Abstract
Sorcin is a penta-EF hand calcium binding protein, which is involved in the resistance to chemotherapeutics in cancer cells, and is overexpressed in various cancer cells. However, tumor relapse combined with the development of drug resistance remains a significant problem. Here, we demonstrated that silencing of Sorcin in chemotherapy resistance myeloma U266/ADM and KM3/DDP cell lines resulted in reduced cell proliferation, cell cycle arrest and cell apoptosis. Sorcin siRNA successfully silenced Sorcin mRNA and protein expression. Silencing of Sorcin also significantly reduced the mRNA and protein expression levels of MDR1, MRP1, GST-π, Survinvin, Livin, Bcl-2, Cyclin-D1, phospho-Src, C-myc, p21, NF-κB and phospho-AKT, while p53 expression and caspase-3 and caspase-8 activity significantly increased when compared with control group. Silencing of Sorcin significantly increased the sensitivity of KM3/DDP cells to cisplatin and the sensitivity of U266/ADM to adriamycin, compared to cells untransfected and transfected with negative control shRNA. In addition, intracellular accumulation of Rhodamine 123 significantly increased in KM3/DDP and U266/ADM cells. In summary, our studies indicate that drug resistance can be effectively reversed in cisplatin-resistance and adriamycin-resistant myeloma cells through delivery of siRNAs targeting Sorcin. Assessment of potential as a target for human myeloma treatment is clearly warranted.
Keywords: Sorcin, shRNA, drug resistance-myeloma
Introduction
Chemotherapy plays an important role in various tumors treatment. However, many cancer patients develop drug resistance, leading to poor treatment outcomes [1]. Multidrug resistance (MDR) is one of the major reasons why chemotherapy-based treatment failure occurs [2,3]. Therefore, resolving the issue of tumor MDR may be a potential source of improved therapies.
Sorcin is a soluble resistance-associated calcium-binding protein widely present in many tissues. When compared with normal tissues, Sorcin is over-expressed in tumor tissues [4]. Studies have shown that Sorcin over-expression is closely related to tumor MDR [5,6]. Therefore, clarifying the mechanisms of drug resistance may provide new targets for the diagnosis and treatment of drug-resistant tumors.
RNA interference (RNAi) is a new technology developed in recent years. Because it can specifically and effectively block the target gene expression. Various RNAi strategies have been applied to reverse MDR in different tumor models in vitro by down-regulating genes associated with MDR, such as multidrug resistance 1 (MDR1), multidrug resistance-associated protein (MRP), it is one of the most effective methods capable of reversing tumor MDR [7,8]. In this study, we employed RNAi technology to silence the Sorcin gene of drug-resistant myeloma KM3/DDP and U266/ADM cell lines in order to not only analyze its effects on cell proliferation, apoptosis and cycle but also further investigate its roles in reversing tumor cell drug resistance.
Materials and methods
Cell culture and transfection
Cisplatin-resistant KM3/DDP cell lines and Doxorubicin-resistant U266/ADM cell lines were established in our laboratory. Myeloma KM3/DDP and U266/ADM cell lines were routinely cultured in DMEM medium containing 10% fetal calf serum, 2 µg/mL cisplatin for KM3/DDP (or 0.1 μM doxorubicin for U266/ADM), 100 U/L penicillin and 100 U/L streptomycin under 37°C and 5% CO2 condition. When the cells reached confluence, the medium was changed regularly. The cells were digested with 0.25% trypsin and passaged in 1:2 ratio.
Cells in logarithmic growth phase were seeded in 6-well plates, 5×104 cells/well, to continue the culture in antibiotics and cisplatin-free DMEM medium. When the cells reached 30%~50% confluence 24 h later, Sorcin shRNA vector was mixed with Lipofactamine 2000 at room temperature to transfect KM3/DDP cells and optimize the transfection condition in accordance with the instructions of Lipofactamine 2000 transfection kit. In the meantime, the negative control group was set.
MTS assay to determine the drug sensitivity of KM3/DDP and U266/ADM
KM3/DDP and U266/ADM cells in logarithmic growth phase were seeded in 96-well plates, 4×103 cells/well, 6 duplicate wells in each group, to culture in a 37°C and 5% CO2 incubator. After culturing for 24 h, different concentration gradient of chemotherapeutic drugs (e.g. 0, 0.1 μg/mL, 0.2 μg/mL, 1 μg/mL, 2 μg/mL, 5 μg/mL, 10 μg/mL, 50 μg/ml and 100 μg/mL for cisplatin; 0, 0.5 μg/mL, 1 μg/ml, 2 μg/mL, 3 μg/mL, 4 μg/mL, 5 μg/mL, 6 μg/mL for doxorubicin) was added to continue the culture for 48 h. After aspirating the medium, MTS reagent was added in accordance with the kit instructions to determine the optical density (A) of each well at 490 nm wavelength with a fully automated ELISA analyzer; the mean of 5 parallel wells was calculated. Based on the formula, the calculate cell inhibition rate = [1-(test group mean A-blank group mean A)/(negative group mean A-blank group mean A) ×100%. The above experiments were repeated 3 times.
