Abstract
As one of the most classic supportive cells, Schwann cells (SCs) have been considered as potential candidates for nerve regeneration. However, SCs cultured in vitro are found with attenuated biological activities, which limits their application. Pulsed magnetic field (PMF) has been demonstrated to be safe and efficient to regulate several cells activities. However, it is still unclear the effect of PMF on proliferation and expression of neurotrophic factors in SCs. Therefore, the present study was designed to examine such possible effects. The tolerance of SCs to PMF was examined by flow cytometry and scanning electron microscopy (SEM). The proliferation of cells was detected by an EdU labeling assay and a Prestoblue assay. The expression and secretion of neurotrophic factors in SCs was assayed by RT-PCR and ELISA. We found that 2.0 mT was the optimal intensity that caused relatively little apoptosis with profound proliferation in SCs. The gene expression and protein level of brain-derived neurotrophic factor (BDNF), glial cell derived neurotrophic factor (GDNF), vascular endothelial growth factor (VEGF) were up-regulated following PMF stimulation, additionally, the gene expression and protein level of neurotrophin-3 (NT-3) was not enhanced by PMF. Our results suggested that PMF could improve SC proliferation and biological function, which might shed a light on the potential utilization of PMF in nerve regeneration via SC activation.
Keywords: Schwann cells, pulsed magnetic field, cell proliferation, neurotrophic factor
Introduction
Nerve injury and regeneration are clinically and experimentally concerned in regenerative medicine. Generally, functional recovery is poor after nerve injuries, which results in the loss of motor, sensory and autonomic functions in both peripheral nervous system (PNS) and central nervous system (CNS) [1]. Nowadays, numerous biologic and cellular strategies have been reported as reparative approaches for anatomical restitution and locomotor recovery after nerve injury, amongst which Schwann cells (SCs) are attractive for its unique neurotrophic and neuroprotective effects [2].
SCs are the myelinating glia of the PNS which are capable of directing axonal migration and outgrowth [3]. Accumulating studies have shown that SCs play a central role in axonal regeneration and elicit remyelination by producing various of trophic factors, as well as the expression of cell adhesion molecules known to influence neurite growth [4,5]. Therefore, the application of SCs has been regarded as a potential source of cell therapy for nerve regeneration. However, SCs cultured in vitro are found with attenuated biological activities, which significantly limits the application of SCs in the repair of nerve injuries. Thus, it is interesting to explore new ways to activate SCs to improve their viability and biological properties in cellular treatment for nerve regeneration.
Pulsed electromagnetic field (PMF) has been demonstrated to be safe and efficient to enhance nerve regeneration in vivo [6-9]. In addition, Studies have been reported that PMF is closely linked with ionic mobilization, protein synthesis and growth factors secretion in various cell types [10,11]. Thus far, a few studies have focused on the effect of PMF on SCs. It has been noted that SCs is impacted by magnetic field in orientation control [12]. However, it still remains unclear with regard to the influence of PMF on SC biological activities. Accordingly, the aim of this study was to address the underlying role of PMF in SCs and to further facilitate the application of PMF in SCs for nerve regeneration.
Methods and materials
SC culture and identification
SCs were prepared and purified following a protocol described previously. All the experimental procedures were performed by the Guide for the Care and Use of Laboratory Animals. In brief, SCs were isolated from the sciatic nerves of newborn Sprague Dawley rats and further selected from fibroblasts. The purity of the SCs was determined by immunofluorescent staining with S-100. The final preparations consisted of a high purity (> 97%) of SCs (Figure 1). SCs were maintained in Dulbecco’s modified Eagle’s medium nutrient mixture F-12 (DMEM/F12; Gibco, USA) containing 10% fetal calf serum (FCS; Gibco), antibiotics, bovine pituitary extract (Biomedical Technologies, USA) and forskolin (Sigma-Aldrich) at 37°C under humidified 5% CO2. SC cultures were passaged no more than five times before conducting experiments.
Figure 1.
