Abstract
Decreased glutathione peroxidase 3 (GPX3) expression has been identified in numerous solid tumors. However, GPX3 expression pattern in acute myeloid leukemia (AML) remains poorly known. Our study was intended to explore GPX3 expression status and further analyze the clinical relevance of GPX3 expression in AML. GPX3 mRNA level was detected by real-time quantitative PCR in 122 de novo AML patients and 44 normal controls. GPX3 transcript level was significantly decreased compared with normal controls (P < 0.001). The patients with low GPX3 expression had significantly higher hemoglobin and platelets than those with high GPX3 expression (P = 0.049 and 0.020). The frequency of low GPX3 expression in favorable karyotype (66%, 23/35) and intermediate karyotype (65%, 45/69) was higher than in poor karyotype (29%, 4/14) (P = 0.017). No significant differences were observed in both complete remission and overall survival between the GPX3 low-expressed and high-expressed patients (P > 0.05). Reduced GPX3 expression is associated with favorable/intermediate karyotypes but not with survival in de novo AML patients.
Keywords: GPX3, expression, karyotype, acute myeliod leukemia
Introduction
Acute myeloid leukemia (AML), the most common adult leukemia, is a group of heterogeneous disorders characterized by a clone of leukemic stem cells (LSCs), and resulted from a failure of hematopoiesis [1,2]. The specific pathogenesis and progression of AML are not well understood. Morphology, immunology, cytogenetics, and molecular biology (MICM) features comprise the main points for the diagnosis of AML. Recent advances in genetic, cellular and molecular methodologies provide new insights into disease pathogenesis and opportunities for therapeutic advances. Many different cytogenetic and molecular abnormalities have been demonstrated to be involved in leukemogenensis [3-5]. Moreover, the genetic or molecular alterations implicated in pathogenesis of the disease are also important to predict response to therapy and survival [6].
Glutathione peroxidase 3 (GPX3), a member of glutathione peroxidase (GPX) family, locates on chromosome 5q23. GPX family plays crucial roles in protecting cell from oxidative damages by reducing hydrogen peroxide or organic hydroperoxides [7]. Recently, GPX inhibition has been identified to be associated with cancer progression by regulating cancer cells proliferation, invasion, migration, angiogenesis and apoptosis [8]. Down-regulation of GPX3 expression has been found in various solid tumors [9-18]. Moreover, increasing studies have revealed the prognostic significance of GPX3 expression in several cancers [10-12]. However, GPX3 expression pattern in AML is rarely known. The present study was aimed to investigate the GPX3 expression pattern and further analyze the clinical significance in de novo AML patients.
Materials and methods
Patients, cell lines and cell culture
After obtaining the written informed consent, BM was collected from 122 patients who had a diagnosis of de novo AML according to the French-America-British (FAB) and the World Health Organization (WHO) criteria [19,20]. All patients were treated at the Affiliated People’s Hospital of Jiangsu University from October 2005 to March 2014. Treatment protocol was described as reported previously [21]. The characteristics of the patients were summarized in Table 1. 44 healthy donors were collected as normal controls. Seven human leukemic cell lines including HL-60, NB4, U937, THP-1, HEL, SHI-1, and K562 cultured in IMDM medium containing 10% fetal calf serum and grown at 37°C in 5% CO2 humidified atmosphere were also included.
Table 1.
