Abstract
Little is known about the role of isorhamnetin on endothelial cell apoptosis and inflammation when insulted by TNF-α injury. In our study, HUVECs were treated with TNF-α for 6 hours. HUVECs apoptosis were detected using flow cytometry. The expressions of ICAM-1, VCAM-1, E-selectin, NF-κB, AP-1 and eNOS were determined with western blotting or flow cytometry. The results showed TNF-α increased of apoptosis and the expression of ICAM-1, VCAM-1 and E-selectin in HUVECs, accompanied by significant augmentation of NF-κB and AP-1 expression. Pretreatment with isorhamnetin significantly reduced apoptosis in TNF-α-treated HUVECs. Moreover, isorhamnetin significantly attenuated TNF-α-induced upregulation of ICAM-1, VCAM-1, AP-1, E-selectin and NF-κB expression. Meanwhile, isorhamnetin also increased the expression of eNOS. So, isorhamnetin could suppress TNF-α-induced apoptosis and inflammation by blocking NF-κB and AP-1 signaling in HUVECs, which might be one of the underlying mechanisms for treatment of coronary heart disease.
Keywords: Isorhamnetin, HUVECs, apoptosis, ICAM-1, VCAM-1, E-selectin, eNOS, NF-κB, AP-1
Introduction
Inflammation plays a pivotal role in both the development of atherosclerosis and the acute activation of the vascular wall with consequent local thrombosis and vasoconstriction [1], although the mechanism remains unknown [2]. In general, the adhesion of the leukocytes to the blood vessel endothelium plays a crucial role in the inflammation [3]. During the inflammation, an array of adhesion molecules expressed by the endothelium mediate the process of leukocytes attachment to the vessel wall. The leukocyte-endothelial interactions require expression of various adhesion molecules such as intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin [4,5]. These endothelial cell adhesion molecules increased in inflamed tissue including vessel endothelium. As one of the inflammatory cytokines, tumor necrosis factor-alpha (TNF-α) is an important risk factor in the process of the arteriosclerosis [6]. The vascular endothelial cell injury induced by the TNF-α promotes the expressing of cell adhesion molecules [7]. The former stimulates the adhesion of leukocytes to the endothelium and mediates the process of leukocyte attachment to the vascular wall [8]. Simultaneously, the adhesion molecules will injury the vascular endothelial cell [9]. E-selectin mediates the initial stage of the leukocyte attachment to vascular wall by slowing the fluidity of the leukocyte [10]. Nuclear factor-kappaB (NF-κB) and activator protein 1 (AP-1) are the transcription factors that regulate many genes related to apoptosis and inflammation at the transcriptional level. NF-κB and AP-1 involved in cellular responses to stimuli such as cytokines controls transcription of DNA, plays a key role in the intracellular regulation of inflammation [11]. It might control genes in the inflammation, which is found to be active in the inflammatory disease, such as sepsis, inflammatory bowel disease, atherosclerosis [12,13]. Endothelial nitric oxide synthase (eNOS) plays an important role in the regulation of cardiovascular function. In general, eNOS expressed in responses to inflammatory stimuli such as cytokines and could generate large quantities of nitric oxide (NO) [14], which acutely vasodilates blood vessels and inhibits platelet aggregation. Chronically, eNOS has a major role in the regulation of prevention of atherosclerosis by decreasing leukocyte adhesion and smooth muscle proliferation [15].
Isorhamnetin, one of the major and active components isolated from the traditional Chinese herb Pollen Typhae Angustifoliae or Hippophae rhamnoides L., is commonly used in the prevention and treatment of ischemic heart disease and circulatory disorders. Recent pharmacological studies have demonstrated that isorhamnetin has anti-inflammatory property [16]. The protective effects of isorhamnetin on endothelial cell line EA.hy926 from ox-LDL-induced cell injuries via inhibition of lectin-like ox-LDL receptor-1 upregulation, interference of ox-LDL-mediated intracellular signaling pathway [17]. Isorhamnetin also inhibits the H(2)O(2)-induced activation of the intrinsic apoptotic pathway via ROS scavenging and ERK inactivation [18]. Isorhamnetin had the potential anti-inflammatory activity in cardiovascular disease [19]. However, little is known about the role of isorhamnetin on endothelial cell in inflammation process when insulted by TNF-α injury.
In this study, we investigated the effects of isorhamnetin on TNF-α-induced apoptosis index and the expressions of ICAM-1, VCAM-1, E-selectin and eNOS in HUVECs. NF-κB and AP-1 were tested to further define the signaling pathway responsible for isorhamnetin regulation of inflammation and apoptosis. Our study may provide a novel therapeutic use of isorhamnetin in vascular inflammatory disorders.
Materials and methods
Materials
HUVECs were purchased from the Chinese Academy of Sciences (Shanghai, China). Chemically pure isorhamnetin was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), and was dissolved and diluted in incubation medium to final concentrations of 100 μg/ml. Human recombinant TNF-α was purchased from R<D systems (USA). The antibodies of NF-κB, eNOS, hVCAM-1, hICAM-1, AP-1 and E-Selectin was purchased from R&;D systems (USA). The antibody of β-actin purchased from Santa Cruz Biotechnology (USA).
HUVECs culture and protocol
The HUVECs were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum and antibiotics in a humidified 37°C incubator with an atmosphere of 95% air and 5% CO2. For experiments, the cells were grown in 24- or 96-well plates in DMEM. All cells used in this study were passage 3. The HUVESs were randomly assigned to 3 groups: control group (Con): HUVECs were cultured with DMEM medium; TNF-α group (TNF): HUVECs were treated with TNF-α at a dose of 200 ng/ml for 6 hours; Isorhamnetin group (Iso): HUVECs were pretreated with isorhamnetin at a dose of 30 µg/ml for 18 hours prior to TNF-α at a dose of 200 ng/ml for 6 hours.