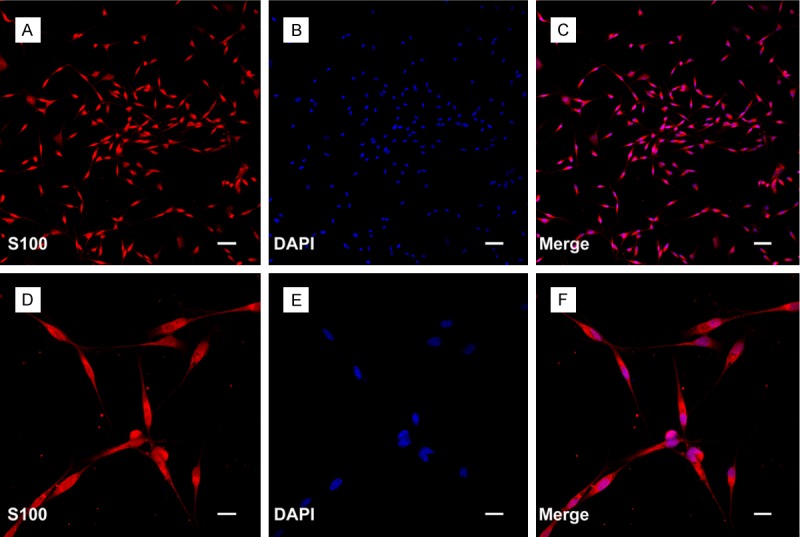
Isolated SCs from sciatic nerve of the Sprague Dawley rats. A and D: The expression of S100 was showed by immunofluorescent staining. B and E: Nuclei of SCs were visualized with DAPI (4’-6-diamidino-2-phenylindole) staining. C and F: Merged file showed that approximately more than 97% cells were positive staining. Scale bars: A-C: 100 μm, D-F: 50 μm.
Magnetic field exposure system
The magnetic stimulation system involved a solenoid and a magnetic field (PMF) generator to produce magnetic field with an adjustable magnetic induction of 0-20 mT and a frequency of 0-100 Hz. A PMF generator (GHY-III, China Patent, Xi’an, China) connected with the solenoid to produce an open-circuit output waveform PMF. The measurement accuracy of the electromagnetic field output was confirmed with a gaussmeter (Model 455 DSP Gaussmeter, Lake Shore Cryotronics, US). The PMF parameter was set to a frequency of 50 Hz as it was described in the previous experiments. Cells cultured in culture plates without solenoid was used as the culture plate group. In the no magnetic field group, the cell culture plate was put correctly in a solenoid that was connected to the PMF generator but no output waveform.
Flow cytometry
The SCs (1 × 105 cell/cm2) were cultured on culture plate for 24 h, and then different PMF gradients (0.5 mT, 1.0 mT, 2.0 mT, 5.0 mT, 10.0 mT; 50 Hz) were applied to the cells cultured on culture plate for 4 h. After PMF exposure, SCs were cultured for another 24 h, and then the percentage of viable cells was analyzed by flow cytometry within 30 min.
CCK-8 assay
The viability of SCs was detected and quantified using the Cell Counting Kit 8 (CCK-8; Dojindo, Japan). Briefly, the SCs inoculated in 96-well plates were under different exposure times of the PMF (2.0 mT). At 24 and 48 hours after PMF exposure, SCs were washed with PBS and inoculated with DMEM/F12. Thereafter, the CCK-8 solution (10 µL) was added to each well of the plate and incubated for 4 hours. The absorbance was measured at 492 nm using a microplate reader.
Morphological characteristics of SCs under PMF
Twenty-four hours after PMF stimulation, the morphological feature of SCs was observed by scanning electron microscopy (SEM; JSM-4800; Hitachi). In brief, SCs were rinsed with PBS and fixed with 4% paraformaldehyde for 30 minutes, washed three times with deionized water, and then dehydrated and later dried in vacuum environment. Thereafter, the specimens were sputter-coated with gold, and then visualized with SEM.
EdU-labeling assay
Cell proliferation assays were performed using the Cell LightTM 5-ethynyl-2-deoxyuridine (EdU) deoxyribonucleic acid (DNA) cell proliferation kit (RiboBio, PRC) In brief, 12 and 24 hours after PMF exposure, EdU was applied 4 hours prior to fixation of SCs and the EdU immunostaining. Then, EdU-labeling index was calculated as the amount of EdU-stained nuclei divided by the amount of DAPI-stained nuclei calculated from five random fields. Assays were done three times using triplicate wells.