Association between GPX3 expression as well as GPX3 promoter methylation and clinical parameters of AML patients
| Patient’s parameters | Status of GPX3 expression | ||
|---|---|---|---|
|
| |||
| Low (n = 73) | High (n = 49) | P value | |
| Sex, male/female | 44/29 | 30/19 | 1.000 |
| Median age, years (range) | 54 (10-93) | 58 (21-81) | 0.496 |
| Median WBC, × 109/L (range) | 12.2 (0.8-528.0) | 6.6 (0.3-185.4) | 0.331 |
| Median hemoglobin, g/L (range) | 78 (34-131) | 68.5 (32-138) | 0.049 |
| Median platelets, × 109/L (range) | 50 (3-264) | 31 (4-447) | 0.020 |
| BM blasts, % (range) | 52.0 (3.0-109.0) | 42.0 (1.0-94.5) | 0.396 |
| FAB | 0.336 | ||
| M0 | 0 (0%) | 1 (2%) | |
| M1 | 4 (5%) | 4 (8%) | |
| M2 | 24 (33%) | 21 (43%) | |
| M3 | 20 (27%) | 7 (14%) | |
| M4 | 16 (22%) | 12 (24%) | |
| M5 | 6 (8%) | 4 (8%) | |
| M6 | 3 (4%) | 0 (0%) | |
| WHO | 0.232 | ||
| AML with t(8;21) | 3 (4%) | 5 (10%) | |
| APL with t(15;17) | 20 (27%) | 7 (14%) | |
| AML with 11q23 | 0 (0%) | 1 (2%) | |
| AML without maturation | 4 (5%) | 4 (8%) | |
| AML with maturation | 21 (29%) | 16 (33%) | |
| Acute myelomonocytic leukemia | 16 (22%) | 13 (26%) | |
| Acute monoblastic and monocytic leukemia | 6 (8%) | 2 (4%) | |
| Acute erythroid leukemia | 3 (4%) | 0 (0%) | |
| No data | 0 (0%) | 1 (2%) | |
| Karyotype classification | 0.026 | ||
| Favorable | 23 (32%) | 12 (24%) | |
| Intermediate | 45 (62%) | 24 (49%) | |
| Poor | 4 (5%) | 10 (20%) | |
| No data | 1 (1%) | 3 (6%) | |
| Karyotype | 0.006 | ||
| Normal | 38 (52%) | 18 (37%) | |
| T(8;21) | 3 (4%) | 5 (10%) | |
| T(15;17) | 20 (27%) | 7 (14%) | |
| 11q23 | 0 (0%) | 1 (2%) | |
| Complex | 2 (3%) | 9 (18%) | |
| Others | 9 (12%) | 6 (12%) | |
| No data | 1 (1%) | 3 (6%) | |
| Gene Mutation | |||
| C/EBPA (+/-) | 5/60 | 6/37 | 0.340 |
| NPM1 (+/-) | 3/62 | 6/37 | 0.151 |
| FLT3-ITD (+/-) | 7/58 | 7/36 | 0.560 |
| C-KIT (+/-) | 1/64 | 0/43 | 1.000 |
| N/K RAS (+/-) | 7/58 | 2/41 | 0.312 |
| IDH1/2 (+/-) | 4/61 | 2/41 | 1.000 |
| DNMT3A (+/-) | 6/59 | 2/41 | 0.473 |
| U2AF1 (+/-) | 2/63 | 4/39 | 0.213 |
| CR (+/-) | 33/30 | 20/27 | 0.339 |
WBC: white blood cells; FAB: French-American-British classification; AML: acute myeloid leukemia; CR: complete remission.
BMMNCs separation, RNA isolation and reverse transcription
BM mononuclear cells (BMMNCs) were extracted from BM specimens by Ficoll solution and washed twice with PBS. Total RNA was isolated from the BMMNCs and the leukemic cell lines using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription reaction with 40 μL volume contained 5 × buffer 10 mM, dNTPs 10 mM, random hexamers 10 μM, RNAsin 80 U, and MMLV reverse transcriptase 200 U (MBI Fermentas, Hanover, USA) was performed on iCycler Thermal Cycler (Eppendorf, Hamburg, Germany). The system of reverse transcription was incubated for 10 min at 25°C, 60 min at 42°C, and then stored at -20°C.