Annexin V assay
After treatment, HUVECs in every group were trypsinized and washed with PBS, then resuspended in annexin V-FITC-added binding buffer and PI-containing binding buffer for 15 minutes under dark conditions. The samples were then analyzed immediately by a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA) using BD CellQuest Pro software (Becton Dickinson) as described previously [20].
Flow cytometry
Expression of AP-1 and NF-κB in the 3 groups was measured by a flow cytometry assay. After different process according to the protocol, the cells were washed with Perm/Wash Butter, and then incubated for 10 minutes with a 5 μl of NF-κB-PE or AP-1-FITC antibodies, after 2 washes with Perm/Wash Butter, the fluorescent cells were analyzed on a FACScan flow cytometer using BD CellQuest Pro software.
Western blot analysis
For the detection of the protein expression, disposed cells were washed and scraped into 50 μl lysis buffer. After being quantified by BCA reagent, equivalent proteins for each sample were resuspended in 10 μl of electrophoresis sample buffer and subjected to SDS-PAGE in 12-15% acrylamide minigels. Proteins were then transferred to PVDF membrane in a transfer buffer. After blocking with 5% skim milk, PVDF membrane was washed in TBS containing 0.1% Tween 20 and incubated with a protein-specific antibody at room temperature followed by a secondary antibody. Primary antibodies were used as follows: antibodies specific to NF-κB, eNOS, hVCAM-1, hICAM-1/CD54, AP-1, β-actin and E-Selectin/CD62E. After the secondary antibody incubation, the membranes were rinsed and the bound antibodies were detected using enhanced chemiluminescence (ECL, Santa Cruz Biotechnology, USA) followed by autoradiography. Image pro plus 5 software was used to semiquantify protein in every lane.
Statistical analysis
Statistical analysis was performed with one-way anova followed by Bonferroni multiple-comparison test. The results were considered to be significant at a value of P < 0.05.
Results
Isorhamnetin decreased TNF-α induced HUVECs apoptosis
TNF-α is an important risk factor in the process of the arteriosclerosis, which could induce endothelial cell apoptosis and inflammation. Recent data suggested that TNF mediated endothelial cell activation. Endothelial cell apoptosis is likely important in the process termed “plaque erosion” that leads to platelet aggregation [21]. Hence, in our study, we first detected whether isorhamnetin could decrease TNF-α induced HUVECs apoptosis. To study the effect of these stimuli, HUVECs were exposed to TNF-α at a dose of 200 ng/ml for 6 hours with or without isorhamnetin were analyzed by flow cytometry. In our mode, about 3.6% of HUVECs in control group were positive (Figure 1A). When HUVECs cultured in for TNF-α at a dose of 200 ng/ml for 6 hours, the apoptotic index was significantly increased (10.005±0.903%, §P < 0.05 vs. control group) (Figure 1B). Compared with TNF-α group, the isorhamnetin pretreatment dramatically decreased cell apoptosis index to 7.954±0.347% (▲P < 0.05 vs. TNF group) (Figure 1C and 1D).
Figure 1.
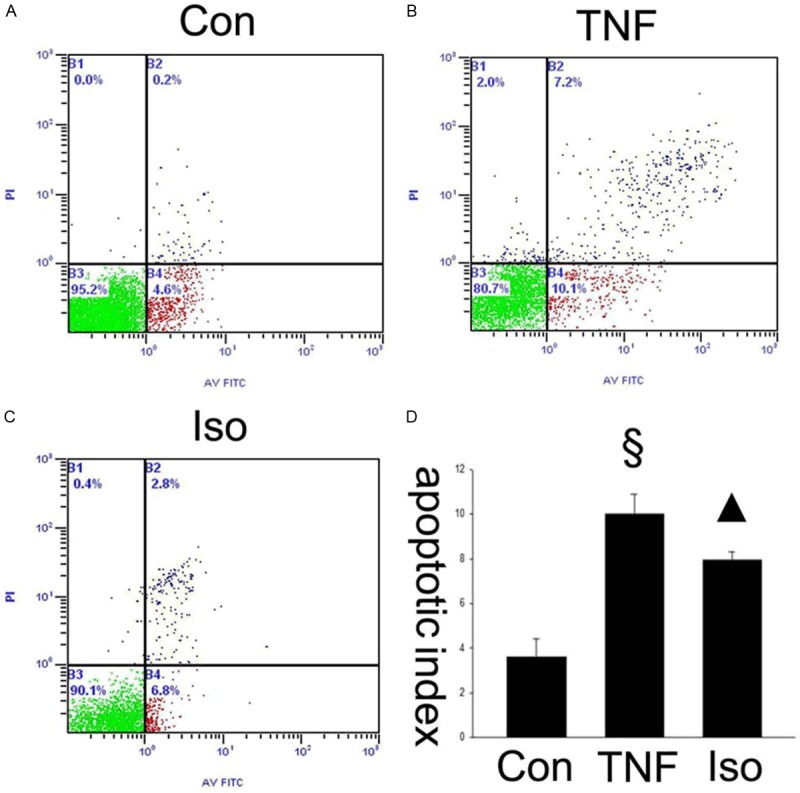
Isorhamnetin decreased TNF- α induced HUVECs apoptosis. Apoptosis index assessed by flow cytometry in HUVECs. A. Percentage of apoptotic cells in control group. B. Percentage of apoptotic cells in TNF-α group (TNF). C. Percentage of apoptotic cells in isorhamnetin group (Iso). D. Quantification of apoptotic HUVECs by flow cytometry. §P < 0.05 vs. control group, ▲P < 0.05 vs. TNF-α group. Data shown are means ± SE representative of 5 independent experiments.