Gene-expression analysis
At 12 and 24 hours after PMF stimulation, SCs were first counted and then homogenized in Trizol reagent (Sigma-Aldrich). Total ribonucleic acid (RNA) was isolated and normalized to cell numbers. Complementary DNA was synthesized using Superscript III reagents (Invitrogen, US). Then, quantitative real-time PCR (QRT-PCR) analysis was performed. The forward (f) and reverse (r) primers are used as follows: fBDNF AGTATTAGCGAGTGGGTC, rBDNF GTTCCAGTGCCTTTTGTC, fGDNF CAGAGGGAAAGGTCGCAGAG, rGDNF ATCAGTTCCTCCTTGGTTTCGTAG, fVEGF-A AACTTCTACCCGTGCCTT, r VEGF-A ACTTAGGTCAGCGTTTCC, fNT-3 GCAGGGTGAAGGGGAAAAC, rNT-3 GCCACGGAGATAAGCAAGAAA, fACTB (internal control) ATGAAGATCCTGACCGAG and rACTB GCTCATTGCCGATAGTGA. QRT-PCR conditions were as follows: denaturation at 95°C, 30 seconds; primer annealing at 59°C, 30 seconds; and elongation at 72°C, 40 seconds. Quantification of PCR products was performed using the 2-∆∆Ct method. Quantities of messenger RNA (mRNA) were normalized to the housekeeping gene ACTB. Assays were done three times using triplicate wells.
ELISA assay
For enzyme-linked immunosorbent assay (ELISA) analysis, the SCs were cultured in fresh DMEM/F12 medium without FCS. At 12, 24, and 36 hours after PMF exposure, the number of SCs was counted, and the amount of BDNF, GDNF, VEGF, and NT-3 secreted by the cultured SCs was determined by culture mediums. Cell culture supernatants were centrifuged, and the assays were carried out using an ELISA kit (Antibodies Online, Germany). The absorbance of each well was measured using a micro-ELISA reader (Multiscan MK3; Thermo Labsystems, Finland) at 450 nm. Then, the amount of BDNF, GDNF, VEGF, and NT-3 secreted by SCs was normalized to cell numbers in each group.
Statistical analysis
All data presented here are expressed as means ± standard error of the mean (SEM). The data were analyzed using one-way analysis of variance with SPSS 13.0 software (SPSS, USA). Values of P < 0.05 were considered statistically significant.
Results
SC survival under different intensities of PMF
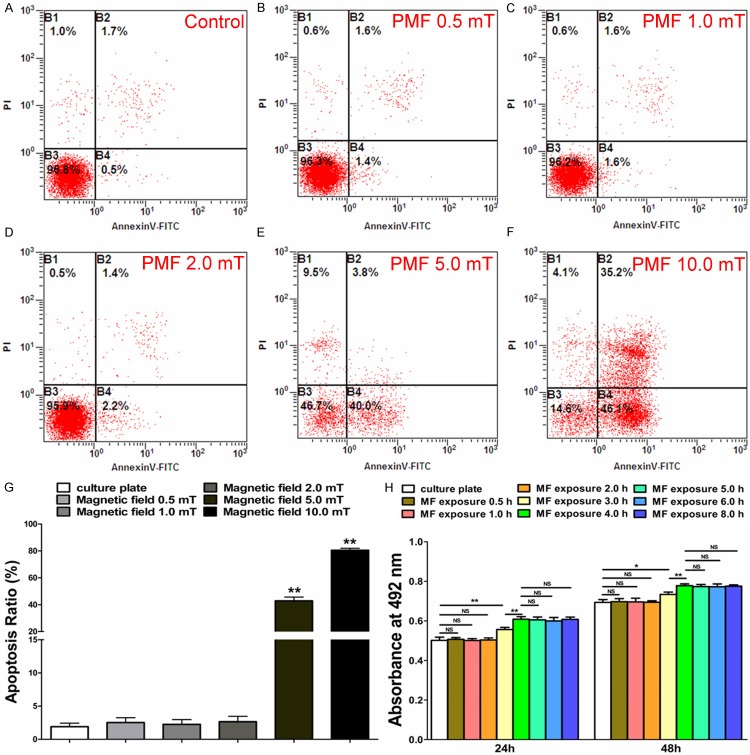
The effect of PMF on the survival of SCs was investigated by flow cytometry. As shown in Figure 2A-G, the apoptosis ratio of SCs after 24 hours PMF exposure was in a similar range to that without PMF exposure or at 0.5 mT, 1.0 mT, and 2.0 mT stimulation. When the PMF was increased to 5.0 mT and 10.0 mT, the percentage of apoptotic cells was significantly increased. Therefore, PMF at 2.0 mT was used in the remaining experiments for the observation that it caused no significant cell apoptosis.
Figure 2.
