Abstract
Purpose: To investigate the effects and mechanism of pollen typhae on spinal cord injury (SCI) in rats. Methods: The SCI model was built and animals were randomly divided into three groups according to different concentrations of pollen typhae. Protein, mRNA, and fluorescence expression levels of light-chain-3 (LC-3) and Beclin-1 were determined by western blotting (WB), real-time PCR and immunofluorescence, along as Akt and mammalian target of rapamycin (mROT) by WB. The demyelination area and integrated optical density (IOD) were analyzed by luxol fast blue (LFB) and Nissl staining, respectively; Behavioral assessments were assessed by Basso Beattie Bresnahan (BBB) scale. Results: Protein, mRNA, and fluorescence expression levels of LC-3 and Beclin-1 were significantly increased after SCI, while were obviously decreased by administration of pollen typhae, along with protein level of Akt and mROT. The demyelination area was significantly reduced, while IOD and BBB were significantly increased compared with the model group. Conclusion: Autophagic activity increased in damaged neural tissue after SCI, and pollen typhae have certain therapeutic effect on SCI, the higher concentration of pollen typhae, the more effective. Besides, pollen typhae also provided neuroprotective effect and improved locomotor function. The effects may be produced by blockade of Akt/mTOR pathway.
Keywords: Spinal cord injury, pollen typhae, Beclin-1, LC-3, autophagy, Akt/mTOR
Introduction
Spinal cord injury (SCI) is a traumatic event, which is a major cause of disability and death in human communities. It is characterized by immediate and severe loss of sensory, motor or autonomic functions below the level of injury [1]. The effects of SCI not only impact on an individual’s health, but also bring an enormous financial burden for families and society [2-5]. It has been reported that the prevalence of SCI is growing worldwide, especially in Asia [6,7]. In our country, the number of SCI has increased ten-fold causing by car crashes, construction and mining accidents in the past decade. Moreover, China has approximate 60000 new cases annually [8].
The pathophysiology of acute SCI consists of primary and secondary mechanisms of injury. The primary injury serves as the nidus, while the secondary injury is responsible for the extension of injury and causes further tissue loss and dysfunction, involving a cascade of stress response [9-11]. Among the stress response, autophagy is a highly pivotal cellular response to injury and influences the development and progression of post-traumatic disease [12,13]. Autophagy is an intracellular degradation system, which can deliver cytoplasmic constituents to the lysosome [14,15]. Multifarious studies have demonstrated that autophagy is associated with SCI [16-18]. Beclin-1 and light-chain-3 (LC-3) are characteristic markers of autophagy and a growing body of evidence suggests these two genes involve in the SCI [19,20]. Moreover, it has been reported that Akt/mammalian target of rapamycin (mTOR) signaling pathway plays a critical role in autophagy [21,22]. In addition, previous studies have confirmed that activation of this pathway is helpful for preventing cell death and enhancing cellular processes related to growth and proliferation [16].
The current treatments for SCI are neurotrophin (NT), however, the molecular weight of NT is relatively large and it is difficult to cross blood brain barrier (BBB). In addition, the therapeutic effect can decrease heavily because of the degradation of enzyme. Besides, the price of NT is very expensive, and the dose and frequency is relatively high. In addition to the above disadvantages, there has been associated with significant side effects. Hence, new therapeutic strategies are needed to explore to treat SCI. Traditional Chinese medicines may have many benefits. Pollen typhae is a traditional Chinese herbal medicine, which has lower toxicity and side effects [23]. Moreover, it can promote circulation and remove stasis, and is widely used to treat the hemorrhagic diseases either by external or oral application. However, there were rare studies to investigate the treatment effect on SCI.
In our study, the effects and mechanism of pollen typhae on inhibiting the expression of autophagy related genes after SCI in rats was investigated. The results in this study might contribute to a better understanding of SCI and provide valid therapy information for SCI.