Effect of isorhamnetin on VCAM-1, ICAM-1 and E-Selectin expression
The adhesion and migration of circulating leukocytes to the vascular endothelium is a crucial step during vascular inflammation. The cell adhesion molecules including VCAM-1, ICAM-1 and E-selectin mediate these processes [22]. Therefore, it is a key question whether isorhamnetin could inhibit these adhesion molecules in TNF-α-mediated HUVECs injury. Firstly, to investigate whether isorhamnetin affects adhesion molecules, the expression of VCAM-1 in HUVECs was studied by western blot. As shown in Figure 2A and 2B, VCAM-1 expression was upregulated in TNF-α group compared with control groups (1.122±0.048 vs. 0.093±0.027, §P < 0.05 vs. control group), whereas isorhamnetin pretreatment reversed the upregulation of the protein VCAM-1 compared with the TNF-α group (0.504±0.030 vs. 1.122±0.048, ▲P < 0.05 vs. TNF-α group). Then, ICAM-1 in HUVECs was studied by western blot. As shown in Figure 3A and 3B, hICAM-1 expression was upregulated in TNF-α group compared with control groups (0.958±0.031 vs. 0.059±0.019, §P < 0.05 vs. control group), whereas isorhamnetin pretreatment reversed the upregulation of the protein ICAM-1 compared with the TNF-α group (0.444±0.026 vs. 0.958±0.031, ▲P < 0.05 vs. TNF-α group). To further determine the effects of isorhamnetin on HUVECs, we tested E-Selectin expression using an antibody specific to E-Selectin. As shown in Figure 4, E-Selectin increased in TNF-α group compared with control groups (1.120±0.060 vs 0.106±0.016, §P < 0.05 vs. control group), whereas isorhamnetin pretreatment reversed the upregulation of the protein E-Selectin compared with the TNF-α group (0.911±0.028 vs. 1.120±0.060, ▲P < 0.05 vs. TNF-α group). In summary, isorhamnetin pretreatment could reverse the upregulation of adhesion molecules including VCAM-1, ICAM-1 and E-selectin induced by TNF-α injury.
Figure 2.
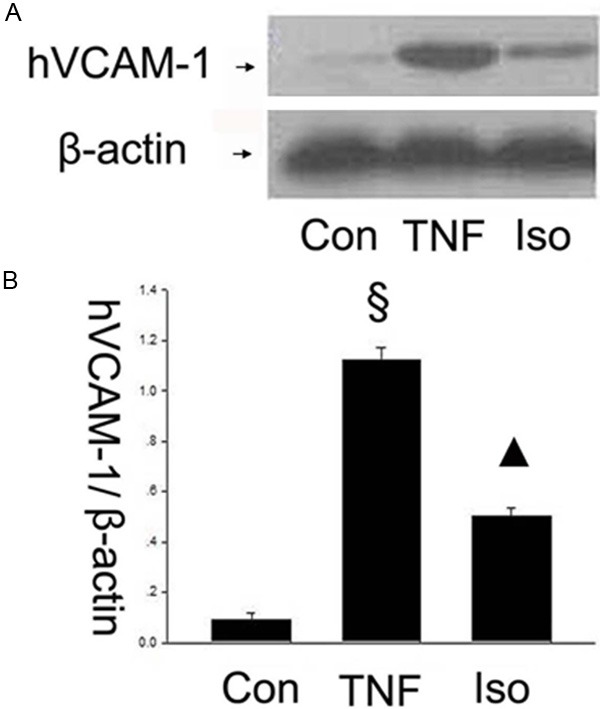
Effect of isorhamnetin on VCAM-1 expression. A. 40 µg HUVECs lysates in control, TNF-α and isorhamnetin groups were loaded onto an SDS-polyacrylamide gel and VCAM-1were detected by Western blotting. Equal loading of protein was confirmed by reprobing blots with antibodies against β-actin. B. Quantitation of VCAM-1 expression of HUVECs. §P < 0.05 vs. control group, ▲P < 0.05 vs. TNF-α group. Data shown are means ± SE representative of 4 independent experiments.
Figure 3.
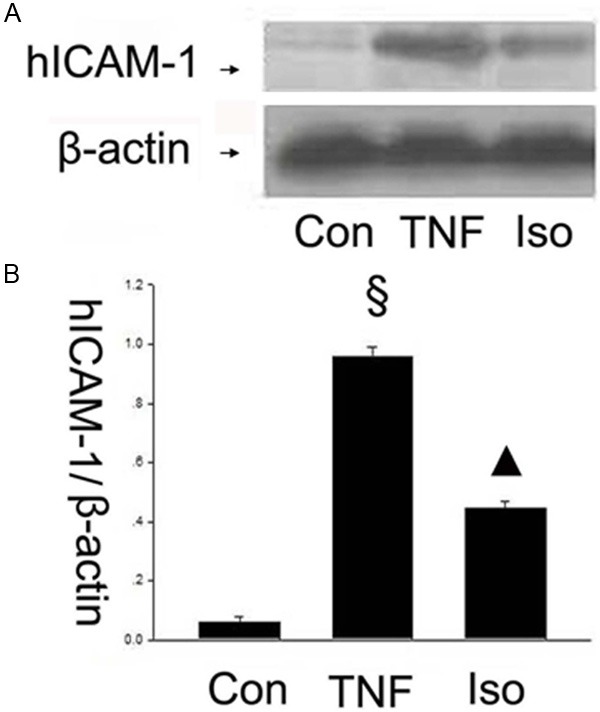
Effect of isorhamnetin on ICAM-1 expression. A. 40 µg HUVECs lysates in control, TNF-α and isorhamnetin groups were loaded onto an SDS-polyacrylamide gel and ICAM-1were detected by Western blotting. Equal loading of protein was confirmed by reprobing blots with antibodies against β-actin; B. Quantitation of ICAM-1 expression of HUVECs. §P < 0.05 vs. control group, ▲P < 0.05 vs. TNF-α group. Data shown are means ± SE representative of 4 independent experiments.
