Abstract
Objective: Fasudil, a Rho kinase inhibitor, has neuroprotection in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-based Parkinson’s disease (PD). This study aims to investigate the mechanism underlying the neuroprotection of fasudil in the PD mice model. Methods: Female MPTP-intoxication C57BL/6 mice were treated with normal saline or fasudil on day 15 after first administration of MPTP. Pole test was used for the behavioral analysis of mice. Expression of interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) in brain tissue were detected by ELISA. Expression of tyrosine hydroxylase (TH), p-MYPT1, p-nuclear transcription factor NF-κB, toll-like receptor 2 (TLR2), arginase1, inducible nitric oxide synthase (iNOS), bromodeoxyuridine (BrdU), glial cell line-derived neurotrophic factor (GDNF), p-GSK-3b, p110-PI3K, p-Akt, WNT1, Fzd1 and β-catenin were determined by western blot and immunofluorescence analysis. Results: Fasudil enhanced the number of TH neurons which was decreased by MPTP treatment. Behavioral test showed that the motor performance of mice was improved after fasudil treatment. The expression of IL-1β, TNF-α, TLR2 and p-NF-κB and iNOS were lower after fasudil treatment (P < 0.05) while the expression of arginase1 was increased (P < 0.05). Further, we could observe the increase of GDNF expression in the microglial cells. The expression of p110-PI3K, p-Akt, WNT1, Fzd1 and β-catenin were increased after fasudil administration (P < 0.05) in MPTP-based mice model. Conclusions: Maybe fasudil protect dopamine neurons from loss in the MPTP mice model of PD through inflammatory inhibition via activation of PI3K/p-Akt and WNT1/Fzd1/β-catenin cell signaling pathways.
Keywords: Parkinson’s disease, MPTP, fasudil, inflammatory response, signaling pathways
Introduction
Parkinson’s disease (PD) is a chronic progressive neurodegenerative movement disorder characterized by loss of dopaminergic neurons [1]. The prevalence of PD was higher in men than in women [2]. Several toxin-based disease paradigms have been widely used to establish PD animal model. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) has been used to induce PD in animal along with the decrease of tyrosine hydroxylase (TH)-immunoreactive fibres in the median forebrain bundle [3,4]. Fasudil, a Rho kinase inhibitor, has neuroprotective effect in the MPTP model of PD, which was firstly described by Tönges et al, demonstrating that dopaminergic cells loss was attenuated after fasudil treatment. Besides, they also showed that Akt survival pathway was involved in the neuroprotection of fasudil in this paradigm [5]. However, the mechanism underlying neuroprotection of fasudil is not fully elucidated in the MPTP model of PD.
Several evidences have shown that chronic inflammation occurs in the brain regions in PD and may continue for many years in spite of the disappearance of MPTP stimuli [6-8]. The pro-inflammatory cytokines such as interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF)-α were expressed in PD patients [9,10]. Inflammatory regulators such as inducible nitric oxide synthase (iNOS), cyclooxygenases-1 and -2 were up-regulated in human Parkinsonism [11]. Some anti-inflammatory agents have been reported to inhibit the cell death in PD model [12]. Neuroinflammation could be characterized by active microglia [13] which includes classically activated M1 phenotype (pro-inflammatory function) and alternatively activated M2 phenotype (anti-inflammatory) [14,15]. The activated microglia may produce large amounts of potential neurotoxins and superoxide anions thought to be responsible for the dopaminergic cell death in PD [16,17]. Glial cell line-derived neurotrophic factor (GDNF, a growth factor) which was released by activated microglia were transplanted to a PD rat model and resulted in the reduction of dopamine neuron loss [18].
Wingless-type MMTV integration site1 (Wnt1) pathway is an essential signaling cascade regulating axonal extension, neuron survival, neurogenesis [19,20]. Wnt ligand binds to the seven-pass transmembrane receptors of the Frizzled (Fzd) family and “canonical” Wnt/β-catenin has been reported to be involved in the cell survival and proliferation [21]. Wnt pathway plays an important role in regulation of inflammation [22]. PI3K-Akt pathway has been reported to be related with inflammation [23,24].
In the present study, we aimed to investigate if the neuroprotective effect of fasudil was involved in the inflammation and signaling pathways. MPTP was used to induce the PD mice model and investigate the effect of fasudil. We further explored if neuroprotective effect of fasudil was involved in the cytokines and inflammatory signaling, such as IL-1β, TNF-α. Then we also investigated if the signaling pathways (p110-PI3K/p-Akt and WNT1/Fzd1/β-catenin) were involved in the neuroprotective effects of fasudil in the PD mice model.