Materials and methods
Experimental animals and model building
Two hundred male Wistar rats (8-12 weeks old, weighing 220-250 g) were purchased from the Central Animal Laboratory of Zhongshan University (Guangzhou, China). Each animal was housed in a single clean cage and provided with liquid diet before the model being built. All animals were subjected to the weight drop model using the New York University (NYU) impactor (W.Young, Rutgers University, NJ) [24,25]. A 10 g rod (2.5 mm in diameter) was centered above the cord at T10 and released from a height of 25 mm onto the exposed spinal cord. Each animal was anesthetized with pentobabital (50 mg/kg, i.p.), and a laminectomy was conducted at the T9-11. If the two hindlimbs and body presented retraction, indicting the model was being successfully built. Finally, a total of 144 rats were subjected to the experiments. In addition, another 36 healthy rats were enrolled as normal control group. During and after recovery, rats were placed overnight in a temperature and humidity-controlled chamber, then were housed in the identical room with a 12-hour light/dark cycle and acquired daily postoperative care. The protocol of using animal was agreed with the Animal Ethics Committee of International Association for the Study of Pain.
Animals were randomly and equally divided into 5 Groups (n = 36): (1) normal control group, the rats received 0.9% normal saline for treatment without modeling; (2) modeling group: the rats were successfully induced into experimental model and received 0.9% normal saline for treatment; (3) low-dose group, the rats were successfully induced into experimental model and received 1 g/kg pollen typhae; (4) medium-dose group, the rats were successfully induced into experimental model and received 5 g/kg pollen typhae; (5) high-dose group, the rats were successfully induced into experimental model and received 10 g/kg pollen typhae; Pollen typhae and 0.9% normal saline were both administrated by intraperitoneal injection for two consecutive weeks.
Detection of LC-3 and Beclin-1 mRNA by quantitative real-time PCR
Rats were sacrificed and the spines were harvested under anesthesia at 1 h, 2 h, 6 h, 24 h, 48 h, and 72 h after SCI. Total RNA was extracted using 1 ml Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA; Cat. 15596- 026) with an RNase protection assay kit according to the manufacturer’s instructions (catalog No. 1414; Ambion). After fully dissociation, 0.2 ml chloroform was mixed, followed by ice-bath for standing 5 min. The mixture was then centrifuged at 12000 rpm for 20 min at 4°C. The supernatant was taken and mixed with the same amount of isopropanol, followed by ice-bath for standing 5 min. Then the mixture was centrifuged at 12000 rpm for 20 min at 4°C (Biofuge pico Centrifuge, Heraeus, Rotor 7500 3325). After centrifugation, supernatant was removed and 1 ml 75% ethanol was added, followed by centrifuging at 10000 rpm for 5 min at 4°C. Then the supernatant was removed and dried 15 min at room temperature. After drying, 20 μl RNase-Free ddH2O was added to dissolve the precipitation. Then the dissociation (1 μl) was taken out to assess the quality of the total RNA using spectrophotometric evaluation (MiniSpin, Eppendorf, Germany, Hamburg) by OD260/OD280 ratio. The remaining dissociation was stored at -70°C. cDNA synthesis was performed according to the protocol of the PrimeScript ™RT reagent Kit (DRR037A, Sigma).
The primers sequences used were as follows: LC-3: upstream, 5’-GATGCGGTCGGATAAGTCCT-3’, downstream, 5’-GCTGAGCGGGTCATCATCCACA-3’. Beclin-1: upstream, 5’-ATCGCTAATTAGATcGTCTGAA-3’, downstream, 5’-TGTCAGGGACTCCGATACGAGTG-3’; β-actin: upstream, 5’-CTt GAG CTGAGTACGTCGTC-3’, downstream, 5’-CAG TCT TCT CTG TGC CAGAG-3’.
PCR was performed as follows: amplification curve, initial denaturing at 95°C for 5 min, denaturing at 95°C for 20 s, annealing at 60°C for 30 s, extension at 72°C for 20 s, total 40 cycles for acquiring the fluorescence signal; dissolution curve, under the condition of 60-95°C, the temperature was raised 0.5°C per cycle and lasted for 20 s, the fluorescence signal was collected and total 71 cycles.
PCR products (10 μl) were analyzed on a 1% agarose gel. All gene expression data were calculated as 2-ΔΔCt.