Figure 4.
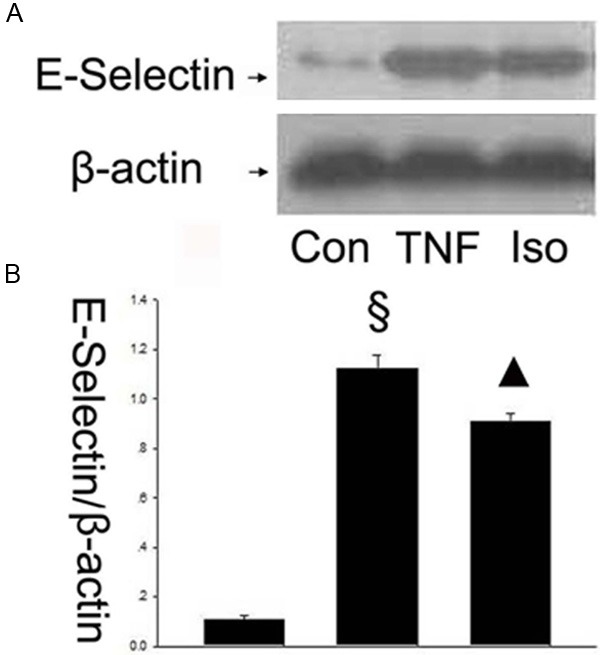
Effect of isorhamnetin on E-Selectin expression. A. 40 µg HUVECs lysates in control, TNF-α and isorhamnetin groups were loaded onto an SDS-polyacrylamide gel and E-Selectin were detected by Western blotting. Equal loading of protein was confirmed by reprobing blots with antibodies against β-actin; B. Quantitation of E-Selectin expression of HUVECs. § P < 0.05 vs. control group, ▲P < 0.05 vs. TNF-α group. Data shown are means ± SE representative of 4 independent experiments.
Isorhamnetin up-regulated eNOS expression
In addition, eNOS catalyzed the formation of NO characterized as an anti-atherosclerosis effect. The eNOS produces the low density of NO, which helps to maintain the normal structure and function of the endothelium. To further determine the underlying mechanism of the effects of isorhamnetin on HUVECs, we tested the expression of eNOS using a specific antibody. The results showed that the eNOS expression decreased in the TNF-α group compared with control groups (0.278±0.049 vs. 0.671±0.018, §P < 0.05 vs. control group), whereas isorhamnetin pretreatment reversed the downregulation of the protein eNOS compared with the TNF-α group (0.606±0.032 vs. 0.278±0.049, ▲P < 0.05 vs. TNF-α group) (Figure 5).
Figure 5.
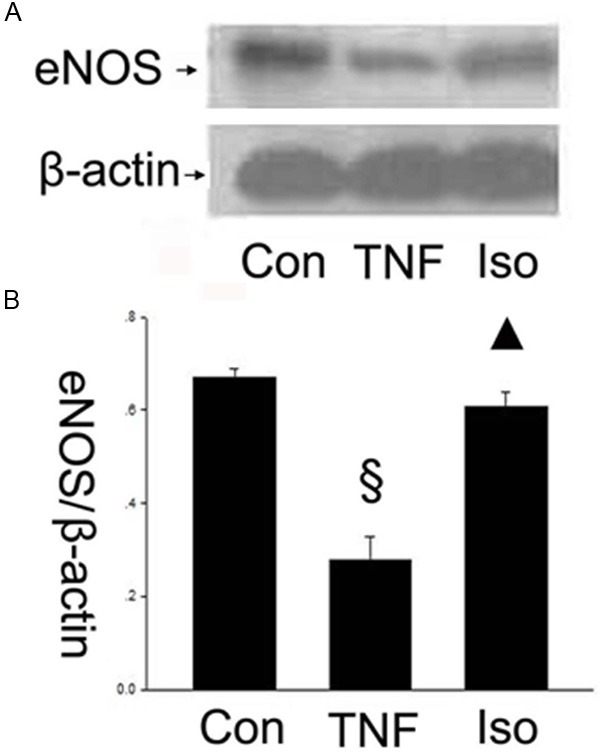
Isorhamnetin up-regulated eNOS expression. A. 40 µg HUVECs lysates in control, TNF-α and isorhamnetin groups were loaded onto an SDS-polyacrylamide gel and eNOS were detected by Western blotting. Equal loading of protein was confirmed by reprobing blots with antibodies against β-actin; B. Quantitation of eNOS expression of HUVECs. §P < 0.05 vs. control group, ▲P < 0.05 vs. TNF-α group. Data shown are means ± SE representative of 4 independent experiments.
Isorhamnetin suppressed NF-κB and AP-1 pathway activation
To elucidate how isorhamnetin plays a role on anti-inflammation, it is necessary to further determine the possible mechanism of the effects of isorhamnetin on HUVECs. The transcription factors, NF-κB and AP-1, play important roles in TNF-α-induced transcriptional regulation. Next, we tested the expression of NF-κB and AP-1 using specific antibodies by flow cytometry and western blot, respectively. In Figure 6, AP-1 increased in TNF-α group compared with control groups (0.919±0.075 vs. 0.275±0.068, §P < 0.05 vs. control group), whereas isorhamnetin pretreatment reversed the upregulation of the proapoptotic protein AP-1 compared with the TNF-α group (0.367±0.103 vs. 0.919±0.075, ▲P < 0.05 vs. TNF-α group). As shown in Figure 7, the results showed that the NF-κB expression increased in the TNF-α group compared with control groups (0.922±0.016 vs. 0.326±0.019, §P < 0.05 vs. control group), however, isorhamnetin pretreatment reversed the upregulation of the protein NF-κB expression compared with the TNF-α group (0.686±0.015 vs. 0.922±0.016, ▲P < 0.05 vs. TNF-α group). This result showed that NF-κB and AP-1 may be involved in isorhamnetin-mediated protection in HUVECs induced by TNF-α injury.