Detection of apoptosis and proliferation in SCs. A-F: Flow cytometry assay of apoptosis in SCs following different PMF gradients (0.5 mT, 1.0 mT, 2.0 mT, 5.0 mT, 10.0 mT and 50 Hz). G: The percentage of apoptotic cells in each group was showed by averaging the results of four flow cytometry assays for each group. H: CCK-8 values of 24 h and 48 h after PMF exposure were obtained by averaging the results of four samples for each group. All data were expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
CCK-8 assay
Twenty-four hours after PMF exposure, the CCK-8 value of SCs less than 4 hours of PMF stimulation was significantly higher than that of fewer hours’ stimulation. No significant difference was observed among stimulation under 4, 5, 6 and 8 hours. The CCK-8 value showed a similar outcome after 48 hours PMF exposure. Therefore, a 4-hour exposure time of PMF was applied in the remaining experiments (Figure 2H).
SC morphology under PMF
Twenty-four hours after PMF stimulation (2.0 mT), SCs exhibited a spindle feature which was in a similar shape to that under 0.5 and 1.0 mT, as well as those without PMF stimulation. When the PMF increased to 5.0 mT, the SCs were changed into round shape, losing its characteristic morphology. In addition, when the PMF increased to 10.0 mT, the SCs were almost broken with damaged membranes. The SEM results were consistent with the apoptosis assay (Figure 3).
Figure 3.
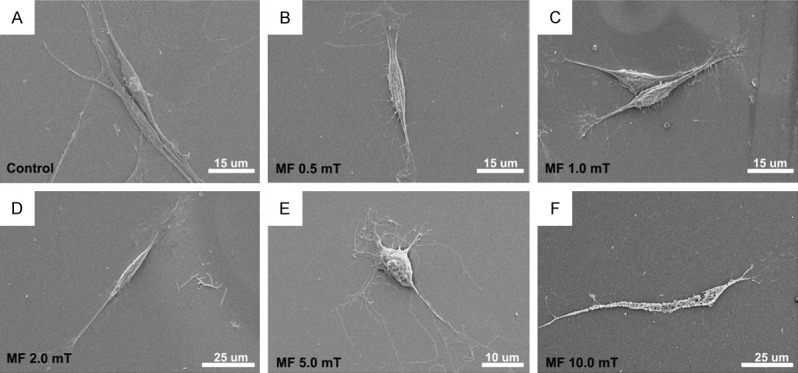
Electron microscopy assay of SCs following different PMF gradients. A-D: SCs exhibited a spindle feature in the control group as well as in 0.5, 1.0 and 2.0 mT PMF stimulation. E: SCs were changed into round shape when the PMF increased to 5.0 mT. F: Damaged membranes were observed when the PMF increased to 10.0 mT.
EdU-labeling assay
The cell proliferation was quantified by an EdU-labeling assay. Twelve hours after PMF exposure, the EdU-labeling index of SCs was significantly higher than the culture plate group and the no magnetic field group, respectively. The EdU-labeling index of SCs was in a similar range to that of the other two groups after 24 hours (Figure 4).
Figure 4.
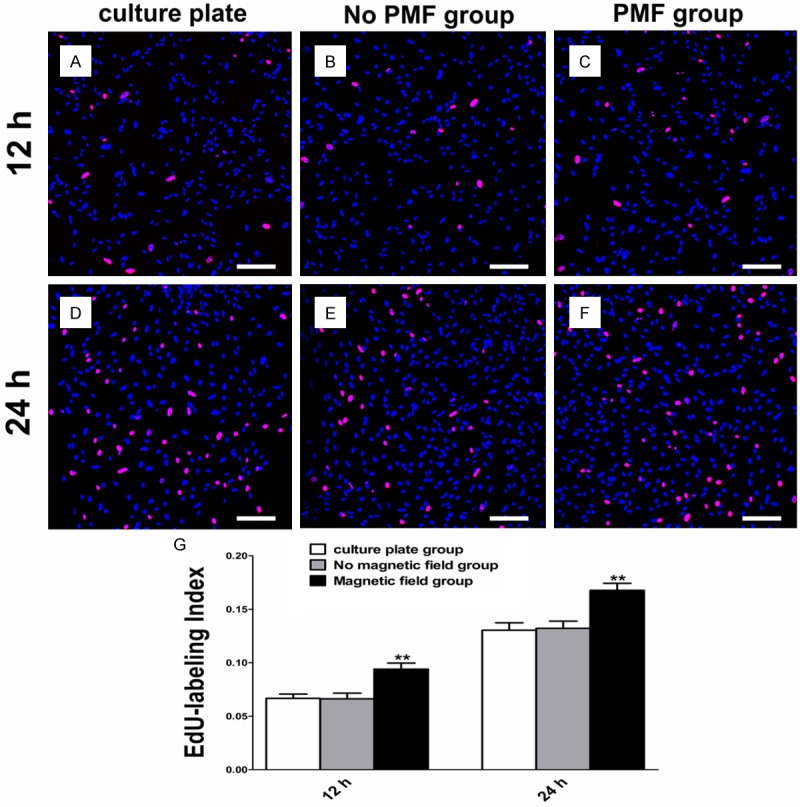
EdU-labeling assay of SCs. A-C: EdU-labeling of SCs after PMF exposure at 12 h. Scale bars: 100 μm. D-F: EdU-labeling of SCs after PMF exposure at 24 h. Scale bars: 100 μm. G: EdU-labeling index of SCs obtained by averaging the results of four samples for each group. All data were expressed as the mean ± SEM. **P < 0.01.