GPX3 mRNA level detection
Real-time quantitative PCR (RQ-PCR) was performed on a 7300 Thermo cycler (Applied Biosystems, CA, USA). The primers of GPX3 expression were 5’-GCCGGGGACAAGAGAAGT-3’ (forward) and 5’-GAGGACGTATTTGCCAGCAT-3’ (reverse) as reported previously [12]. The RQ-PCR reaction with a volume of 20 μL was composed of cDNA 20 ng, 0.8 μM of primers, 10 μM of AceQTMqPCR SYBR Green Master Mix (Vazyme Biotech Co., Piscataway, NJ, USA), and 0.4 μM of ROX Reference Dye 1 (Invitrogen, Carlsbad, CA, USA). The RQ-PCR were carried out at 95°C for 5 min, followed by 45 cycles at 95°C for 10 s, 63°C for 30 s, 72°C for 30 s, and 80°C for 30 s to collect fluorescence, finally followed by 95°C for 15 s, 60°C for 60 s, 95°C for 15 s, and 60°C for 15 s. Both positive (GPX3-plasmid) and negative (water) controls were included in each assay. ABL was used to calculate the abundance of GPX3 mRNA. Relative GPX3 transcript levels were calculated by the following equation: NGPX3 = (EGPX3)ΔCT GPX3 (control-sample) ÷ (EABL)ΔCT ABL (control-sample). The parameter efficiency (E) was counted by the formula E = 10(-1/slope) (the slope referred to CT versus cDNA concentration plot).
Gene mutation detection
DNMT3A, U2AF1, IDH1/2, N/K-RAS, and C/EBPA mutations were detected as reported previously [22-26]. NPM1 and c-KIT mutations were detected by PCR and high-resolution melting analysis (HRMA). All positive samples were confirmed by DNA direct sequencing. FLT3-ITD mutation was detected by PCR and direct DNA sequencing.
Statistical analysis
Statistical analyses were performed on SPSS 18.0 software package (SPSS, Chicago, IL). Pearson Chi-square analysis or Fisher exact test were employed to compare the difference of categorical variables. Mann-Whitney’s U test was carried out to compare the difference of continuous variables between two groups. Receiver operating characteristic curve (ROC) and area under the ROC curve (AUC) were conducted to assess the value of GPX3 expression in distinguishing AML patients from normal controls. Kaplan-Meier analysis and multivariate analysis were performed to analyze the impact of GPX3 expression on survival respectively. For all analyses, a two-tailed P value of 0.05 or less was determined as statistically significant.
Results
GPX3 expression in normal controls, AML patients and cell lines
GPX3 mRNA level in normal controls ranged from 0.069 to 9.258 with a median level of 0.348. However, the level of GPX3 transcript in de novo AML patients (0.000-12.600, median 0.037) was significantly down-regulated as compared with normal controls (P < 0.001, Figure 1). In addition, all the seven leukemic cell lines presented decreased GPX3 expression ranging from 0.000 to 0.047 (Figure 2).
Figure 1.
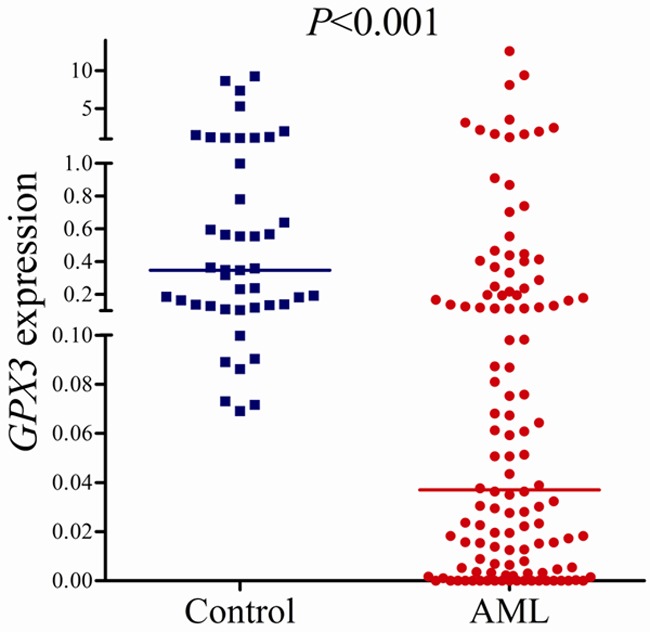
Relative expression levels of GPX3 in AML patients and controls.