Materials and methods
Subacute MPTP mice model of PD with fasudil treatment
All the experiments were approved by ethic committee of Fudan University. C57BL/6 mice, weighing 18 to 20 g, were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. The age of mice was from 8 to 10 weeks. Mice were randomly divided into 3 groups: control group, MPTP + fasudil group and MPTP + NS (normal saline) group (n = 8 per experimental group). Mice were intraperitoneally injected with MPTP every day, lasting for 7 days. On the first day, dosage was 15 mg/kg body weight. On the second day, the dosage was 20 mg/kg body weight. From day 3 to 7, dosage was 30 mg/kg body weight. MPTP was dissolved in 200 μL NS. In control group, mice were intraperitoneally injected with 200 μL NS every day, lasting for 7 days. In MPTP + Fa group, mice were intraperitoneally injected with 400 μL fasudil (1 μg/μL dissolved in NS) on day 15 after first administration of MPTP, which was performed twice a day for 7 days. For MPTP + NS group and control group, mice were given equal volume of NS on day 15 instead. Mice were sacrificed on day 40 after first application of MPTP.
Behavioral analysis
Behavioral analysis of mice was performed 37 days from the first MPTP treatment. Mice were put on the top of vertical iron with 12 mm diameter and 80 cm height. The time mice climbed from top to ground was recorded. The time longer than 120 s was recorded as 120 s. The training was performed for 2 days before test which was then performed every day for 3 days.
Enzyme-linked immunosorbent assay (ELISA)
Mice were performed anesthesia by intraperitoneal injection of 10% chloral hydrate at dosage of 3 mL/kg body weight. The chest was opened and perfused with warm saline through ventriculus sinister until liver became white. Fresh brain tissue was lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology, China) on ice for 20 min and then the mixture was centrifuged at 4°C for 30 min. The supernatants were collected and frozen at -80°C for further analysis.
The content of IL-1β and TNF-α in the brain tissue were tested using ELISA method according to manufacture’s instruction (PEPROTECH, USA). Briefly, samples were plated in the 96-well plate and the corresponding antibodies (anti-IL-1β, anti-TNF-α) were added for incubation overnight at 4°C. Then 1% BSA (bovine serum albumi, Serotec, Bicester, UK) was used to block for 1 h at room temperature. Subsequently, secondary antibody with biotin labeled was added to incubate for 1 h and then 50 μL horseradish peroxidase (HRP) for 45 min incubation at room temperature. Finally, the plate was observed by microplate reader at 450 nm.
Western blot
The content of protein in the samples was determined using bicinchoninic acid (BCA) method according to manufacture’s instruction (Beyotime Institute of Biotechnology, China) and was separated on a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Then 40 μg protein were transferred to nitrocellulose filter membrane and blocked by 5% defatted milk for 2 h. Then membrane was incubated with primary antibody (anti-p-NF-κB, anti-toll-like receptor 2 (TLR2), anti-arginase1, anti-iNOS,anti-p110-PI3K, anti-p-GSK-3β, anti-p-Akt, anti-WNT1, anti-Fzd1, anti-β-catenin, anti-GDNF) at 4°C overnight and was washed by 1 × Tris-buffered saline/Tween 20 (TBST) for 10 min for 3 times. Then membrane was incubated with corresponding HRP-coupled secondary antibodies at 1:5000 dilution for 1 h and was washed for 10 min by 1 × TBST for 3 times. The membrane was observed in Bio-Rad and images were analyzed by ImageLab software. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control.
Immunofluorescence
Immunofluorescence detection of bromodeoxyuridine (BrdU) requires intraperitoneally injection of Brdu in mice every day for 3 days before sacrifice. Brain tissue were postfixed overnight, cryoprotected and frozen, and microtome sectioned at 10 μm thickness. Every tenth section was processed for immunohistochemistry. Frozen brain tissue were dried at room temperature and washed with 1 × PBS for 5 min. Tissue were permeabilized by 0.2% TritonX-100/PBS and blocked by 5% BSA for 1 h, and then incubated with primary antibody (1:100, anti-TH, anti-Brdu, anti-GDNF, anti-NF-κB-p65, anti-iNOS, anti-arginase1, anti-CD11b, anti-glial fibrillary acidic protein (GFAP)) at 4°C overnight. PBS was used as negative control. After that, tissue was incubated for 60 min at room temperature with fluorescence secondary antibody (1:1000). Positive cells were counted in a total of 5 sections for each brain. The total number of positive cells in the 5 sections for each brain was the presented value. The sections were observed under fluorescence microscope (Olympus, Japan) and immunofluorescence images were analyzed by Image-Pro Plus 6.0 software. Number of TH immune positive cells was counted in the substantia nigra region; number of Brdu positive cells was counted in the ventricular zone; the number of GDNF, NF-κB-p65, iNOS and arginase1 positive cells was respectively counted in the striatum region.