Western blotting assay for detection of LC-3, Beclin-1, mROT and Akt expression
In each group, six rats were killed under anesthesia at 2 h after administrating pollen typhae or 0.9% normal saline. Under sterile conditions, 1 cm segment of spinal cord was dissected out the central zone of the injured spinal cord, and was then washed with 0.1% DEPC water and flashly frozen and stored at -80°C until used. Protein density was calculated by the BCA method. Protein samples (15 μL) from each group were resolved in a standard electrophoresis sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gel and transferred onto polyvinylidene difluoride (PDVF) membranes (Nihon Millipore Kogyo, Tokyo, Japan). The blots were blocked with 2% defatted milk powder for 1 h at room temperature and incubated overnight at 4°C with the following antibodies: anti-LC-3 (1:1000, 11546, Sigma), anti-Beclin-1 (1:1000, 2026-1, Sigma), anti- Akt (1:1000, 2014-2, Sigma), and anti-mTOR (1:1000, 12532, Sigma). The second antibody was biotinylated goat anti-mouse After incubation with an goat anti-mouse (1:500, A22110, Beijing Zhongshan Golden Bridge Biotechnology Co., Beijing, China) as the secondary antibody, the samples were carried out to enhanced chemiluminescence and densitometric analysis. Band densitometric analysis was performed in ImageJ 1.44p software (NIH, Bethesda, MD, USA).
Immunofluorescence detection of LC-3 and Beclin-1 expression
After 2 h administrating pollen typhae or 0.9% normal saline, the tissues of lesion center were prepared for frozen sections to analyze by immunofluorescence. The frozen sections were rinsed with 0.01 M PBS and then blocked with 1% BSA (Sigma) at room temperature. The sections were then incubated with anti-LC3 (1:1000) and anti-Beclin-1 (1:1000) at 4°C overnight. Sections were then washed with PBS and incubated with secondary antibodies (goat anti-mouse, 1:500). After rinsing with PBS, the sections were observed under confocal laser scanning microscope (CLSM, Fluoview FV-300, Olympus Optical Co., Ltd., Tokyo, Japan) with DAPI immunofluorescence.
Luxol-fast-blue (LFB) staining for spinal cord myelin
After SCI 2 h, the spinal tissue was harvested and was prepared for frozen sections (5 μm). The sections were displaced in 95% alcohol for 5 min and overnight in 0.1% LFB at 57°C. After rinsing in 95% alcohol and double-distilled water, the sections were put into 0.05% aqueous lithium carbonate and washed through several changes of 70% alcohol, and then placed in double-distilled water. Nine slices which included lesion center with spanning 4 mm were evaluated (×40). The LFB-positive area of white matter was calculated using ImageJ 1.44p software. The ratio between positive area and the total area of normal tissue slice was calculated.
Nissl staining for motor neurons of anterior spinal cord
Nine slices which included lesion center were immerged into 1:1 alcohol/chloroform overnight. After rinsing in 95% alcohol and double-distilled water, the sections were bathed in a 0.1% Cresyl violet solution for 10 min at 37°C. The slices were washed again with double-distilled water, and differentiated in 95% ethanol for 1 min. The number of motor neurons of anterior spinal cord was calculated by using Olympus microscope BX51 and digital camera DP70 (Olympus, Tokyo, Japan). Integrated optical density (IOD) of Nissl body was counted using ImageJ 1.44p software.
Behavioral assessments
Behavioral assessments were assessed using Basso Beattie Bresnahan (BBB) Locomotor Rating Scale. BBB testing began at the post injury once a week for 6 consecutive weeks. To ensure the accuracy of scoring, 3 individuals who blinded to the experimental groups gave the scoring. Then the average was taken.
Statistical analysis
Statistical analysis was performed by SPSS 13.0 software (SPSS, Chicago, IL, USA). The measurement data were expressed as mean ± standard deviation. Multiple comparisons were conducted by one-way analysis of variance (ANOVA). There is significantly statistical difference when P < 0.05.
Results
LC-3 and Beclin-1 expression by quantitative real-time PCR and western blotting
The mRNA level of LC-3 and Beclin-1 was showed in Figure 1A and 1B. The figure showed that the mRNA level of LC-3 and Beclin-1 reached their peak 2 h after SCI. In addition, the level was significantly decreased after receiving different doses of pollen typhae compared with the model group. In the model group, the mRNA level of LC-3 and Beclin-1 was 2.3 and 2.1, respectively. As shown in Figure 2, the protein levels of LC-3, Beclin-1, Akt, and mTOR were the highest and the lowest in the model group 2 h after SCI and in the normal control group, respectively. The levels were obviously decreased in the low, medium and high dose group compared with the model group. Besides, the levels reached the lowest level in the high dose group.
Figure 1.