Figure 6.
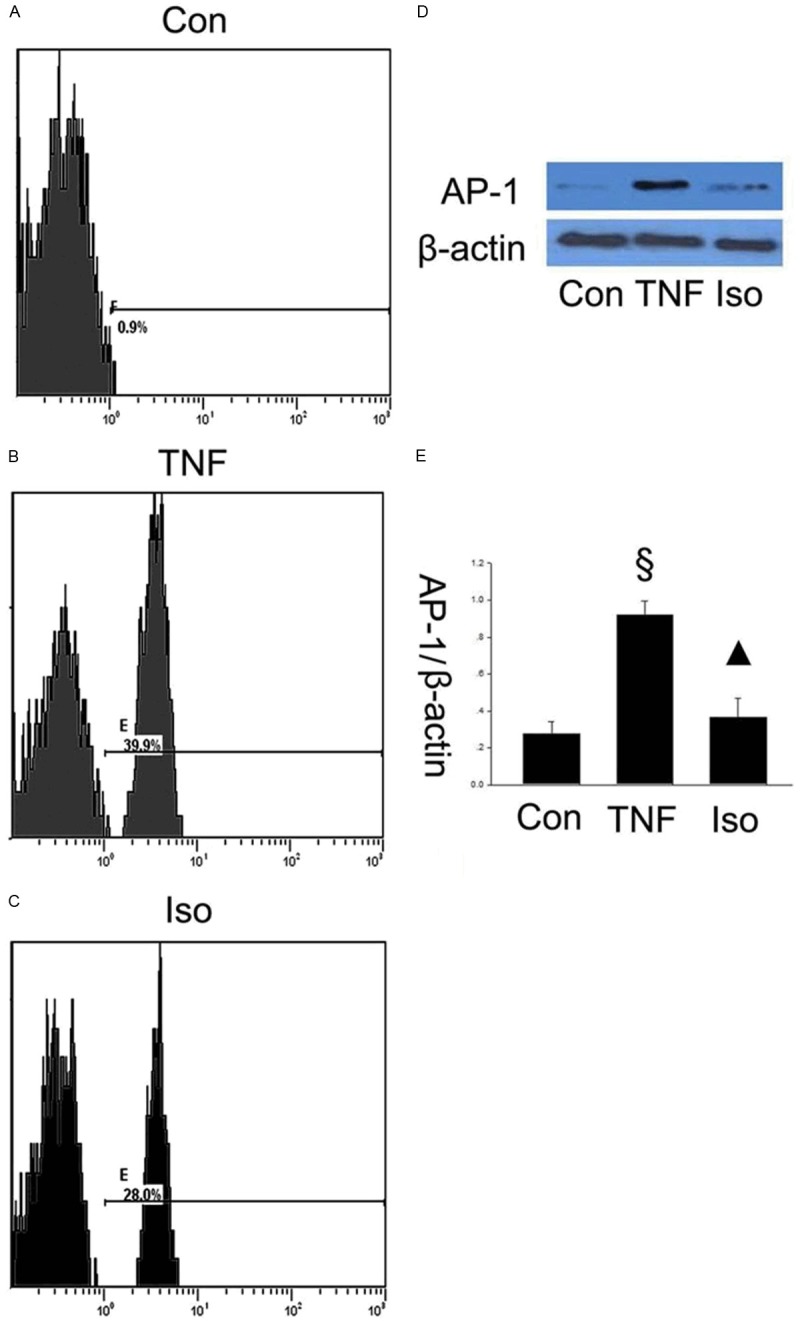
Isorhamnetin suppressed AP-1 pathway activation. A. Expression of AP-1 in control group was measured by a flow cytometry assay; B. Expression of AP-1 in TNF-α group was measured by a flow cytometry assay; C. Expression of AP-1 in isorhamnetin group was measured by a flow cytometry assay; D. 40 µg HUVECs lysates in control, TNF-α and isorhamnetin groups were loaded onto an SDS-polyacrylamide gel and AP-1 were detected by Western blotting. Equal loading of proteins were confirmed by reprobing blots with antibodies against β-actin. E. Quantitation of AP-1 expression of HUVECs. §P < 0.05 vs. control group, ▲P < 0.05 vs. TNF-α group. Data shown are means ± SE representative of 4 independent experiments.
Figure 7.