Flow cytometry to determine cell cycle and apoptosis
After adjusting cell density, KM3/DDP and U266/ADM cells in logarithmic growth phase were seeded in 6 cm culture dishes. Then, the cells were digested with 0.25% trypsin and centrifuged at 2000 r/min for 5 min to collect the cells. The cells were washed twice with PBS and centrifuged again at 2000 r/min for 5 min to collect the cells. The cells were fixed with 500 μL of 70% ethanol at 4°C overnight. On the next day, the ethanol-fixed cells were centrifuged at 2000 r/min, 4°C, for 5 min. After discarding the upper-layer ethanol, the cells were washed twice with cold PBS. In each tube, 500 μL of 50 g/L PI solution was added to re-suspend the cell pellets and stain at room temperature away from light for 30 min. The distribution of cell cycle was determined with BD flow cytometry.
Apoptosis was determined with PI/Annexin V-FITC double-staining technique in accordance with the kit instructions, using negative control plasmid-transfected tumor cells as the control.
Flow cytometry to determine P-glycoprotein expression and rhodamine 123 content
KM3/DDP and U266/ADM cells in logarithmic growth phase of various treatment groups were digested with trypsin and centrifuged. Then, the cells were fixed with 70% volume fraction of ethanol at 4°C for 1 h. After washing twice with PBS, appropriate amount of P-gP monoclonal antibody, approximately 50 μL, was added to incubate at 4°C overnight. After the cells were centrifuged and washed twice with PBS, fluorescein-labeled goat anti-mouse IgG (H+L) secondary antibody was added to allow standing at 4°C for 60 min. After washing twice with PBS, the cells were re-suspended with 0.5 mL PBS to determine P-gP protein expression level with flow cytometry. Cancer cells in logarithmic growth phase of various treatment groups were cultured with 10 μmol/L Rh-123 staining solution for 1 h before the cells were harvested. After the cell density was adjusted to 106 cells/mL, flow cytometry was used to measure Rh-123 fluorescence intensity in the cells (excitation at 488 nm, emission at 560 nm) in order to determine intracellular Rh-123 content.
Western blot analysis
Cell lysate of KM3/DDP and U266/ADM cells in logarithmic growth phase of various treatment groups was collected to extract the proteins. The protein content in cell lysate was determined with BCA assay. Equal amount of proteins were separated in 12% SDS-PAGE and transferred onto a PVDF membrane. The membrane was incubated with monoclonal antibodies (MDR1, MRP1, GST-π, Survivin, Livin, Bcl-2, Cyclin-D1, C-myc, p21, p53, NF-κB, phospho-Src, and phospho-Akt) at 4°C overnight to determine the target proteins. After washing off the primary antibodies, the membrane was incubated with HRP-conjugated secondary antibodies at room temperature for 2 h. After several washes, ECL kit was used to the display immunoblotting bands. The β-actin was used as an internal reference.
Real time-PCR
RNA was routinely extracted from KM3/DDP and U266/ADM cells in logarithmic growth phase of various treatment groups with TRIzol reagent (Invitrogin). The cDNA was synthesized in accordance with the M-MLV reverse transcription kit instructions. The expression of various genes was determined on SYBR Green I dye-based ABI 7500HT fluorescence quantitative PCR using GAPDH as an internal reference. Reaction program: after denaturation at 94°C for 3 min, 35 cycles of amplification was carried out under the following conditions: 95°C 5 s, 60°C annealing temperature 30 s, 72°C 95 s, and 72°C extension for 5 min. The differences in relative expression of genes in various samples were analyzed with 2-ΔΔCT method. The primer sequence of each gene was given in the following Table 1. The primers were synthesized and purified by Sangon Biotech (Shanghai) Co., Ltd.
Table 1.
Primer sequences for real-time PCR study
| Gene | Sequences (5’-3’) |
|---|---|
| Sorcin | Forward: ATCAAGAGGAAGCTGCATGA |
| Reverse: ATTACCGAAGGCAAAGAGGA | |
| MDR1 | Forward: AAATTGGCTTGACAAGTTGTATATGG |
| Reverse: CACCAGCATCATGAGAGGAAGTC | |
| MRP1 | Forward: TCTACCTCCTGTGGCTGAATCTG |
| Reverse: CCGATTGTCTTTGCTCTTCATG | |
| Survivin | Forward: GGACCACCGCATCTCTACAT |
| Reverse: CAACGCGAAAGGAAAGACAG | |
| Bcl-2 | Forward: GGCTGGGATGCCTTTGTG |
| Reverse: GCCAGGAGAAATCAAACAGAGG | |
| GST-π | Forward: CTGGAAGGAGGAGGTGGTG |
| Reverse: GACGCAGGATGGTATTGGAC | |
| Livin | Forward: TCTGAGGAGTTGCGTCTGG |
| Reverse: GCACCTCACCTTGTCCTGAT | |
| Cyclin-D1 | Forward: TATTGCGCTGCTACCGTTGA |
| Reverse: TATTGCGCTGCTACCGTTGA | |
| P21 | Forward: CGCTAATGGCGGGCTG |
| Reverse: CGGTGACAAAGTCGAAGTTCC | |
| MYC | Forward: GCCACGTCTCCACACATCAG |
| Reverse: TGGTGCATTTTCGGTTGTTG | |
| TP53 | Forward: TCAACAAGATGTTTTGCCAACTG |
| Reverse: ATGTGCTGTGACTGCTTGTAGATG | |
| β-actin | Forward: TGACGTGGACATCCGCAAAG |
| Reverse: CTGGAAGGTGCACAGAGAGG |
Caspase-3 and caspase-8 activation analysis
Cancer cells (U266/ADM: 1×106/ml, KM3/DDP: 4×105/ml) in logarithmic growth phase of various treatment groups were treated with reference to the instructions for caspase-3 and caspase-8 activity assay kit. The absorbance at OD405 was determined with a microplate reader to represent the relative activity of caspase-3 and caspase-8.
