger K, Sproles AA, Yagita H, Hamid Q, Wills-Karp M. PD-L2 modulates asthma severity by directly decreasing dendritic cell IL-12 production. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oflazoglu E, Swart DA, Anders-Bartholo P, Jessup HK, Norment AM, Lawrence WA, Brasel K, Tocker JE, Horan T, Welcher AA, Fitzpatrick DR. Paradoxical role of programmed death-1 ligand 2 in Th2 immune responses in vitro and in a mouse asthma model in vivo. Eur J Immunol. 2004;34:3326–3336. doi: 10.1002/eji.200425197. [DOI] [PubMed] [Google Scholar]
- 22.Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One. 2008;3:e3879. doi: 10.1371/journal.pone.0003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsushima F, Iwai H, Otsuki N, Abe M, Hirose S, Yamazaki T, Akiba H, Yagita H, Takahashi Y, Omura K, Okumura K, Azuma M. Preferential contribution of B7-H1 to programmed death-1-mediated regulation of hapten-specific allergic inflammatory responses. Eur J Immunol. 2003;33:2773–2782. doi: 10.1002/eji.200324084. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki T, Akiba H, Koyanagi A, Azuma M, Yagita H, Okumura K. Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-gamma-induced nitric oxide production. J Immunol. 2005;175:1586–1592. doi: 10.4049/jimmunol.175.3.1586. [DOI] [PubMed] [Google Scholar]
- 25.Lewkowich IP, Rempel JD, HayGlass KT. In vivo IgE levels in exogenous antigen stimulated responses: measurement of total IgE as a valid, simple surrogate for Ag-specific IgE. J Immunol Methods. 2004;286:123–132. doi: 10.1016/j.jim.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Finkelman FD, Morris SC. Development of an assay to measure in vivo cytokine production in the mouse. Int Immunol. 1999;11:1811–1818. doi: 10.1093/intimm/11.11.1811. [DOI] [PubMed] [Google Scholar]
- 27.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaudhary D. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 28.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P, Punt CJ, Jacobs JF, Schalken JA, Oosterwijk E, van Eenennaam H, Boots AM. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35:169–178. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- 30.Tousif S, Singh Y, Prasad DV, Sharma P, Van Kaer L, Das G. T cells from Programmed Death-1 deficient mice respond poorly to Mycobacterium tuberculosis infection. PLoS One. 2011;6:e19864. doi: 10.1371/journal.pone.0019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, Sayegh MH, Guleria I. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179:5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 32.Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR., Jr Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 35.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, Kurahara C, Lott F, Sun N, Welcher AA, Dong C. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Addio F, Riella LV, Mfarrej BG, Chabtini L, Adams LT, Yeung M, Yagita H, Azuma M, Sayegh MH, Guleria I. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol. 2011;187:4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Werf N, Redpath SA, Azuma M, Yagita H, Taylor MD. Th2 cell-intrinsic hypo-responsiveness determines susceptibility to helminth infection. PLoS Pathog. 2013;9:e1003215. doi: 10.1371/journal.ppat.1003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang SC, Greenwald RJ, Latchman YE, Rosas L, Satoskar A, Freeman GJ, Sharpe AH. PD-L1 and PD-L2 have distinct roles in regulating host immunity to cutaneous leishmaniasis. Eur J Immunol. 2006;36:58–64. doi: 10.1002/eji.200535458. [DOI] [PubMed] [Google Scholar]
- 40.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita M, Ukai-Tadenuma M, Kimura M, Omori M, Inami M, Taniguchi M, Nakayama T. Identification of a conserved GATA3 response element upstream proximal from the interleukin-13 gene locus. J Biol Chem. 2002;277:42399–42408. doi: 10.1074/jbc.M205876200. [DOI] [PubMed] [Google Scholar]
- 45.Hofer T, Nathansen H, Lohning M, Radbruch A, Heinrich R. GATA-3 transcriptional imprinting in Th2 lymphocytes: a mathematical model. Proc Natl Acad Sci U S A. 2002;99:9364–9368. doi: 10.1073/pnas.142284699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerzerho J, Maazi H, Speak AO, Szely N, Lombardi V, Khoo B, Geryak S, Lam J, Soroosh P, Van Snick J, Akbari O. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol. 2013;131:1048–1057. 1057 e1041–1042. doi: 10.1016/j.jaci.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 48.Cui W, Taub DD, Gardner K. qPrimerDepot: a primer database for quantitative real time PCR. Nucleic Acids Res. 2007;35:D805–809. doi: 10.1093/nar/gkl767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.