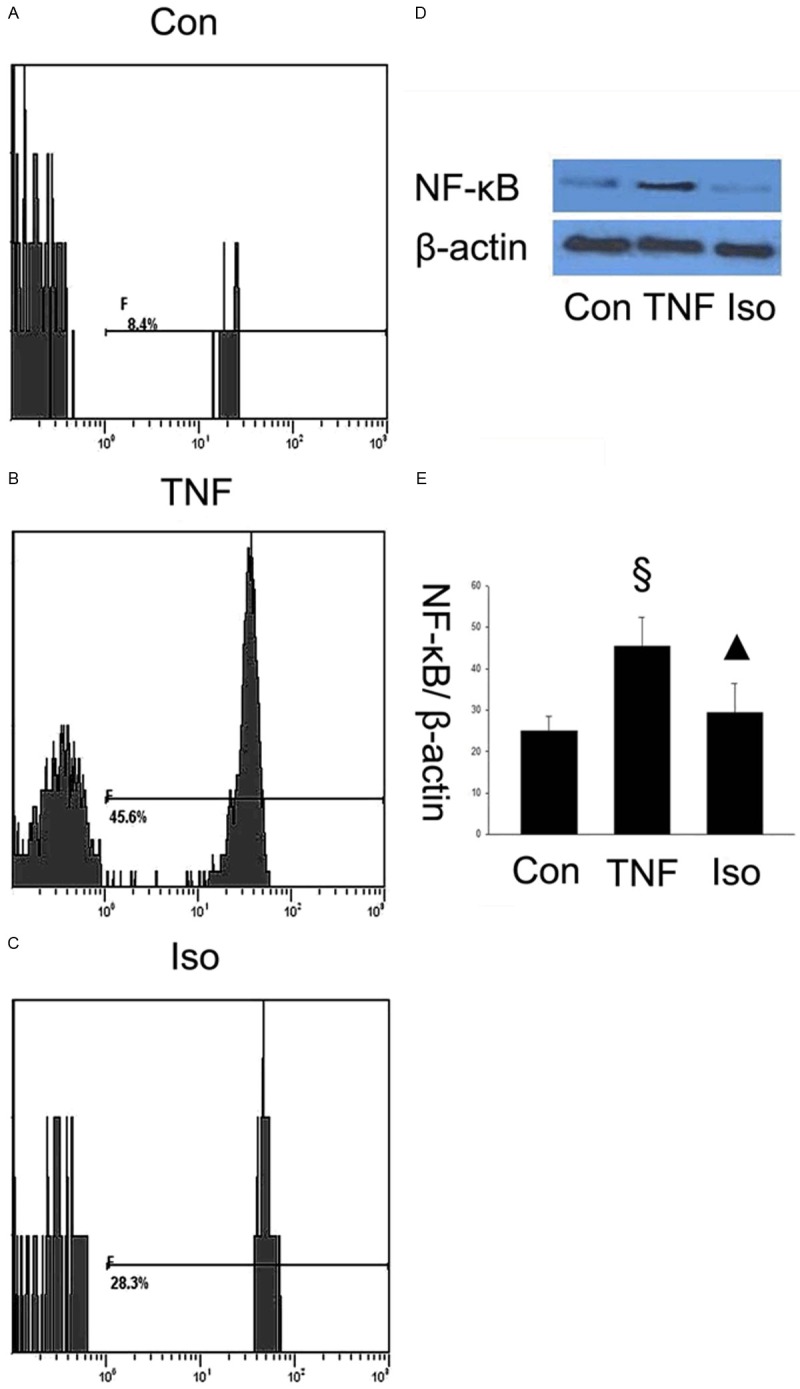
Isorhamnetin suppressed NF-κB pathway activation. A. Expression of NF-κB in control group was measured by a flow cytometry assay; B. Expression of NF-κB in TNF-α group was measured by a flow cytometry assay: C. Expression of NF-κB in isorhamnetin group was measured by a flow cytometry assay; D. 40 µg HUVECs lysates in control, TNF-α and isorhamnetin groups were loaded onto an SDS-polyacrylamide gel and NF-κB were detected by Western blotting. Equal loading of proteins were confirmed by reprobing blots with antibodies against β-actin; E. Quantitation of NF-κB expression of HUVECs. §P < 0.05 vs. control group, ▲P < 0.05 vs. TNF-α group. Data shown are means ± SE representative of 4 independent experiments.
Discussion
Endothelial inflammation plays important roles in the development of atherosclerotic process, which is the leading cause of death in cardiovascular disease. The initial inflammatory response was regarded as a defense reaction and its further speeded up atherosclerosis [23]. In this study, TNF-α induced apoptosis and up-regulated the expression of ICAM-1, VCAM-1 and E-selectin in HUVECs, the expression of these adhesion molecules could lead to vascular inflammation and the development of atherosclerosis [24,25]. Interestingly, isorhamnetin has a significant protective effects on HUVECs, which showed that isorhamnetin had the ability to inhibit of apoptosis and inflammation in HUVECs. In our model, we found that isorhamnetin could reduce the apoptotic index and regulate these adhesion molecules expression in HUVECs induced by TNF-α. Isorhamnetin pretreatment reduced TNF-α-induced apoptotic damage in HUVECs. In our study, NF-κB downregulation might participate in isorhamnetin-mediated protection of apoptosis [26]. Recent study had showed that NF-κB could mediate endothelial cells apoptosis and endothelial NF-κB pathway could switch to AP-1 pathway in endothelial injury and repair [27,28]. Whether other pathways contribute to the apoptosis need to be further studied. In H9c2 cardiomyocytes, isorhamnetin did not inhibit the death receptor dependent pathways. Instead, isorhamnetin specifically suppressed the mitochondria-dependent pathways, as characterized by maintenance of the mitochondrial membrane potential (ΔΨm) and regulation of a series of Bcl-2 family genes upstream of ΔΨm [18].