Immunohistological analysis
All tumor tissues were fixed in neutral buffered 4% formaldehyde and embedded in paraffin. After being dewaxed and rehydrated, slides were incubated in 0.3% H2O2 solution and dried in a microwave in citric acid buffer for 15 min to retrieve antigens. The slices were blocked with normal goat serum for 30 min at 37°C and probed with a rabbit monoclonal antibody to Cox-2 and mouse monoclonal antibodies to Sorcin at 4°C overnight (diluted at 1:300). Biotinylated anti-mouse IgG were added and incubated at 37°C for 30 min before enzyme conjugated HRP-streptavidin was added. In addition, 3, 3’-diaminobenzidine (DAB) was used as the chromogen. Slides were counterstained with hematoxylin, dehydrated in alcohol, and mounted on neutral balsam. Controls were prepared using secondary antibodies only. Two independent, blinded investigators examined all tumor slides randomly. Five views were examined per slide, and 100 cells were observed per view at 400× magnification.
Statistical analysis
The experimental data were expressed as mean±standard deviation. SPSS 12.0 software was used for the analysis. One-way ANOVA was used for the comparison; P<0.05 indicated the statistical significance of the difference.
Results
shRNA suppressed Sorcin mRNA and protein levels in myeloma cell lines
First, we explored the effect of shRNA on Sorcin gene expression in KM3/DDP and U266/ADM cells by qRT-PCR and Western blot analysis. Sorcin gene expression was calculated in relation to the negative control group, which was considered as 100%. As shown in Figure 1, Sorcin shRNA effectively reduced of both Sorcin mRNA and protein levels (P<0.05; relative to the negative control) (Figure 1).
Figure 1.
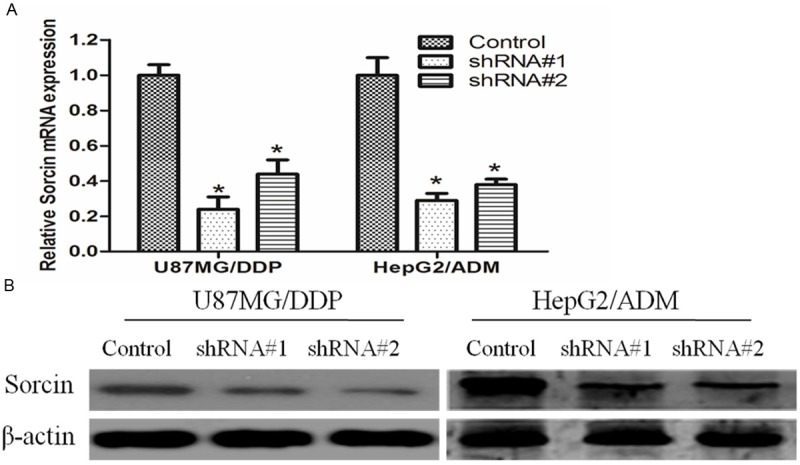
Suppression of Sorcin mRNA and protein expression in KM3/DDP or U266/ADM cells. KM3/DDP or U266/ADM cells were transfected with negative control (NC) siRNA or Sorcin shRNA for 48 h, mRNA and protein expression levels were measured by qRT-PCR and Western blotting. A. The mRNA levels of Sorcin expression were measured by qRT-PCR. Using 2(-ΔΔCt) method. The results are expressed a mean ± SD (n=3); *P<0.05 versus negative control; B. the protein levels of Sorcin in KM3/DDP or U266/ADM cells were measured and normalized to β-actin by western blotting.
Effect of Sorcin silencing on drug sensitivity in human myeloma cell lines
To evaluate whether the change in Sorcin expression effect on drug sensitivity of cancer cell, we examined the drug sensitivity of each treatment group by MTS assay. MTS assay showed that, when compared with the control group, the sensitivity of KM3/DDP cells to cisplatin and the sensitivity of U266/ADM cells to adriamycin significantly increased after Sorcin expression were silenced. The reversal fold of shRNA #1 group and shRNA #2 group was 3.2 and 4.1 in KM3/DDP cells, 2.8 and 3.3 in U266/ADM cells respectively (Figure 2).
Figure 2.
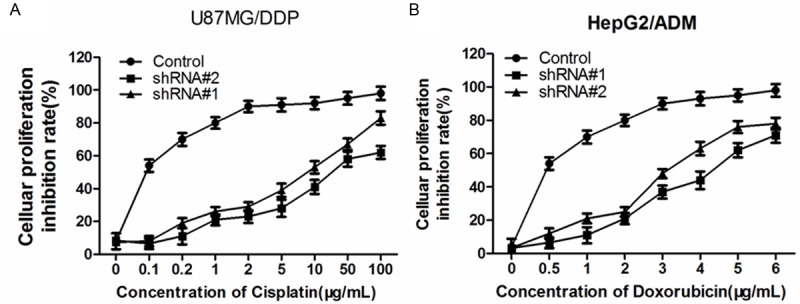
Effect of Sorcin silencing on cell proliferation in human myeloma cell lines. Cells were untransfected (Control), or transfected with Sorcin shRNA#1 or shRNA#2, and then incubated with different concentrations of cisplatin, as indicated for an additional 48 h. Cell survival was determined using the MTS assay. Data are mean ± SD of three independent experiments; *P<0.05 compared with untransfected control cells.