Statistical analysis
Data were presented as mean ± SEM. One-way analysis of variance was applied for comparison between two groups using GraphPad v5.0 (La Jolla, CA). ELISA results were analyzed by unilateral t test. *P < 0.05, **P < 0.01 and ***P < 0.001 were represented as significance.
Results
Effect of fasudil on the dopamine neurons in MPTP mice model of PD
We fist studied the neuroprotection effects of fasudil in the MPTP induced model. Fasudil was treated for MPTP model of PD on the day 15 after the first administration of MPTP. Western blot analysis showed that the expression of p-MYPT1 was higher in MPTP + NS group comparing with control group and its expression was decreased after fasudil administration (P < 0.01, Figure 1A). Figure 1B showed that the time spent climbing was shorter in MPTP + Fa group (18.96 ± 1.224 s) comparing with MPTP + NS group (15.58 ± 1.088 s, P < 0.05). The content of TH in the substantia nigra significantly decreased after MPTP application compared with control group (P < 0.01) and increased after fasudil treatment (P < 0.05, Figure 1C). The immunofluorescence analysis showed that number of TH immune positive cells increased after fasudil administration comparing with MPTP + NS group (5638 ± 564.9 vs. 3239 ± 245.9 cells, P < 0.01, Figure 1D). Analysis of BrdU+ cells in combination of TH+ cells demonstrated that the number of TH+ cells in the ventricular zone was increased after fasudil treatment (Figure 2). Thus, fasudil confer dopaminergic neuron protection from the MPTP induced intoxication in mice.
Figure 1.
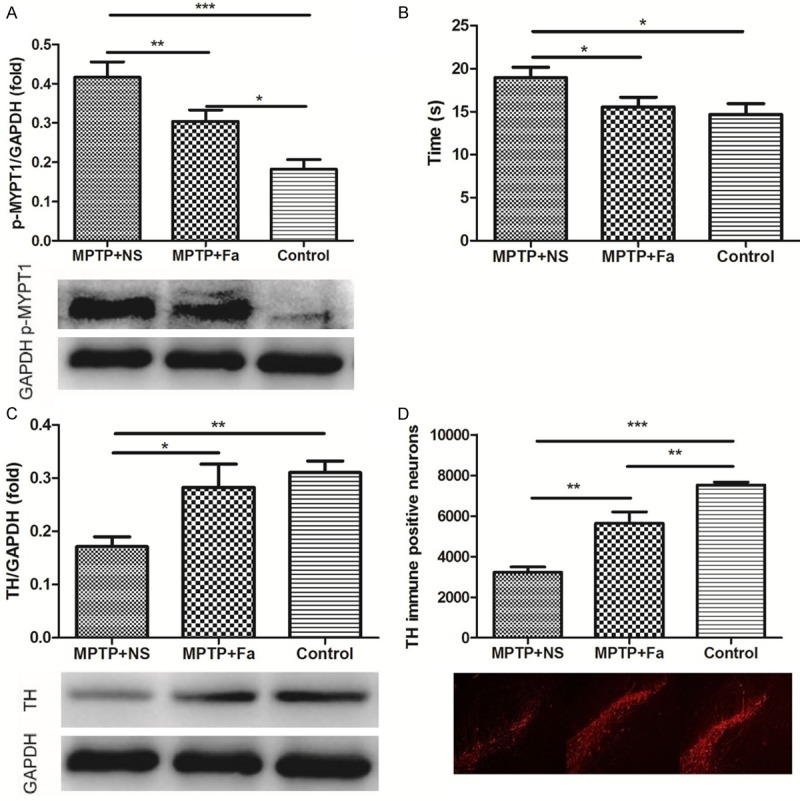
Dopamine neuronal protection by Rho kinase inhibitor fasudil in MPTP-treated mice. The expression of p-MYPT1 (A), time of climbing pole (B), expression of tyrosine hydroxylase (TH, C) by Western blot analysis and quantification of immunopositive nigral cells for TH (D) by immunofluorescence analysis were assessed in control group, MPTP + NS group (administration of MPTP and normal saline), and MPTP + Fa group (administration of MPTP and fasudil). *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 2.