TNF-α-mediated inflammation responses are achieved through activating multiple signaling pathways. The expression of the adhesion molecules and the adhesion of leukocytes to the injury endothelium are the important steps of the inflammation. The current study found that isorhamnetin could reduce TNF-α-induced up-regulation of ICAM-1, VCAM-1 and E-selectin, suggesting that isorhamnetin might downregulate adhesion molecules expression and reduce the leukocytes adhesion. The activation of transcription factors NF-κB and AP-1 plays a critical role in mediating HUVECs responses, as shown in Figures 6 and 7, NF-κB and AP-1 expression significantly increased in TNF-α group. Whereas, isorhamnetin downregulated NF-κB and AP-1 expression, hence, downregulation of NF-κb and AP-1 were involved in the protective process. There are some crosstalk between ICAM-1, VCAM-1, E-selectin and NF-κB or AP-1 pathways. NF-κB plays a pivotal role in the coordinated transactivation of cytokine and adhesion molecule genes involved in endothelial activation [11,29,30]. Previous studies [13] showed that VEGF-stimulated expression of ICAM-1, VCAM-1 and E-selectin mRNAs was mainly through NF-κB activation. NF-κB mediated TNF-α-induced activation of endothelial cell via inducing the genes coding-the genes for adhesion molecules ICAM-1, VCAM-1and E-selectin [14,15]. Though increased NF-κB expression in macrophages could secrete and produce TNF-α, TNF-α significantly activate NF-κB signaling pathway in a positive feedback [16]. NF-κB is the control and induction of the pro-inflammatory cytokines induced by TNF-α, and it plays a major role in TNF-α expression. The pathway activating NF-κB is the primarily involved in the initiation of inflammatory responses. NF-κB modulated the production of proinflammatory mediators, and pretreatment with puerarin attenuated the inflammatory responses in rats via inhibition of NF-κB [17], which showed the effective treatment with inflammation by the NF-κB signaling pathway. Previous study suggested that activation of redox-sensitive transcription factors such as NF-κB and AP-1, which in turn stimulate the expression of an array of inflammatory cytokines [31]. Consistent with these observations, our data show that isorhamnetin inhibits TNF-α-induced inflammation, as evidenced by reduced adhesion molecules and decreased NF-κB and AP-1 activation.
In our study, isorhamnetin also could increase the expression of eNOS induced by TNF-α injury. eNOS produces the low density of NO, which helps to maintain the normal structure and function of the endothelium. NO is one of the vasoactive-substances. It is synthesized by the Nitric Oxide Synthase. NO also has the ability of anti-inflammation by inhibition of the adhesion molecules [9]. NO might decrease endothelial cell activation by the mechanism of reducing NF-κB activation in vascular endothelial cells. Exogenous NO has been showed to inhibit NF-κB activation by reducing expression and prolonging the half-life of the NF-κB inhibitory subunit IκBα [18].
In conclusion, adhesion molecules had an important role in the process of atherosclerosis, isorhamnetin inhibited TNF-α-induced apoptosis and up-regulation adhesion molecules ICAM-1, VCAM-1, E-selectin by suppress the NF-κB and AP-1 expression. Simultaneously, up-regulating the expression of eNOS by isorhamnetin was involved in the protective process which could help to keep vascular function. This study suggests that isorhamnetin has the potential effects of anti-apoptosis and anti-inflammation on TNF-α induced HUVECs, which might be one of the underlying mechanisms for the treatment of coronary heart disease.
Acknowledgements
All authors had full access to all of the data and take responsibility for the integrity of the data in the study and the accuracy of the analysis. This work was supported by the grant from Hangzhou Health Science and technology project (2008Z008); Hangzhou Science and Technology Bureau project (20080333Q23); Traditional Chinese medicine of Zhejiang province science and technology plan project (2008CBO54) and Zhejiang Province Natural Science Foundation (No. Y2081035).
Disclosure of conflict of interest
None.
References
- 1.Liuzzo G. Atherosclerosis: an inflammatory disease. Rays. 2001;26:221–230. [PubMed] [Google Scholar]
- 2.Williams KJ, Tabas I. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:1928. [PubMed] [Google Scholar]
- 3.Hossain M, Qadri SM, Su Y, Liu L. ICAM-1-mediated leukocyte adhesion is critical for the activation of endothelial LSP1. Am J Physiol Cell Physiol. 2013;304:C895–904. doi: 10.1152/ajpcell.00297.2012. [DOI] [PubMed] [Google Scholar]
- 4.Siasos G, Tousoulis D, Tsigkou V, Kokkou E, Oikonomou E, Vavuranakis M, Basdra EK, Papavassiliou AG, Stefanadis C. Flavonoids in atherosclerosis: an overview of their mechanisms of action. Curr Med Chem. 2013;20:2641–2660. doi: 10.2174/0929867311320210003. [DOI] [PubMed] [Google Scholar]
- 5.Debefve E, Mithieux F, Perentes JY, Wang Y, Cheng C, Schaefer SC, Ruffieux C, Ballini JP, Gonzalez M, van den Bergh H, Ris HB, Lehr HA, Krueger T. Leukocyte-endothelial cell interaction is necessary for photodynamic therapy induced vascular permeabilization. Lasers Surg Med. 2011;43:696–704. doi: 10.1002/lsm.21115. [DOI] [PubMed] [Google Scholar]
- 6.Gillham JC, Myers JE, Baker PN, Taggart MJ. TNF-alpha alters nitric oxide-and endothelium-derived hyperpolarizing factor-mediated vasodilatation in human omental arteries. Hypertens Pregnancy. 2008;27:29–38. doi: 10.1080/10641950701825796. [DOI] [PubMed] [Google Scholar]