Expression of regeneration-related genes
The effect of PMF on expression of BDNF, GDNF, VEGF, and NT-3 of SCs was examined by RT-PCR 12 and 24 hours after PMF exposure. Under PMF stimulation, the mRNA levels of BDNF, GDNF and VEGF were higher than that without PMF at 12 and 24 hours. The NT-3 mRNA level at 12 and 24 hours was in a similar range to that without PMF (Figure 5).
Figure 5.
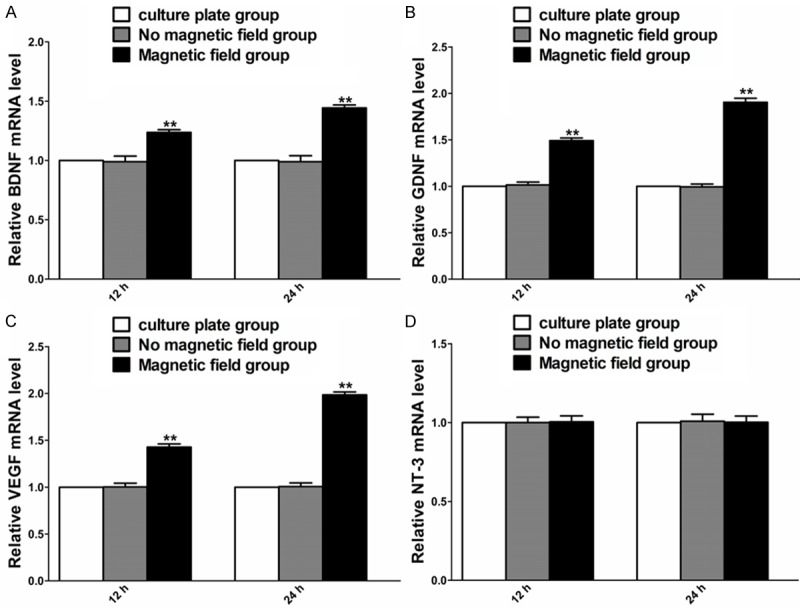
Gene expression of BDNF, GDNF, VEGF and NT-3 in each cultured group at different time points after PMF exposure. A-D: The mRNA levels of BDNF, GDNF, VEGF and NT-3 were determined at 12 and 24 h. Each test was repeated three times. All data were expressed as the mean ± SEM. **P < 0.01.
Secretion of regeneration-related protein
The effect of PMF on the secretion of BDNF, GDNF, VEGF and NT-3 was tested 12, 24 and 36 hours after PMF stimulation. Under PMF exposure, the secretion of BDNF, GDNF and VEGF was higher than that without PMF at 12, 24 and 36 hours (Figure 4E-G). The secretion of NT-3 at 12, 24 and 36 hours was in a similar range to that without PMF (Figure 6).
Figure 6.