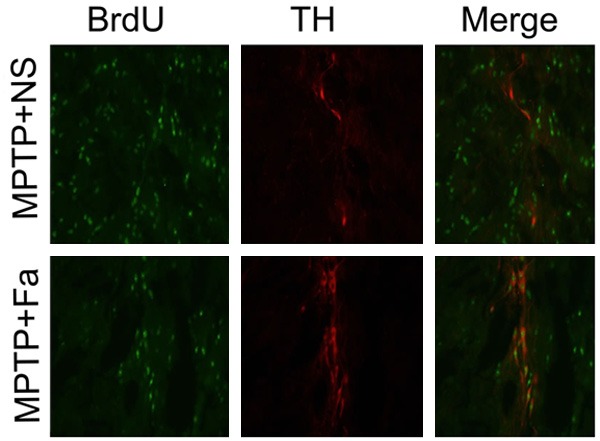
Administration of fasudil promotes the proliferation of tyrosine hydroxylase (TH) positive cells in midbrain nigra by immunofluorescence analysis.
Cellular survival after fasudil is mediated the inhibition of inflammatory response
We further studied the inflammatory response to the fasudil treatment in MPTP mice model of PD. In the brain tissue, the expression of inflammatory cytokines IL-1β and TNF-α were decreased (328.0 ± 16.56 pg/mL and 356.8 ± 20.73 pg/mL) by fasudil treatment comparing with the NS treatment (435.1 ± 51.23 pg/mL and 465.1 ± 18.43 pg/mL, Figure 3A and 3B). Besides, the expression of TLR2 (toll-like receptor 2) and p-NF-κB were both decreased in the MPTP + Fa group comparing with the MPTP + NS group (Figure 3C and 3D). Analysis of p-NF-κB expression in combination with CD11b which is a marker for the microglia population revealed that p-NF-κB positive cells were increased after MPTP treatment and decreased following fasudil administration (Figure 3E), which were consistent with the number of p-NF-κB positive astrocytes after fasudil treatment (GFAP as marker of astrocyte, Figure 3F). Analysis of inducible nitric oxide synthase (iNOS) and arginase1 expression in combination with CD11b demonstrated