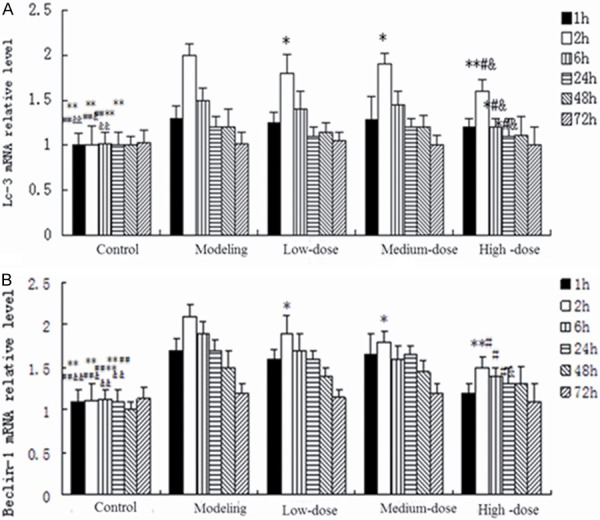
The mRNA level of LC-3 and Beclin-1 by real-time PCR. A. The mRNA level of LC-3; B. The mRNA level of Beclin-1 **P < 0.01 vs. modeling group; *P < 0.05 vs. modeling group; ##P < 0.01 vs. low-dose group; #P < 0.05 vs. low-dose group; &P < 0.05 medium-dose group; $P < 0.05 vs. medium-dose group LC-3, light-chain-3.
Figure 2.
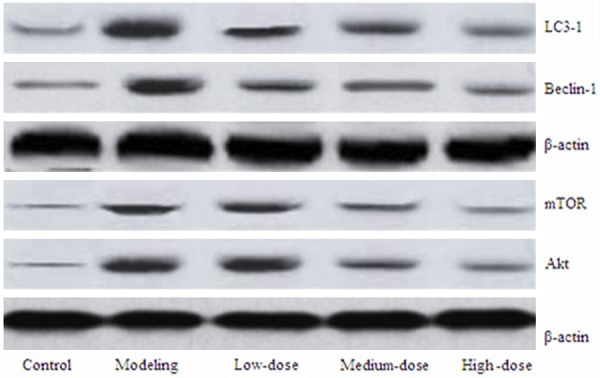
The protein levels of LC-3, Beclin-1, Akt, and mROT. LC-3, light-chain-3; mROT mammalian target of rapamycin mROT.
LC-3 and Beclin-1 immunofluorescence
As shown in Figure 3A and 3B, the protein accumulation of LC-3 and Beclin-1 was both significantly increased in the model group, while was decreased by pollen typhae administration.
Figure 3.
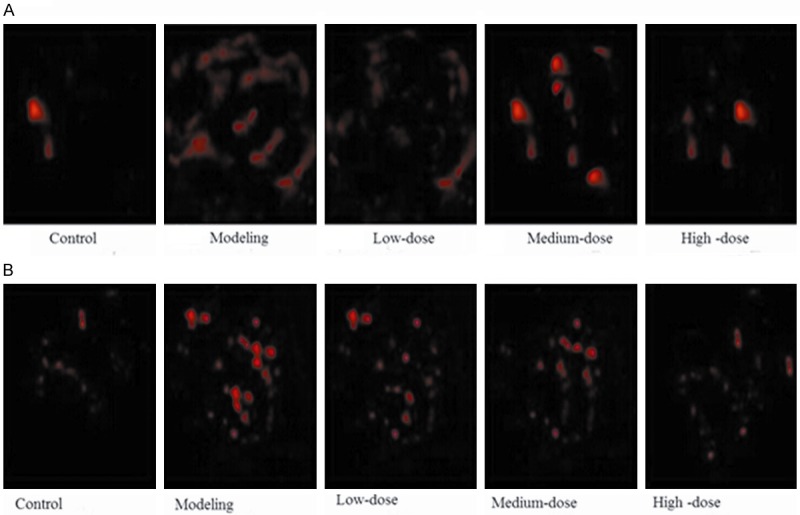
The protein accumulation of LC-3 and Beclin-1 by immunofluorescence. A. The protein accumulation of LC-3; B. The protein accumulation of Beclin-1 LC-3, light-chain-3.
LFB staining and Nissl staining
The results of LFB staining were showed in Figure 4A and 4B. The area of demyelination was 17%, 21%, 27% and 34% in the model group, low-dose group, medium-dose group, and high-dose group. Compared with the model group, the area was significantly reduced after receiving different concentrations of pollen typhae. The results of Nissl staining were showed in Figure 5. The IOD was 2.5 ± 0.20, 3.3 ± 0.23, 3.4 ± 0.26, 3.5 ± 0.25 in the model, low-dose, medium-dose and high-dose group, respectively. The IOD was significantly increased after receiving different concentrations of pollen typhae, and the higher dose, the more effective.