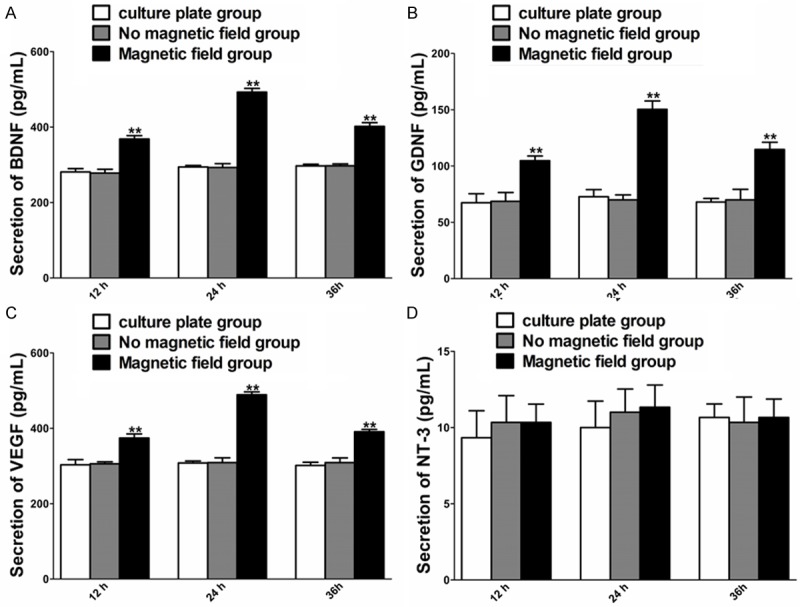
Concentration of BDNF, GDNF, VEGF and NT-3 in each cultured group at different time points after PMF exposure. A-D: Secretion of BDNF, GDNF, VEGF and NT-3 in culture supernatants was determined at 12, 24 h and 36 h. Each test was repeated three times. All data were expressed as the mean ± SEM. **P < 0.01.
Discussion
With its convenient and practical property, PMF is identified as an ideal option that can be used easily in patients with nervous system injuries. Until now, several lines of evidence have demonstrated that PMF plays an important role in never regeneration [13,14]. Despite PMF has been shown promising in the stimulation of axonal regenerative processes, the underlying cellular and molecular mechanism is still unclear. On the other hand, SCs can be activated to expand the production of neurotrophic factors in response to various external stimuli. In this field, we have previously proven that electrical stimulation is capable of promoting SCs neurotrophic function [15]. Considering the close relationship between electrical currents and magnetic field, we hypothesized that controlled PMF could activate SCs to enhanced biological function, which is thought to be beneficial for nerve regeneration.
To our knowledge, this study is the first report addressing the application of PMF in the activation and functional influence of SCs in vitro. Here, we found that PMF was capable of stimulating SCs to enhanced viability and biological properties. A magnetic field (50 Hz, 2.0 mT) was detected to improve viability and proliferation of SCs with almost no impact on apoptosis. Furthermore, we demonstrated that neurotrophic factors were up-regulated by PMF in SCs. These findings might shed a light on the potential utilization of PMF in nerve regeneration via SCs activation.
Different intensities of PMF have been used in the studies of biological properties in various cell types [16,17]. In the present study, we applied a range of magnetic gradients to detect an optimal intensity. We found that 2.0 mT was the maximum intensity that promoted proliferation and caused relatively little apoptosis in SCs. Higher magnetic gradients were found to cause dramatically increased apoptotic ratio, suggesting that high magnetic stimulation is detrimental for SCs proliferation. These data was confirmed by SEM analysis, which showed different morphology in the applied magnetic gradients. When SCs were subjected to lower magnetic gradients than 2.0 mT, the morphology of SCs stayed unchanged. However, the SCs shrunk and broken membrane fragment was detected in higher magnetic gradients. It has been reported that long-term cultured SCs failed to support regeneration and neuronal survival, which might be attributable to the low survival rate of SCs [18,19]. Our data suggested that 2.0 mT might be an optimal intensity to improve SCs proliferation with less detrimental effect, indicating that this magnetic gradient might be used in further SCs stimulation for sufficient nerve regeneration application.
SCs has been shown to be capable of enhancing nerve regeneration in both PNS and CNS [20]. It has been indicated that one of this beneficial effects is partly due to the synthesis and release of neurotrophic factors [21,22]. However, SCs cultured in vitro was found to lose their neuroprotective effect with the reduction of neurotrophic factors. Therefore, we examined the neurotrophic factors level in SCs following PMF stimulation. We found that the expression of BDNF, GDNF and VEGF was up-regulated in gene level after PMF stimulation (2.0 mT). Moreover, their secretion was observed in the culture medium. BDNF has been shown to promote survival and outgrowth of motor neurons in both PNS and CNS [23]. GDNF is a classical factor that facilitates the survival, growth, and differentiation of motoneurons and central noradrenergic neurons [24]. Moreover, VEGF is an effective protein that support SCs proliferation and axonal outgrowth [25]. Therefore, our study showed that the simultaneously increased expression of these factors may help to establish a supportive environment for nerve regeneration. On the other hand, it has been demonstrated that PMF can regulate neurotrophic factor activity and levels in neurons [26,27]. However, the underlying mechanism is unclear. Our results suggested that the PMF-induced secretion of neurotrophic factors in SCs and their crosslink with neurons might contribute to this phenomenon. Interestingly, we found that the expression of the neurotrophic factors achieved the highest level at 24 h and decreased over time afterwards. This information suggests that PMF exerted a delayed function on SCs in neurotrophic factors secretion and provides information in therapeutic time window for further PMF application clinically.