that iNOS positive cells were decreased and arginase1 positive cells increased following fasudil treatment (Figure 4A and 4B). Western bolt analysis confirmed the results that fasduil inhibited the expression of iNOS and increased the expression of arginase1 (Figure 4C and 4D). Western blot and immunofluorescence analysis showed that GDNF was highly expressed after fasudil administration in the microglial cells in the subventricular zone (Figure 5). In addition, western blot analysis showed that the expression of glycogen synthase kinase-3β (p-GSK-3β) was decreased after fasudil treatment (Figure 6A).
Figure 3.
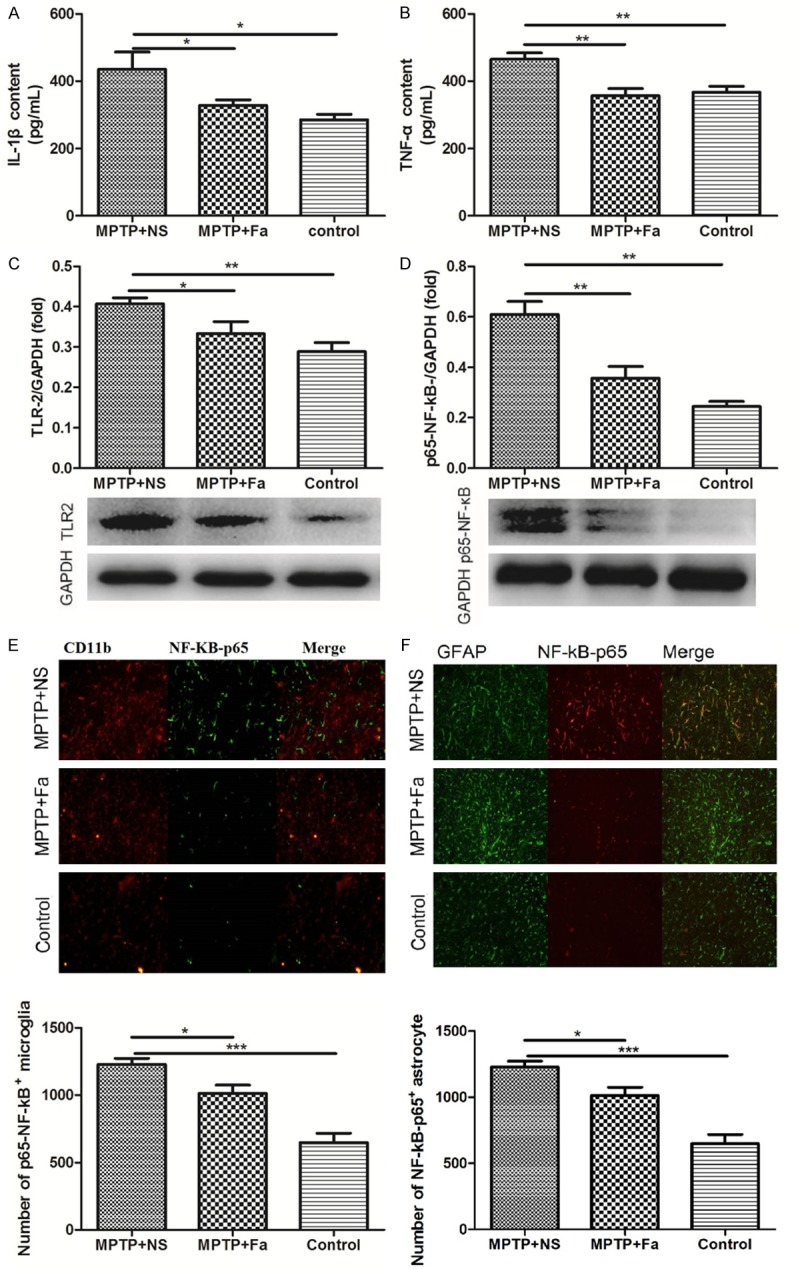
Expression of IL-1β (A), TNF-α (B), TLR-2 (C), p-NF-κB (D-F) were detected in control group, MPTP + NS group (administration of MPTP and normal saline), and MPTP + Fa group (administration of MPTP and fasudil) by Western and immunofluorescence analysis. *P < 0.05; **P < 0.01.
Figure 4.
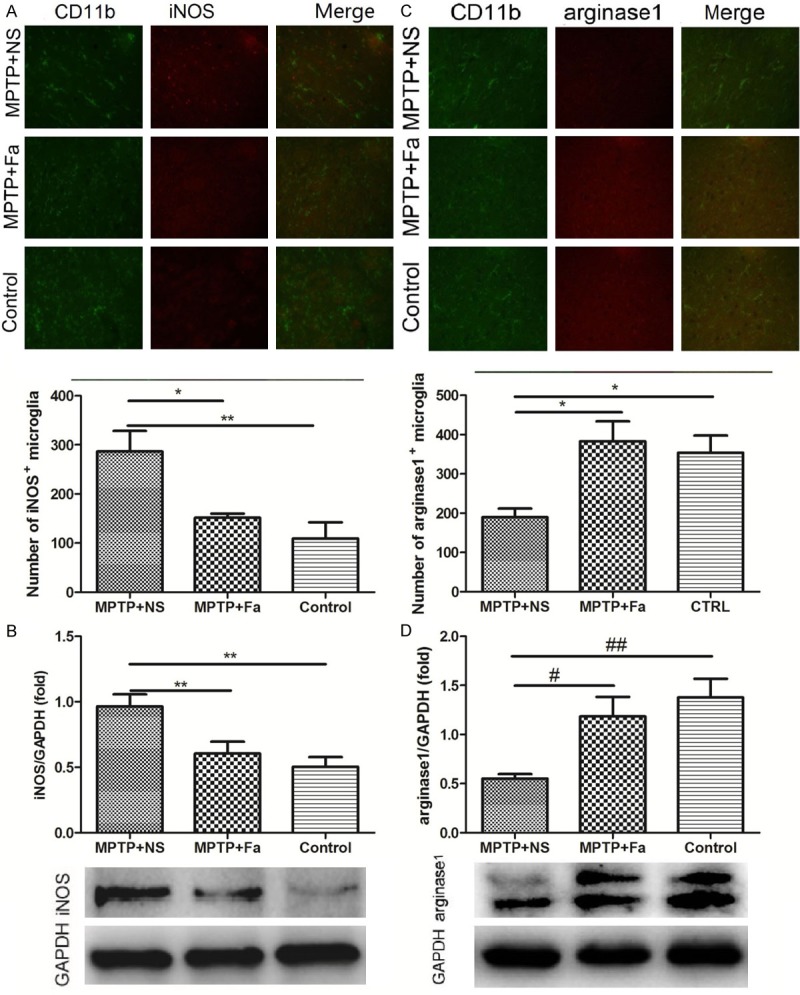
Expression of iNOS and arginase1 in the CD11b positive microglia by immunofluorescence (A and C) and Western blot analysis (B and D) in control group, MPTP + NS group (administration of MPTP and normal saline), and MPTP + Fa group (administration of MPTP and fasudil). *P < 0.05; **P < 0.01.