Figure 4.
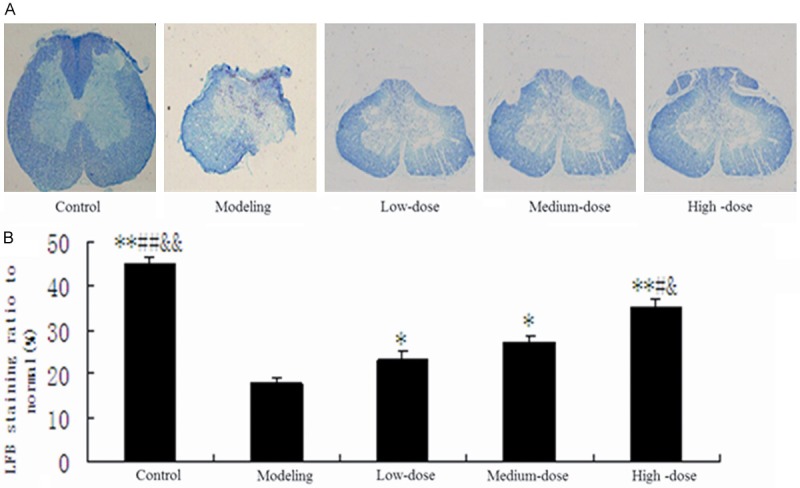
The results of LFB staining. A. The photos of LFB staining for spinal cord myelin; B. LFB staining ratio to normal. **P < 0.01 vs. modeling group; *P < 0.05 vs. modeling group; ##P < 0.01 vs. low-dose group; #P < 0.05 vs. low-dose group; &&P < 0.01 vs. medium-dose group; &P < 0.05 vs. medium-dose group LFB, Luxol-fast-blue.
Figure 5.
The results of Nissl staining.
Results for behavioral assessments
The results of behavioral assessments using BBB test were showed in Figure 6. The results demonstrated that there were no significant differences between the model group and the treatment group ranging from the first week to the third week. The scores were significantly increased compared with the model group in the fourth week: high-dose group was 10; both medium-dose and low-dose group were 8; model group was 6. While in the fifth week, the scores were 15, 13, 12 and 7 in the high-dose group, medium-dose group, low-dose group 8 and model group, respectively. The scores were obviously increased compared with the model group, but were lower than the normal control group. In the sixth week, although behavioral function did not go back to normal in both model group and treatment group, the scores of the three treatment groups were significantly higher than the model group: high-dose group was 17; medium-dose group was 14, low-dose group was 13 and model group was 8.
Figure 6.
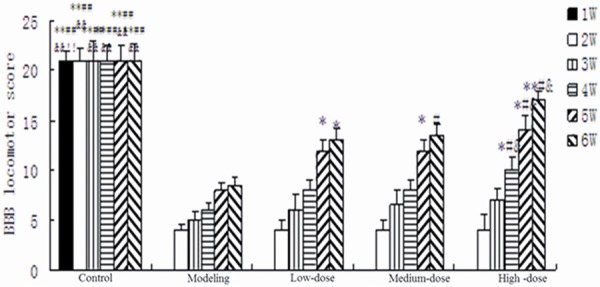
The results of behavioral assessments using BBB test. **P < 0.01 vs. modeling group; *P < 0.05 vs. modeling group; ##P < 0.01 vs. low-dose group; #P < 0.05 vs. low-dose group; &P < 0.05 vs. medium-dose group; $P < 0.05 vs. medium-dose group; BBB, Basso Beattie Bresnahan.
Discussion
In the present study, the effects and mechanism of pollen typhae on SCI in rats was investigated. The results showed that autophagic activity was increased in damaged neural tissue after SCI, and different concentrations of pollen typhae had certain therapeutic effects on SCI by inhibiting the expression of autophagy-related genes, the higher concentration, and the more effect. Besides, pollen typhae also provided neuroprotective effect and improved locomotor function following SCI. The effects may be produced by blockade of Akt/mTOR pathway.