Notwithstanding our study deepen the understanding of SCs in PMF stimulation; we acknowledge that the mechanism by which PMF regulates the functions of SCs is unclear. PMF has been shown to affect cell in metabolism activation, mitochondrial membrane potential and ATP synthesis in different cell types [28]. In addition, it has been noted that PMF plays an important role in Ca+ flux, which is thought to crucially participate in nerve system [29,30]. However, whether these were involved in the PMF-mediated effect on SCs is still unclear. Further studies are needed to elucidate the coupling process between the PMF energy and conformational changes in biomolecules and how the “electromagnetic” information transfers to certain signaling pathways.
In conclusion, the current study provides evidence in the influence of PMF on SCs proliferation and function for the first time. We found the viability and biological properties of SCs were enhanced by PMF with up-regulated neurotrophic factors secretion. Consequently, PMF might be used as an activator in further application of SCs for nerve regeneration.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81201389 and 30973052), the National Basic Research Program of China (973 Program No. 2014CB542206), the Program for Changjiang Scholar and Innovative Research Team in University (IRT1053 and IRT13051), and A Foundation for the Author of National Excellent Doctoral Dissertation of PR China (201480).
Disclosure of conflict of interest
None.
References
- 1.Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242:S19–21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg AF, Isaacman-Beck J, Franzini-Armstrong C, Granato M. Schwann cells and deleted in colorectal carcinoma direct regenerating motor axons towards their original path. J Neurosci. 2014;34:14668–14681. doi: 10.1523/JNEUROSCI.2007-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis GM, Kucenas S. Perineurial glia are essential for motor axon regrowth following nerve injury. J Neurosci. 2014;34:12762–12777. doi: 10.1523/JNEUROSCI.1906-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito H, Bassett CA. Effect of weak, pulsing electromagnetic fields on neural regeneration in the rat. Clin Orthop Relat Res. 1983:283–290. [PubMed] [Google Scholar]
- 7.Kanje M, Rusovan A, Sisken B, Lundborg G. Pretreatment of rats with pulsed electromagnetic fields enhances regeneration of the sciatic nerve. Bioelectromagnetics. 1993;14:353–359. doi: 10.1002/bem.2250140407. [DOI] [PubMed] [Google Scholar]
- 8.De Pedro JA, Perez-Caballer AJ, Dominguez J, Collia F, Blanco J, Salvado M. Pulsed electromagnetic fields induce peripheral nerve regeneration and endplate enzymatic changes. Bioelectromagnetics. 2005;26:20–27. doi: 10.1002/bem.20049. [DOI] [PubMed] [Google Scholar]
- 9.Walker JL, Evans JM, Resig P, Guarnieri S, Meade P, Sisken BS. Enhancement of functional recovery following a crush lesion to the rat sciatic nerve by exposure to pulsed electromagnetic fields. Exp Neurol. 1994;125:302–305. doi: 10.1006/exnr.1994.1033. [DOI] [PubMed] [Google Scholar]
- 10.Chalidis B, Sachinis N, Assiotis A, Maccauro G. Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: biologic responses and clinical implications. Int J Immunopathol Pharmacol. 2011;24:17–20. doi: 10.1177/03946320110241S204. [DOI] [PubMed] [Google Scholar]
- 11.Gan JC, Glazer PA. Electrical stimulation therapies for spinal fusions: current concepts. Eur Spine J. 2006;15:1301–1311. doi: 10.1007/s00586-006-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eguchi Y, Ogiue-Ikeda M, Ueno S. Control of orientation of rat Schwann cells using an 8-T static magnetic field. Neurosci Lett. 2003;351:130–132. doi: 10.1016/s0304-3940(03)00719-5. [DOI] [PubMed] [Google Scholar]
- 13.Sisken BF, Kanje M, Lundborg G, Kurtz W. Pulsed electromagnetic fields stimulate nerve regeneration in vitro and in vivo. Restor Neurol Neurosci. 1990;1:303–309. doi: 10.3233/RNN-1990-13419. [DOI] [PubMed] [Google Scholar]