- 7.Rajan S, Ye J, Bai S, Huang F, Guo YL. NF-kappaB, but not p38 MAP kinase, is required for TNF-alpha-induced expression of cell adhesion molecules in endothelial cells. J Cell Biochem. 2008;105:477–486. doi: 10.1002/jcb.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicha I, Urschel K, Daniel WG, Garlichs CD. Telmisartan prevents VCAM-1 induction and monocytic cell adhesion to endothelium exposed to non-uniform shear stress and TNF-alpha. Clin Hemorheol Microcirc. 2011;48:65–73. doi: 10.3233/CH-2011-1394. [DOI] [PubMed] [Google Scholar]
- 9.Bharadwaj AS, Schewitz-Bowers LP, Wei L, Lee RW, Smith JR. Intercellular adhesion molecule 1 mediates migration of Th1 and Th17 cells across human retinal vascular endothelium. Invest Ophthalmol Vis Sci. 2013;54:6917–6925. doi: 10.1167/iovs.13-12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Telen MJ. Cellular adhesion and the endothelium: E-selectin, L-selectin, and pan-selectin inhibitors. Hematol Oncol Clin North Am. 2014;28:341–354. doi: 10.1016/j.hoc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Celec P. Nuclear factor kappa B--molecular biomedicine: the next generation. Biomed Pharmacother. 2004;58:365–371. doi: 10.1016/j.biopha.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 13.Harris TR, Hammock BD. Soluble epoxide hydrolase: gene structure, expression and deletion. Gene. 2013;526:61–74. doi: 10.1016/j.gene.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponnuswamy P, Schrottle A, Ostermeier E, Gruner S, Huang PL, Ertl G, Hoffmann U, Nieswandt B, Kuhlencordt PJ. eNOS protects from atherosclerosis despite relevant superoxide production by the enzyme in apoE mice. PLoS One. 2012;7:e30193. doi: 10.1371/journal.pone.0030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gielis JF, Lin JY, Wingler K, Van Schil PE, Schmidt HH, Moens AL. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Radic Biol Med. 2011;50:765–776. doi: 10.1016/j.freeradbiomed.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Dou W, Zhang J, Li H, Kortagere S, Sun K, Ding L, Ren G, Wang Z, Mani S. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem. 2014;25:923–933. doi: 10.1016/j.jnutbio.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao M, Lou Y. Isorhamnetin prevent endothelial cell injuries from oxidized LDL via activation of p38MAPK. Eur J Pharmacol. 2006;547:22–30. doi: 10.1016/j.ejphar.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Sun B, Sun GB, Xiao J, Chen RC, Wang X, Wu Y, Cao L, Yang ZH, Sun XB. Isorhamnetin inhibits H(2)O(2)-induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation. J Cell Biochem. 2012;113:473–485. doi: 10.1002/jcb.23371. [DOI] [PubMed] [Google Scholar]
- 19.Chirumbolo S. Anti-inflammatory action of isorhamnetin. Inflammation. 2014;37:1200–1201. doi: 10.1007/s10753-014-9846-9. [DOI] [PubMed] [Google Scholar]
- 20.Ling S, Nheu L, Dai A, Guo Z, Komesaroff P. Effects of four medicinal herbs on human vascular endothelial cells in culture. Int J Cardiol. 2008;128:350–358. doi: 10.1016/j.ijcard.2007.05.111. [DOI] [PubMed] [Google Scholar]
- 21.Zhu YP, Shen T, Lin YJ, Chen BD, Ruan Y, Cao Y, Qiao Y, Man Y, Wang S, Li J. Astragalus polysaccharides suppress ICAM-1 and VCAM-1 expression in TNF-alpha-treated human vascular endothelial cells by blocking NF-kappaB activation. Acta Pharmacol Sin. 2013;34:1036–1042. doi: 10.1038/aps.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TH, Ku SK, Lee IC, Bae JS. Anti-inflammatory functions of purpurogallin in LPS-activated human endothelial cells. BMB Rep. 2012;45:200–205. doi: 10.5483/BMBRep.2012.45.3.200. [DOI] [PubMed] [Google Scholar]
- 23.Manduteanu I, Simionescu M. Inflammation in atherosclerosis: a cause or a result of vascular disorders? J Cell Mol Med. 2012;16:1978–1990. doi: 10.1111/j.1582-4934.2012.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aitken SL, Corl CM, Sordillo LM. Pro-inflammatory and pro-apoptotic responses of TNF-alpha stimulated bovine mammary endothelial cells. Vet Immunol Immunopathol. 2011;140:282–290. doi: 10.1016/j.vetimm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Azcutia V, Abu-Taha M, Romacho T, Vazquez-Bella M, Matesanz N, Luscinskas FW, Rodriguez-Manas L, Sanz MJ, Sanchez-Ferrer CF, Peiro C. Inflammation determines the pro-adhesive properties of high extracellular d-glucose in human endothelial cells in vitro and rat microvessels in vivo. PLoS One. 2010;5:e10091. doi: 10.1371/journal.pone.0010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C, Cheng W, Chang M, Su Y, Chen C, Hsieh F. Hypoxia-induced apoptosis in endothelial cells and embryonic stem cells. Apoptosis. 2005;10:887–894. doi: 10.1007/s10495-005-2946-0. [DOI] [PubMed] [Google Scholar]
- 27.Liu G, Ye X, Miller EJ, Liu SF. NF-kappaB-to-AP-1 switch: a mechanism regulating transition from endothelial barrier injury to repair in endotoxemic mice. Sci Rep. 2014;4:5543. doi: 10.1038/srep05543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsou TC, Tsai FY, Wu MC, Chang LW. The protective role of NF-kappaB and AP-1 in arsenite-induced apoptosis in aortic endothelial cells. Toxicol Appl Pharmacol. 2003;191:177–187. doi: 10.1016/s0041-008x(03)00239-4. [DOI] [PubMed] [Google Scholar]
- 29.Saito T, Hasegawa Y, Ishigaki Y, Yamada T, Gao J, Imai J, Uno K, Kaneko K, Ogihara T, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Importance of endothelial NF-kappaB signalling in vascular remodelling and aortic aneurysm formation. Cardiovasc Res. 2013;97:106–114. doi: 10.1093/cvr/cvs298. [DOI] [PubMed] [Google Scholar]
- 30.Wu S, Gao X, Yang S, Liu L, Ge B, Yang Q. Protective effects of cariporide on endothelial dysfunction induced by homocysteine. Pharmacology. 2013;92:303–309. doi: 10.1159/000356318. [DOI] [PubMed] [Google Scholar]
- 31.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