SCI is defined as an acute injury caused by a direct or indirect force to the neural elements in the spinal canal (spinal cord and cauda equina), leading to temporary or permanent sensory, motor deficit, or bladder/bowel disorders [26]. An increasing progress has been made in mechanisms on the decrease of motor functions. In recent years, severe systemic stress response after trauma has been demonstrated, especially on central nervous system (CNS). Autophagy is an important systemic stress response and plays key role in maintaining homeostasis by degradation of organelles and protein [27]. However, it serves as a double-edged sword. Numerous studies have shown that autophagy increases in traumatic SCI, and influences functional recovery of spinal cord [16]. Pollen typhae, one of traditional Chinese medicines, can promote circulation and remove stasis, and the action is similar to that of notoginsenoside.
In our study, the SCI model was successfully built by the weight drop model using the NYU impactor. This model is stable and has high success rate. It can produce a reliable contusion injury to the exposed spinal cord by quickly employing a force-defined impact with a stainless steel-tipped impounder. Besides, the impactor can keep the integrity of the sinal dura mater and prevent the leakage of spinal cord and cerebrospinal fluid. Different weights and heights can cause different impact strength, eventually resulting in different types of damage and different segments of SCI. This model can well simulate the thoracic lumbar segment of SCI. Most studies are inclined to select the segments of T8-12. Because the vertebral plate of this segment was easy to resect and the distribution of stress was the minimum. Hence, impact on physiological curvature and mechanical support of entire spine was little. Similarly, a 10 g rod (2.5 mm in diameter) was dropped onto the spinal cord at T10 segment from the height of 25 mm to induce the model of SCI. Moreover, a simple laminectomy had no effect on feeding, walking or other daily activities in rats.
Plenty of studies have shown that autophagy plays key role in CNS diseases [28-30]. While in the SCI, autophagy and apoptosis cells are also found in the lesion center. Although the occurrence of necrosis is relatively late, it can contribute to a secondary cell death. Previous studies indicated that Beclin-1 and LC-3 increased in SCI [19,20,31]. Beclin-1, a protein with a molecular weight of 60 KDa, was found and named by Liang et al. in rats with fatal Sinbis viral encephalitis [32]. Beclin-1 is a unique autophagy-related protein, which plays a critical role in regulating the development of precursors of autophagosomes from other autophagy-related protein. Therefore, it is characteristic markers of autophagy. LC-3, known as the homolog of yeast Atg8, is also essential for autophagy. It has a crucial function in the formation of autophagosome and thus is considered as anther specific marker protein to monitor autophagy [33]. Kanno et al. [31] found that molecular markers of autophagy such as Beclin-1 and LC3 were significantly upregulated in SCI and administration of rapamycin (specific inhibitor of mTOR) induced neuroprotective effect in SCI. Walker et al. found that administration bisperoxovanadium activated Akt/mTOR, reduced autophagy, and enhanced recovery following cervical SCI [16]. In accordance with previous studies, our results also demonstrated that the expression levels of Beclin-1 and LC-3, either mRNA or protein levels, were significantly increased in the model group, in addition to above results, the levels reached their peak 2 h after SCI. However, the pollen typhae administration reduced neural tissue damage and improved locomotor function, the higher concentration and the more effective. The level of mTOR and Akt also reduced by administrating pollen typhae, indicating pollen typhae may produce effect by blockade of Akt/mTOR pathway.
In conclusion, autophagic activity plays important roles in SCI, and pollen typhae administration has certain therapeutic effect on SCI and provides neuroprotective effect to improve locomotor function.
Disclosure of conflict of interest
None.