- 14.Hannemann PF, Gottgens KW, van Wely BJ, Kolkman KA, Werre AJ, Poeze M, Brink PR. The clinical and radiological outcome of pulsed electromagnetic field treatment for acute scaphoid fractures: a randomised double-blind placebo-controlled multicentre trial. J Bone Joint Surg Br. 2012;94:1403–1408. doi: 10.1302/0301-620X.94B10.28844. [DOI] [PubMed] [Google Scholar]
- 15.Luo B, Huang J, Lu L, Hu X, Luo Z, Li M. Electrically induced brain-derived neurotrophic factor release from Schwann cells. J Neurosci Res. 2014;92:893–903. doi: 10.1002/jnr.23365. [DOI] [PubMed] [Google Scholar]
- 16.Hulme J, Robinson V, DeBie R, Wells G, Judd M, Tugwell P. Electromagnetic fields for the treatment of osteoarthritis. Cochrane Database Syst Rev. 2002;12:CD003523. doi: 10.1002/14651858.CD003523. [DOI] [PubMed] [Google Scholar]
- 17.Bachl N, Ruoff G, Wessner B, Tschan H. Electromagnetic interventions in musculoskeletal disorders. Clin Sports Med. 2008;27:87–105. viii. doi: 10.1016/j.csm.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 18.White FV, Ceccarini C, Georgieff I, Matthieu JM, Costantino-Ceccarini E. Growth properties and biochemical characterization of mouse Schwann cells cultured in vitro. Exp Cell Res. 1983;148:183–194. doi: 10.1016/0014-4827(83)90198-2. [DOI] [PubMed] [Google Scholar]
- 19.Schlosshauer B, Muller E, Schroder B, Planck H, Muller HW. Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Res. 2003;963:321–326. doi: 10.1016/s0006-8993(02)03930-6. [DOI] [PubMed] [Google Scholar]
- 20.Carriel V, Garzon I, Alaminos M, Cornelissen M. Histological assessment in peripheral nerve tissue engineering. Neural Regen Res. 2014;9:1657–1660. doi: 10.4103/1673-5374.141798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dey I, Midha N, Singh G, Forsyth A, Walsh SK, Singh B, Kumar R, Toth C, Midha R. Diabetic Schwann cells suffer from nerve growth factor and neurotrophin-3 underproduction and poor associability with axons. Glia. 2013;61:1990–1999. doi: 10.1002/glia.22570. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HY, Jin XB, Lue TF. Three important components in the regeneration of the cavernous nerve: brain-derived neurotrophic factor, vascular endothelial growth factor and the JAK/STAT signaling pathway. Asian J Androl. 2011;13:231–235. doi: 10.1038/aja.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 25.Hillenbrand M, Holzbach T, Matiasek K, Schlegel J, Giunta RE. Vascular endothelial growth factor gene therapy improves nerve regeneration in a model of obstetric brachial plexus palsy. Neurol Res. 2015;37:197–203. doi: 10.1179/1743132814Y.0000000441. [DOI] [PubMed] [Google Scholar]
- 26.Longo FM, Yang T, Hamilton S, Hyde JF, Walker J, Jennes L, Stach R, Sisken BF. Electromagnetic fields influence NGF activity and levels following sciatic nerve transection. J Neurosci Res. 1999;55:230–237. doi: 10.1002/(SICI)1097-4547(19990115)55:2<230::AID-JNR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Zhang Z, Su Y, Kang L, Geng D, Wang Y, Luan F, Wang M, Cui H. Magnetic stimulation modulates structural synaptic plasticity and regulates BDNF-TrkB signal pathway in cultured hippocampal neurons. Neurochem Int. 2013;62:84–91. doi: 10.1016/j.neuint.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi-Sekino S, Sekino M, Ueno S. Biological effects of electromagnetic fields and recently updated safety guidelines for strong static magnetic fields. Magn Reson Med Sci. 2011;10:1–10. doi: 10.2463/mrms.10.1. [DOI] [PubMed] [Google Scholar]
- 29.Valdez LB, Zaobornyj T, Boveris A. Mitochondrial metabolic states and membrane potential modulate mtNOS activity. Biochim Biophys Acta. 2006;1757:166–172. doi: 10.1016/j.bbabio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Belyavskaya NA. Biological effects due to weak magnetic field on plants. Adv Space Res. 2004;34:1566–1574. doi: 10.1016/j.asr.2004.01.021. [DOI] [PubMed] [Google Scholar]