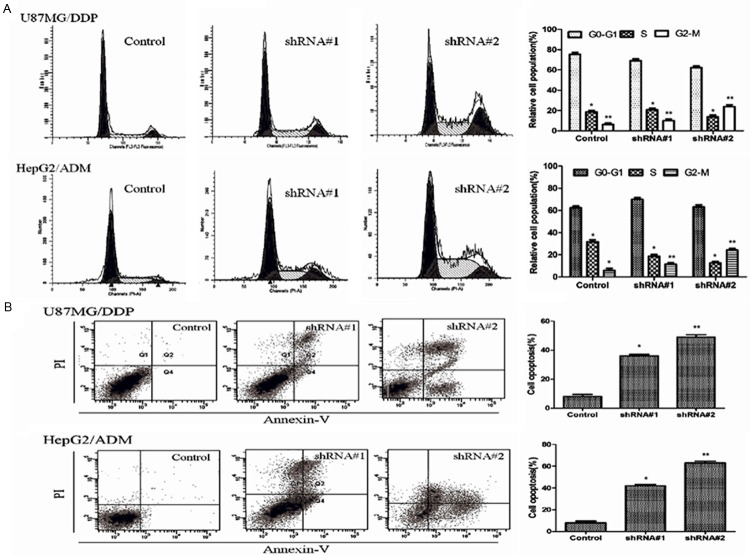
Effects of silencing Sorcin expression on cell cycle and apoptosis
To analyze whether the observed sensitizing effect of Sorcin shRNA was linked to cell cycle distribution and the enhancement of apoptosis, the effects of Sorcin shRNA were evaluated by Flow cytometry. Results showed that when compared with the control group, the cell counts in G2 phase were higher while the cells in G0/G1 phase and G2/M phase significantly increased both in KM3/DDP and U266/ADM cells after Sorcin expression was silenced (Figure 3A). Apoptosis test results showed that KM3/DDP and U266/ADM cells apoptosis rate significantly increased after Sorcin expression was silenced. When compared with the control group, the apoptosis rate in treatment groups was 390 and 602%, in KM3/DDP cells, 510% and 785% in U266/ADM cells respectively (Figure 3B).
Figure 3.
Sorcin silencing induced cell cycle arrest and apoptosis in human myeloma cell lines. A. Cells were treated with negative control and shRNA#1 and shRNA#2 for 24 h. PI staining was used to analyze the cell cycle distribution; B. Apoptosis of treated cells assessed by Annexin V-fluorescein isothiocyanate and propidium iodide (PI) double staining and fluorescence-activated cell sorter analysis; The statistical significance was considered as *P<0.05 and **P<0.01 where compared with control.
Knockdown of Sorcin decreased P-gP content and Rh-123 concentration
The fluorescent dye Rh-123 is a substrate of P-gp, and its cellular retention has been shown to reflect P-gp function. Rh-123 efflux was measured by counting cells in the M1 region of the plot. As showed in Table 2, after Sorcin expression was silenced, P-gP fluorescence intensity significantly decreased by 63.5% and 43.6% in KM3/DDP, and 68.9% and 54.7% in U266/ADM cells respectively when compared with the control group. While the absorption of fluorescent dye Rh-123 in KM3/DDP and U266/ADM cells significantly increased; the intercellular Rh-123 content increased by 5.6 and 4.8 folds, 3.1 and 2.7 folds respectively when compared with the control group, suggesting that intracellular Rh-123 retention increased and P-GP efflux function decreased over the data are mean ± SD of three independent experiments; P value shows shRNA#1 group (or shRNA#2 group) compared with negative control group.
Table 2.
Fluorescence intensity values of P-pg and Rh123 by intracellular accumulation assay
| Group | P-pg | Rh123 | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| KM3/DDP | P | U266/ADM | P | KM3/DDP | P | U266/ADM | P | |
| Control | 251.25±13.18 | 232.31±27.24 | 38.46±2.93 | 62.34±11.14 | ||||
| shRNA#1 | 91.61±12.41 | 0.004 | 72.53±10.74 | 0.015 | 216.32±11.63 | 0.004 | 196.16±9.43 | 0.021 |
| shRNA#2 | 141.63±2.79 | 0.009 | 105.27±11.25 | 0.022 | 186.13±12.24 | 0.009 | 168.78±11.51 | 0.035 |
Silencing of Sorcin down-regulated mRNA and protein expression of MDR1 and MRP1
MDR frequently results from the incongruous expression of drug-resistant genes, such as multi-drug-resistance gene (MDR1). MDR1 and MRP1 are two major membrane transporter proteins that involved in efflux activities and lead to multiple drug resistance. So we would like to investigate whether Sorcin Silenced could downregulate the expression of these proteins to restore the intercellular accumulation of Rhodamine-123. RT-PCR and western blotting results showed that, the expression of MDR1 and MRP1 were decreased in KM3/DDP and U266/ADM cells after Sorcin knockdown (Figure 4).