References
- 1.Galea MP. Spinal cord injury and physical activity: preservation of the body. Spinal Cord. 2012;50:344–351. doi: 10.1038/sc.2011.149. [DOI] [PubMed] [Google Scholar]
- 2.Pickelsimer E, Shiroma EJ, Wilson DA. Statewide investigation of medically attended adverse health conditions of persons with spinal cord injury. J Spinal Cord Med. 2010;33:221–231. doi: 10.1080/10790268.2010.11689699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budh CN, Osteraker AL. Life satisfaction in individuals with a spinal cord injury and pain. Clin Rehabil. 2007;21:89–96. doi: 10.1177/0269215506070313. [DOI] [PubMed] [Google Scholar]
- 4.Furlan JC, Noonan V, Singh A, Fehlings MG. Assessment of impairment in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma. 2011;28:1445–1477. doi: 10.1089/neu.2009.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noonan VK, Fingas M, Farry A, Baxter D, Singh A, Fehlings MG, Dvorak MF. Incidence and prevalence of spinal cord injury in Canada: a national perspective. Neuroepidemiology. 2012;38:219–226. doi: 10.1159/000336014. [DOI] [PubMed] [Google Scholar]
- 6.Ning GZ, Wu Q, Li YL, Feng SQ. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J Spinal Cord Med. 2012;35:229–239. doi: 10.1179/2045772312Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu J. China Spinal Cord Injury Network: changes from within. Lancet Neurol. 2009;8:606–607. doi: 10.1016/S1474-4422(09)70162-0. [DOI] [PubMed] [Google Scholar]
- 9.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 10.Baranov D, Neligan P. Trauma and aggressive homeostasis management. Anesthesiol Clin. 2007;25:49–63. viii. doi: 10.1016/j.atc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Neligan PJ, Baranov D. Trauma and aggressive homeostasis management. Anesthesiol Clin. 2013;31:21–39. doi: 10.1016/j.anclin.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Gabryel B, Kost A, Kasprowska D. Neuronal autophagy in cerebral ischemia--a potential target for neuroprotective strategies? Pharmacol Rep. 2012;64:1–15. doi: 10.1016/s1734-1140(12)70725-9. [DOI] [PubMed] [Google Scholar]
- 13.Xu F, Gu JH, Qin ZH. Neuronal autophagy in cerebral ischemia. Neurosci Bull. 2012;28:658–666. doi: 10.1007/s12264-012-1268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 15.Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22:177–180. doi: 10.1016/j.ceb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Walker CL, Walker MJ, Liu NK, Risberg EC, Gao X, Chen J, Xu XM. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7:e30012. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanno H, Ozawa H, Sekiguchi A, Itoi E. The role of autophagy in spinal cord injury. Autophagy. 2009;5:390–392. doi: 10.4161/auto.5.3.7724. [DOI] [PubMed] [Google Scholar]
- 18.Chen HC, Fong TH, Lee AW, Chiu WT. Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine (Phila Pa 1976) 2012;37:470–475. doi: 10.1097/BRS.0b013e318221e859. [DOI] [PubMed] [Google Scholar]
- 19.Kanno H, Ozawa H, Sekiguchi A, Itoi E. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis. 2009;33:143–148. doi: 10.1016/j.nbd.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976) 2011;36:E1427–1434. doi: 10.1097/BRS.0b013e3182028c3a. [DOI] [PubMed] [Google Scholar]
- 21.Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K, Imoto M, Hattori N. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 2011;7:176–187. doi: 10.4161/auto.7.2.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkura N, Tamura K, Tanaka A, Matsuda J, Atsumi G. Experimental study on the hemostatc activity of Pollen Typhae: a traditional folk medicine used by external and oral application. Blood Coagul Fibrinolysis. 2011;22:631–636. doi: 10.1097/MBC.0b013e328349a22c. [DOI] [PubMed] [Google Scholar]
- 24.Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000;17:1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- 25.Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]
- 26.Sedgwick JD. Immune surveillance and autoantigen recognition in the central nervous system. Aust N Z J Med. 1995;25:784–792. doi: 10.1111/j.1445-5994.1995.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 27.Casella GT, Bunge MB, Wood PM. Endothelial cell loss is not a major cause of neuronal and glial cell death following contusion injury of the spinal cord. Exp Neurol. 2006;202:8–20. doi: 10.1016/j.expneurol.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Xilouri M, Stefanis L. Autophagy in the central nervous system: implications for neurodegenerative disorders. CNS Neurol Disord Drug Targets. 2010;9:701–719. doi: 10.2174/187152710793237421. [DOI] [PubMed] [Google Scholar]
- 29.Coupe B, Bouret SG. Weighing on autophagy: a novel mechanism for the CNS regulation of energy balance. Cell Cycle. 2012;11:1477–1478. doi: 10.4161/cc.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P, Miao CY. Autophagy in the disorders of central nervous system: vital and/or fatal? CNS Neurosci Ther. 2012;18:955–956. doi: 10.1111/cns.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanno H, Ozawa H. Neuroprotection and Regeneration of the Spinal Cord. Springer; 2014. Autophagy in Spinal Cord Injury: Pathogenic Roles and Therapeutic Implications; pp. 19–30. [Google Scholar]
- 32.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 33.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]