Figure 5.
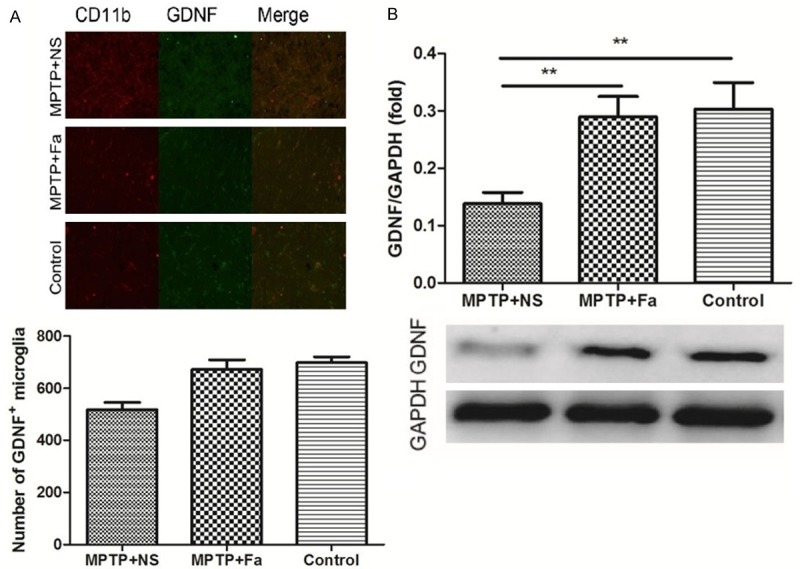
Effect of fasudil on the expression of GDNF in the activated microglia by immunofluorescence (A) and Western blot (B) analysis in control group, MPTP + NS group (administration of MPTP and normal saline), and MPTP + Fa group (administration of MPTP and fasudil). *P < 0.05; **P < 0.01.
Figure 6.
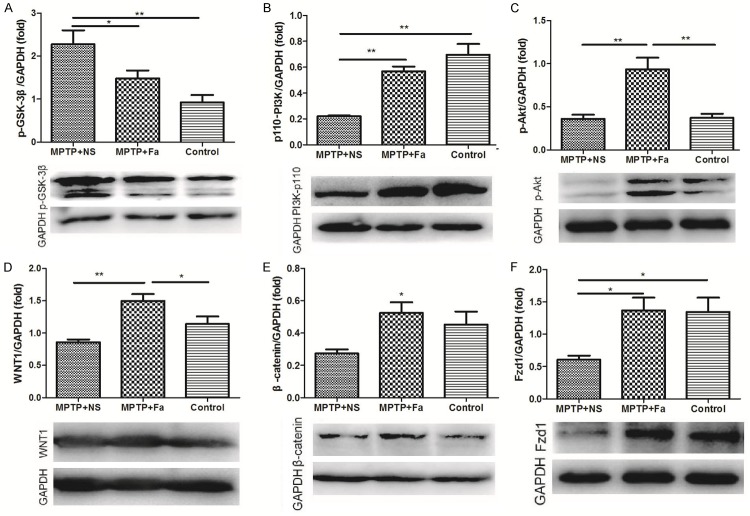
The expression of p-GSK-3β (A), PI3K-p110 (B), p-Akt (C), WNT1 (D), β-catenin (E) and Fzd1 (F) were analyzed by Western blot analysis in control group, MPTP + NS group (administration of MPTP and normal saline), and MPTP + Fa group (administration of MPTP and fasudil). *P < 0.05; **P < 0.01.
Signaling pathways involved in the neuron protection of fasudil
The protein expression of the p110-PI3K, p-Akt, WNT1, β-catenin and Fzd1 were demonstrated by the western blot analysis. After MPTP administration, the expression of p110-P13K and Fzd1 were significantly decreased (Figure 6B and 6F). The expression of all the proteins was significantly increased after fasudil treatment comparing with normal saline (Figure 6B-F).