Figure 4.
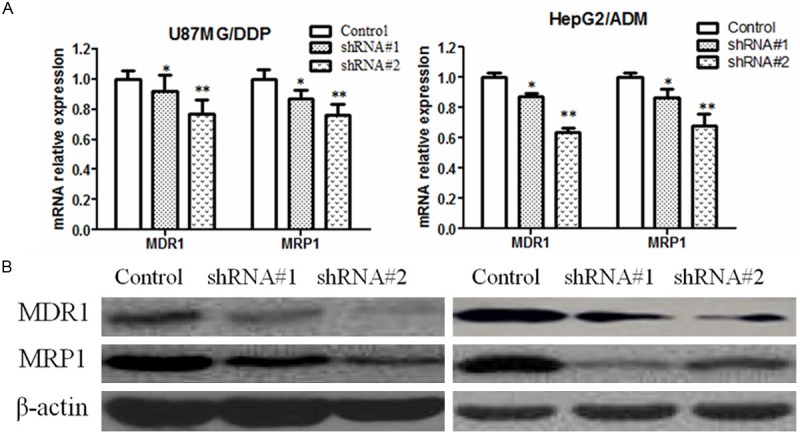
Sorcin silencing reduces the mRNA and protein expression of MDR1 and MRP1 in human myeloma cell lines. A. RT-PCR analysis MDR1 and MRP1 mRNA expression in: Sorcin siRNA#1 and siRNA#2-transfected cells and negative control cells at 48 h after transfection. GAPDH was analyzed in parallel as a loading control; B. Western blot analysis MDR1 and MRP1 mRNA expression in: Sorcin siRNA#1 and siRNA#2-transfected cells and negative control cells at 48 h after transfection. β-actin was analyzed in parallel as a loading control; Values are mean ± SD of three independent experiments; *P<0.05 and **P<0.01 compared with negative control cells.
Silencing of Sorcin down-regulated the expression of other MDR related genes
Except MDR1 and MRP1, other MDR related genes such as GST-π, Livin, Src, Survivin, Bcl-2, Cyclin-D1, C-myc, p21 and TP53 might also get involved in cancer cell MDR. Real-time PCR and Western blotting analysis results showed that, after Sorcin expression was silenced, GST-π, Livin, phospho-Src, Survivin, Bcl-2, Cyclin-D1, C-myc and p21 expression significantly decreased, while p53 expression increased when compared with control group (Figure 5).
Figure 5.
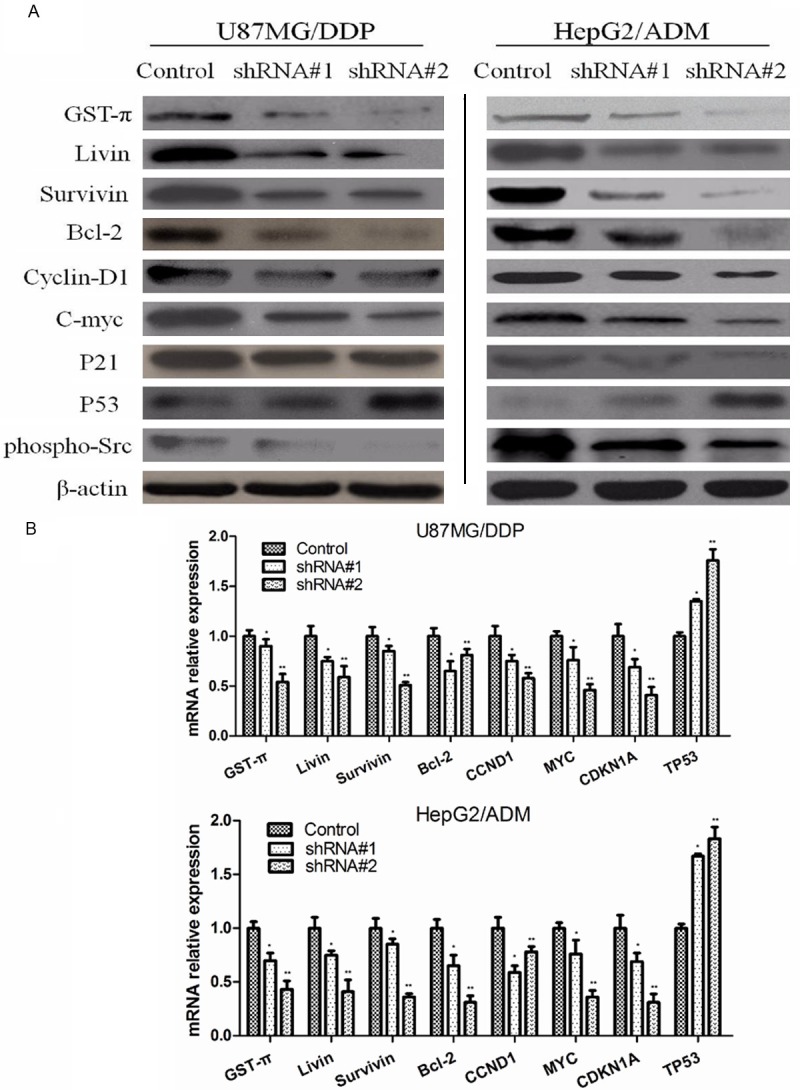
Effect of sorcin silencing on expression of drug resistance related gene in human myeloma cell lines. A. Western blot analysis of protein extracts obtained from KM3/DDP and U266/ADM cells; B. mRNA expressions of GST-π, Livin, Src, Survivin, Bcl-2, Cyclin-D1, C-myc, p21 and TP53 genes in KM3/DDP and U266/ADM cells. Data presented are means ± SD values, N=3. Bars indicate SD. Significant differences from control were indicated by P<0.05 (*) and P<0.01 (**).
Silencing of Sorcin increased the activity of caspase-3 and caspase-8
We also examined the activity of caspase-3 and caspase-8 after Sorcin knockdown. As showed in Table 3, caspase-3 and caspase-8 activity significantly increased when compared with control group.
Table 3.
Changes in caspase-3 and caspase-8 activity
| Group | Caspase-3 | Caspase-8 | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| KM3/DDP | P | U266/ADM | P | KM3/DDP | P | U266/ADM | P | |
| Control | 112.65±7.65 | 78.36±17.48 | 181.51±12.27 | 66.47±19.88 | ||||
| shRNA#1 | 345.52±23.41 | 0.025 | 201.36±13.53 | 0.029 | 403.07±10.74 | 0.023 | 214.07±19.51 | 0.021 |
| shRNA#2 | 291.44±21.63 | 0.030 | 189.81±14.73 | 0.037 | 345.57±3119 | 0.028 | 139.45±28.67 | 0.025 |
Data are mean ± SD of three independent experiments; P value shows shRNA#1 group (or shRNA#2 group) compared with negative control group.
Silencing of Sorcin Inhibits AKT/NF-κB signaling pathway in KM3/DDP and U266/ADM cells
It has been reported that activation of NF-κB followed by Akt phosphorylation plays a role in regulation of cell survival, apoptosis and drug resistance, we wondered if intracellular AKT/NF-κB signaling pathway was involved in the action of Sorcin. As showed in Figure 6, expressions of the proteins AKT and NF-κB were evaluated by Western blotting. Results indicated that annonaceous acetogenins could decrease phosphorylation of AKT and NF-κB expression levels were decreased after Sorcin knockdown.
Figure 6.
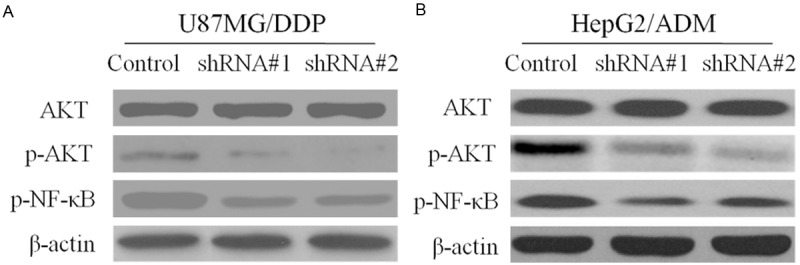
Effect of Sorcin Silencing in NF-κB nuclear translocation and Akt activation. Cells were transfected with shRNA#1 and shRNA#2 and negative control for 48 h. After treatment cell extracts were prepared and Western blot analysis the expression of Akt and NF-κB in KM3/DDP and U266/ADM. Beta-Actin was used as an internal loading control.
Expression of sorcin in glimas and breast cancer patient samples
To clarify the function of sorcin in gliomas and liver Cancer, immunohistochemistry was performed on 10 Gliomas and 12 liver cancer tissue samples, and 6 benign tumors (7% of the tumors) and 8 normal lesions tissue samples following the manufacturer’s instructions. Samples showed Sorcin expnression, while all benign tumors and normal lesions exhibited normal expression. Thus, an increase in immunoexpression of Sorcin was apparent from non-tumorous issues (Figure 7).
Figure 7.
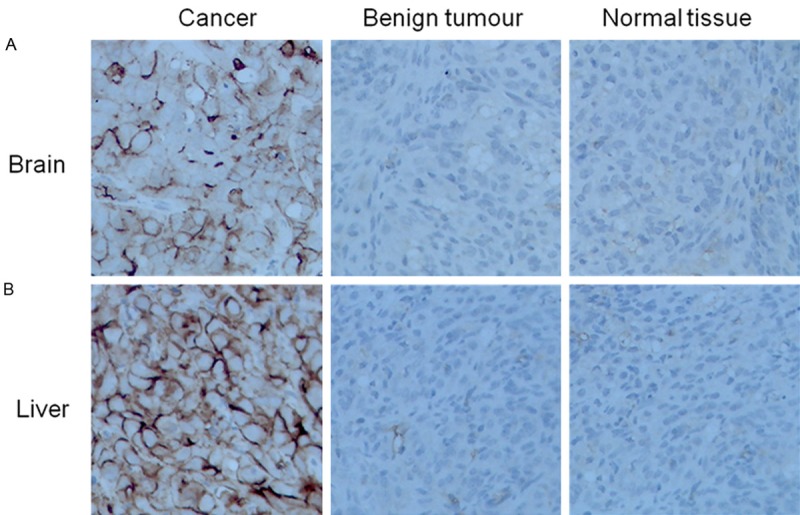
Expression of Sorcin in Glimas and Breast Cancer Patient Samples. By immunohistochemistry (×400). A. Immunohistochemical analysis of Sorcin innormal brain tissues, benign and malignant tumors. B. Immunohistochemical analysis of Sorcin in normal liver tissues, benign and malignant tumors.