Discussion
The Rho kinase inhibitor fasudil has the neuroprotection in the MPTP based mice model [5], however the mechanism is not fully elucidated. In this study, we could observe that expression of TH increased after fasudil treatment in the MPTP-based mice model of PD. Further, the expression of proteins involved in the inflammatory response were increased following administration of fasudil.
C57BL/6 mice were intraperitoneally administrated with MPTP, which induced the decrease of TH expression and number of TH immune positive cells, and both of them were significantly increased after fasudil administration on day 15 after first administration of MPTP. In addition, analysis of BrdU+ cells in combination of TH+ cells demonstrated that the number of TH+ cells in the midbrain nigra was increased after fasudil treatment. Besides, the time of climbing the pole was shorter after fasudil treatment. Li et al demonstrated that number of TH-immunoreactive fibres in the median forebrain bundle significantly decreased, followed by dopaminergic neuron demise [3]. It has been reported that MYPT1 was phosphorylated by Rho-kinase [25,26]. In this study, p-MYPT1 was inhibited by fasudil, demonstrating that the activity of Rho kinase was inhibited by fasudil. In Tönges’ report, MPTP-based mice model was used to investigate the neuroprotective effect of fasudil, showing that the number of TH was higher and motor performance was improved after fasudil treatment [5]. Thus, fasudil, a Rho kinase inhibitor, has the neuroprotection effect in the MPTP intoxication mice model of PD.
Expression of cytokines (IL-1β, TNF-α and TLR2) and nuclear factor p-NF-κB which were both released in response to inflammation were decreased after administration of fasudil. It has been reported that activation of IL-1β and TLR led to the degeneration of dopamine neurons in the substantia nigra pars compacta [8]. The expression of TNF-α was increased in the brain from parkinsonian patients [10] and expression of TLR2 was elevated in the mice model of PD [22]. It has been reported that p-NF-κB was activated in astrocyte in the mice with trigeminal neuropathic pain [20]. In Parkinson, NF-κB death pathway was activated following microglia activation which resulted in the increase of dopamine neurons [8]. PD can be characterized by accumulation of microglia cells and the proteins released by activated microglia including NF-κB, iNOS and arginase1 [8]. The number of p-NF-κB positive cells in the CD11b activated microglia and in GFAP activated astrocyte were both decreased after fasudil administration in this study. Immunofluorescence analysis showed that number of iNOS positive cells was decreased and number of arginase1 positive cells was increased, in the CD11b+ activated microglia following fasudil treatment comparing with normal saline administration. The iNOS was as the indicator of proinflammatory M1 phenotype microglia activation and arginase1 was as the indicator of immunomodulatory M2 phenotype microglia as previous report [27]. Microglial activation and associated induction of iNOS and arginase1 have been well documented in Parkinson’s disease [28,29]. Hou et al demonstrated that the therapeutic effect of fasudil in the chronic-progressive experimental autoimmune encephalomyelitis in B6 mice was through inhibition of p-NF-κB, IL-1, TNF-α, and enhancement of arginase/iNOS ratio [21]. The GDNF positive microglia cells were highly expressed in the fasudil group comparing with NS group. In the injured striatum, GDNF is produced by activated microglia [25] and plays neuroprotection role after spinal cord injury [26]. In PD, lentiviral delivery of GDNF prevent nigrostriatal degeneration and induce regeneration in MPTP-treated monkeys [28]. There were many other studies reporting the effective treatment of GDNF for PD [29-31]. Thus, we conclude that the administration of fasudil may be involved the expression of GDNF in activated microglia for neuroprotection. The activated astrocytes, microglia and inflammatory processes have been reported to be tightly related to the pathogenesis of PD [32]. In summary, fasudil may reduce TH loss through inhibition of inflammatory response and promotion of GDNF release in MPTP-based PD mice model.