Discussion
MDR refers to the cross-drug resistance to a variety of anti-tumor drugs with different structure and mechanisms of actions after the tumor cells have developed drug resistance following exposure to one antitumor drug. MDR is a common challenge hindering the treatment of a variety of tumors and a major cause of treatment failure of cancer chemotherapy [9,10]. Therefore, reversing tumor drug resistance has become an important topic in myeloma research. Studies showed that the over-expression of MDR1 gene-encoded P-gP on tumor cell surface was the main mechanism of tumor cell MDR. MDR1 and MRP1 are important drug resistance genes; the MDR pathway mediated by MDRl, MRP1 and GST-π is also known as the classical MDR pathway. Numerous studies confirmed that blocking or inhibiting the MDR1 or MRP1 expression was able to effectively reverse the MDR of tumor cells. Many scholars regard MDR1 as a desirable target for the reversal of tumor cell drug resistance [11]. Studies in recent years also showed that IAP family, Bcl-2, P53, bax and other apoptosis-related molecules present in tumor cytoplasm/nucleus could mediate tumor resistance to apoptosis and represented another main reason for the development of drug-resistance [12]. This experiment designed and synthesized siRNA to silence Sorcin gene expression in order to determine not only its inhibitory effect on MDRl gene and P-gP protein expression in KM3/DDP and U266/ADM cells but also the reversal of the drug-resistant phenotype. The purpose was to search new and effective gene targets for reversing the MDR of myeloma cells.
Because Sorcin is basically not expressed in terminally differentiated mature tissues, but over-expressed in a vast majority of tumor tissues, it is regarded as a high-value target for tumor treatment. Increasingly more studies demonstrated the close relationship of Sorcin with tumor resistance. When Sorcin is over-expressed in the cells, the activity of caspase protease and a series of apoptosis-related molecules significantly decreased, leading to development of apoptosis resistance in tumor cells.
Src kinase has tyrosine protein kinase activity. Its abnormal expression and activation can cause abnormalities of cell survival, proliferation, migration and adhesion, leading to abnormal regulation of the cells [13]. It is not only involved in the occurrence and development of tumors but also closely related to tumor drug resistance. Inhibiting Src kinase activity can significantly increase the sensitivity of drug-resistant myeloma KM3/DDP cells to cisplatin chemotherapy. In this study, Sorcin was found overexpression in malignant tumors, compare to benign tumor and normal tissues of brain or liver. Sorcin siRNA fragment was transfected to silence cisplatin-resistant Sorcin gene in myeloma KM3/DDP cells and U266/ADM cells in order to explore whether silencing Sorcin could reverse myeloma drug resistance. Western blot and real-time PCR results showed that siRNA effectively inhibited protein and mRNA expression of Sorcin gene. MTS assay results showed that the inhibition of drug on cell proliferation increased in Sorcin silence group than in control group, suggesting that silencing Sorcin gene can reverse the drug resistance of KM3/DDP cells. Flow cytometry results showed the followings: cells in G0/G1 phase and G2/M phase significantly increased; apoptosis rate significantly increased; Survivin and Livin expression and Src kinase activity significantly decreased; and p53 expression was significantly up-regulated; suggesting that the reversal of drug resistance by silencing Sorcin expression may be related to the regulation of apoptosis-related gene expression and Src kinase activity.
Although the main cause of MDR is that P-gP relies on ATP to pump out anti-tumor drugs, recent studies found that P-gP was also able to inhibit apoptosis and cause MDR cells to develop apoptosis resistance by inhibiting the activity of caspase family [14]. Caspase is the main executor of apoptosis. Studies showed that Sorcin inhibited the activity of caspase-3 and caspase-8 mainly through binding to their activated forms, leading to the inhibition of apoptosis [15]. Caspase-3 and caspase-8 are important members in caspase family. Sorcin exerts anti-apoptotic effect by inhibiting caspase-3 and caspase-8 activity. Caspase activity test results showed, when compared with the control group, caspase-3 and caspase-8 activity significantly increased after Sorcin gene expression was silenced.
P21, cyclin D1 and c-Myc are all key molecules regulating the transition of cell cycle from G1 phase to S phase; these molecules are not only involved in cell proliferation and apoptosis but also closely related to the occurrence and development of tumors [16]. Currently, a large number of studies have confirmed that the inhibition of P21, cyclin D1 and c-Myc expression can increase the sensitivity of some tumors to chemotherapeutic drugs to certain extent. The results of this study showed that the protein and mRNA expression levels of apoptosis-related genes P21, cyclin D1 and c-Myc were significantly down-regulated after Sorcin gene was silenced.
In summary, we successfully silenced Sorcin gene using RNAi technology in this study. Silencing Sorcin gene effectively increased the sensitivity of drug-resistant human myeloma cell lines to chemotherapeutic drugs. Silencing Sorcin gene induced caspase-dependent apoptosis. Its mechanisms of reversing drug resistance involved direct or indirect down-regulation of NF-κB/AKT pathway activity, MDR-related gene expression, and apoptosis-related gene expression, and regulation of caspase-dependent apoptosis, suggesting that Sorcin can be used as a new therapeutic target.
Disclosure of conflict of interest
None.
References
- 1.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanwar J, Das S, Fatima Z, Hameed S. Multidrug resistance: an emerging crisis. Interdiscip Perspect Infect Dis. 2014;2014:541340. doi: 10.1155/2014/541340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lespine A, Ménez C, Bourguinat C, Prichard RK. P-glycoproteins and other multidrug resistance transporters in the pharmacology of anthelmintics: Prospects for reversing transport-dependent anthelmintic resistance. Int J Parasitol Drugs Drug Resist. 2011;2:58–75. doi: 10.1016/j.ijpddr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng BB, Zhang P, Jia WW, Jia WW, Yu LG, Guo XL. Sorcin, a potential therapeutic target for reversing multidrug resistance in cancer. J Physiol Biochem. 2012;68:281–287. doi: 10.1007/s13105-011-0140-0. [DOI] [PubMed] [Google Scholar]
- 5.He Q, Zhang G, Hou D, Xu Y, Gao S, Deng L, He W, Yang Y, Guo X, Wang X. Overexpression of sorcin results in multidrug resistance in gastric cancer cells with up-regulation of P-gp. Oncol Rep. 2011;25:237–243. [PubMed] [Google Scholar]
- 6.Zhu Y, Zhu L, Lu L. Role and mechanism of the alkylglycerone phosphate synthase in suppressing the invasion potential of human glioma and hepatic carcinoma cells in vitro. Oncol Rep. 2014;1:431–6. doi: 10.3892/or.2014.3189. [DOI] [PubMed] [Google Scholar]
- 7.Kasinathan RS, Morgan WM, Greenberg RM. Genetic knockdown and pharmacological inhibition of parasite multidrug resistance transporters disrupts egg production in Schistosoma mansoni. PLoS Negl Trop Dis. 2011;5:e1425. doi: 10.1371/journal.pntd.0001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu D, Xiao Z, Wang W, Xu Y, Gao S, Deng L, He W, Yang Y, Guo X, Wang X. Down regulation of CIAPIN1 reverses multidrug resistance in human breast cancer cells by inhibiting MDR1. Molecules. 2011;17:7595–7611. doi: 10.3390/molecules17067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinzi L, Contino M, Cantore M, Capparelli E, Leopoldo M, Colabufo NA. ABC transporters in CSCs membranes as a novel target for treating tumor relapse. Front Pharmacol. 2014;5:163. doi: 10.3389/fphar.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kibria G, Hatakeyama H, Harashima H. Cancer multidrug resistance: mechanisms involved and strategies for circumvention using a drug delivery system. Arch Pharm Res. 2014;37:4–15. doi: 10.1007/s12272-013-0276-2. [DOI] [PubMed] [Google Scholar]
- 11.Yamagishi N, Nakao R, Kondo R, Nishitsuji M, Saito Y, Kuga T, Hatayama T, Nakayama Y. Increased expression of sorcin is associated with multidrug resistance in leukemia cells via up-regulation of MDR1 expression through cAMP response element-binding protein. Biochem Biophys Res Commun. 2014;448:430–436. doi: 10.1016/j.bbrc.2014.04.125. [DOI] [PubMed] [Google Scholar]
- 12.Kim CW, Lu JN, Go SI, Jung JH, Yi SM, Jeong JH, Hah YS, Han MS, Park JW, Lee WS, Min YJ. p53 restoration can overcome cisplatin resistance through inhibition of Akt as well as induction of Bax. Int J Oncol. 2014;43:1495–1502. doi: 10.3892/ijo.2013.2070. [DOI] [PubMed] [Google Scholar]
- 13.Liu ST, Pham H, Pandol SJ, Ptasznik A. Src as the link between inflammation and cancer. Front Physiol. 2014;4:416. doi: 10.3389/fphys.2013.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LH, Yang JY, Yang SN, Li Y, Ping GF, Hou Y, Cui W, Wang ZZ, Xiao W, Wu CF. Suppression of NF-κB signaling and P-glycoprotein function by gambogic acid synergistically potentiates adriamycin-induced apoptosis in lung cancer. Curr Cancer Drug Targets. 2014;14:91–103. doi: 10.2174/1568009613666131113100634. [DOI] [PubMed] [Google Scholar]
- 15.Maddalena F, Laudiero G, Piscazzi A, Secondo A, Scorziello A, Lombardi V, Matassa DS, Fersini A, Neri V, Esposito F, Landriscina M. Sorcin induces a drug-resistant phenotype in human colorectal cancer by modulating Ca(2+) homeostasis. Cancer Res. 2011;71:7659–69. doi: 10.1158/0008-5472.CAN-11-2172. [DOI] [PubMed] [Google Scholar]
- 16.Martino T, Magalhães FC, Justo GA, Coelho MG, Netto CD, Costa PR, Sabino KC. The pterocarpanquinone LQB-118 inhibits tumor cell proliferation by downregulation of c-Myc and cyclins D1 and B1 mRNA and upregulation of p21 cell cycle inhibitor expression. Bioorg Med Chem. 2014;22:3115–3122. doi: 10.1016/j.bmc.2014.04.025. [DOI] [PubMed] [Google Scholar]