GSK-3β is activated by the phosphorylation of tyrosin216 residue [33] and the inhibition of GSK-3β has been shown to protect dopaminergic neurons from MPTP toxicity [34]. Wang et al showed that GSK-3β was activated by phosphorylation of tau in TH positive cells after MPTP treatment [34]. In this study, the expression of p-GSK-3β (phosphorylation of tau) was increased after MPTP administration and decreased after fasudil treatment. Thus, fasudil may paly the neuroprotection role involving the inhibition of p-GSK-3β. The expression of p110-PI3K, p-Akt, WNT1, Fzd1 and β-catenin were increased after fasudil administration. PI3K/Akt has been reported to be neuroprotecive for the substantia nigra pars compacta dopamine neurons in rats model of PD [35]. Dopamine neurons could be activated by caffeine which prevented the MPTP induced apoptosis in PD model of SH-SY5Y cells [36]. Similarly, it has been reported that phosphorylated Akt levels were elevated after fasudil treatment in the MPTP mice model of PD [5]. Wnt1/Fzd1/catenin signaling pathway participates in the protective response in PD when exposure to MPTP [37]. Thus, in the MPTP induced PD model, the neuroprotection of fasudil may be involved in the activation of p110-PI3K/Akt and WNT1/Fzd1/β-cateninsignaling pathways.
In summary, the Rho inhibitor fasudil has the neuroprotection for the mice with PD induced by MPTP. Fasudil inhibited the Rho kinase possibly involving the inhibition of inflammatory response via p110-PI3K/Akt and WNT1/Fzd1/β-catenin signaling pathways, thereby promoting the proliferation of dopaminergic neurons.
Acknowledgements
This study was supported by The National Natural Science Foundation of China (grant no. 81070956 and 81371414).
Disclosure of conflict of interest
None.
References
- 1.McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988;24:574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- 2.Wooten G, Currie L, Bovbjerg V, Lee J, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon JR, Greenamyre JT. Neurotoxic in vivo models of Parkinson’s disease: recent advances. Prog Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Qin H, Wang J, Wang J, Wang X, Gao G. Axonal degeneration of nigra-striatum dopaminergic neurons induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in mice. J Int Med Res. 2009;37:455–463. doi: 10.1177/147323000903700221. [DOI] [PubMed] [Google Scholar]
- 5.Tönges L, Frank T, Tatenhorst L, Saal KA, Koch JC, Szegő ÉM, Bähr M, Weishaupt JH, Lingor P. Inhibition of rho kinase enhances survival of dopaminergic neurons and attenuates axonal loss in a mouse model of Parkinson’s disease. Brain. 2012;135:3355–3370. doi: 10.1093/brain/aws254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 8.Phani S, Loike JD, Przedborski S. Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:S207–S209. doi: 10.1016/S1353-8020(11)70064-5. [DOI] [PubMed] [Google Scholar]
- 9.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1β, IL-2, IL-4, IL-6 and transforming growth factor-α levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neuroscience Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 10.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 11.Knott C, Stern G, Wilkin G. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and-2. Mol Cell Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Zhang SM, Hernán MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 13.Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson’s disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol. 2010;31:7–17. doi: 10.1016/j.it.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2007;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 15.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 16.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53:S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 18.Ebert AD, Beres AJ, Barber AE, Svendsen CN. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson’s disease. Exp Neurol. 2008;209:213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2009;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 21.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 22.George SJ. Wnt pathway a new role in regulation of inflammation. Arterioscler Thromb Vasc Biol. 2008;28:400–402. doi: 10.1161/ATVBAHA.107.160952. [DOI] [PubMed] [Google Scholar]
- 23.Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng H, Wu JP, Tzeng SF. Neuroprotection of glial cell line-derived neurotrophic factor in damaged spinal cords following contusive injury. J Neurosci Res. 2002;69:397–405. doi: 10.1002/jnr.10303. [DOI] [PubMed] [Google Scholar]
- 27.Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- 28.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen E, Palfi S, Roitberg BZ. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 29.Opacka-Juffry J, Ashworth S, Hume SP, Martin D, Brooks DJ, Blunt SB. GDNF protects against 6-OHDA nigrostriatal lesion: in vivo study with microdialysis and PET. Neuroreport. 1995;7:348–352. [PubMed] [Google Scholar]
- 30.Kearns CM, Gash DM. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672:104–111. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- 31.Biju K, Zhou Q, Li G, Imam SZ, Roberts JL, Morgan WW, Clark RA, Li S. Macrophage-mediated GDNF delivery protects against dopaminergic neurodegeneration: a therapeutic strategy for Parkinson’s disease. Mol Ther. 2010;18:1536–1544. doi: 10.1038/mt.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teismann P, Schulz JB. Cellular pathology of Parkinson’s disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- 33.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, You Y, Han Y, Chen R, Wang Y. Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakaso K, Ito S, Nakashima K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci Lett. 2008;432:146–150. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 37.Marchetti B, Pluchino S. Wnt your brain be inflamed? Yes, it Wnt! Trends Mol Med. 2013;19:144–156. doi: 10.1016/j.molmed.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]