Figure 2.
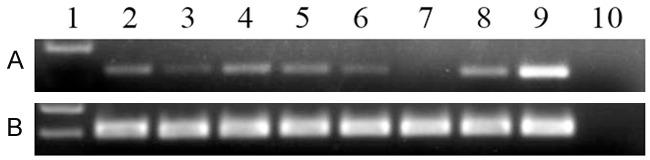
Electrophoresis results of RQ-PCR products in leukemic cell lines. 1: Gene RulerTM 100bp DNA ladder; 2: K562; 3: SHI-1; 4: U937; 5: THP1; 6: NB4; 7: HL60; 8: HEL; 9: positive control; 10: negative control. A: GPX3; B: ABL.
Differentiating value of GPX3 expression
ROC curve indicated that GPX3 expression could serve as a promising biomarker for distinguishing whole AML patients from normal controls (AUC = 0.800, 95% CI: 0.735-0.864, P < 0.001) (Figure 3A). ROC curve analysis disclosed that at the cut-off value less than 0.067, the sensitivity and the specificity were 60% and 100%, respectively. Moreover, significant differentiating value was also revealed in cytogenetically normal AML (CN-AML) patients (AUC = 0.825, 95% CI: 0.741-0.909, P < 0.001) (Figure 3B).
Figure 3.
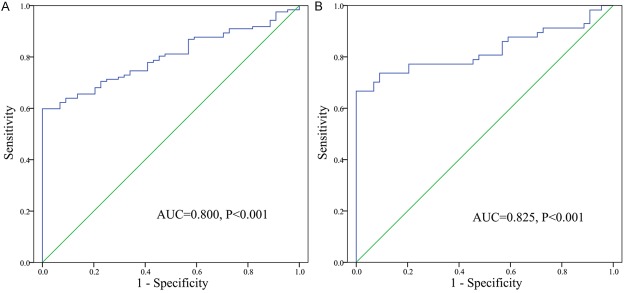
ROC curve analysis using GPX3 for discriminating AML patients. A: All patients; B: Cytogenetically normal patients.
Association between GPX3 expression and clinical parameters of AML patients
The whole AML patients were divided into two groups: GPX3 low-expressed (GPX3 low) (≤ 0.067) and GPX3 high-expressed (GPX3 high) (> 0.067) according to the cut-off value of 0.067. There were no significant differences in age, white blood cell (WBC), BM blasts, and sex distribution between the two group patients (P > 0.05, Table 1). However, GPX3 low cases had significantly higher hemoglobin (HB) and platelets (PLT) than GPX3 high cases (P = 0.049 and 0.020). There were no significant differences in the distribution of FAB as well as WHO classifications between the two groups (P > 0.05). However, significant differences were observed between GPX3 low and GPX3 high cases in the distribution of both karyotype and karyotype classification (P = 0.006 and 0.026). The frequency of low GPX3 expression in favorable karyotype (66%, 23/35) and intermediate karyotype (65%, 45/69) was higher than in poor karyotype (29%, 4/14) (P = 0.017). The level of GPX3 transcript in the patients with favorable (median 0.043) and intermediate karyotypes (median 0.030) was significantly lower than in controls (P < 0.001), whereas those with poor karyotypes (median 0.207) had similar level of GPX3 mRNA as controls (P = 0.291) (Figure 4). The level of GPX3 expression among different karyotypes was shown in Figure 5. Due to the GPX3 gene locates on chromosome 5, we further analyzed GPX3 expression in the patients with and without -5/5q-. Interestingly, patients with -5/5q- (20%, 1/5) trended to have higher incidence of low GPX3 expression than those without -5/5q- (62%, 68/109) (P = 0.078). Additionally, no significant correlations were observed between GPX3 expression and ten gene mutations (P > 0.05, Table 1).
Figure 4.
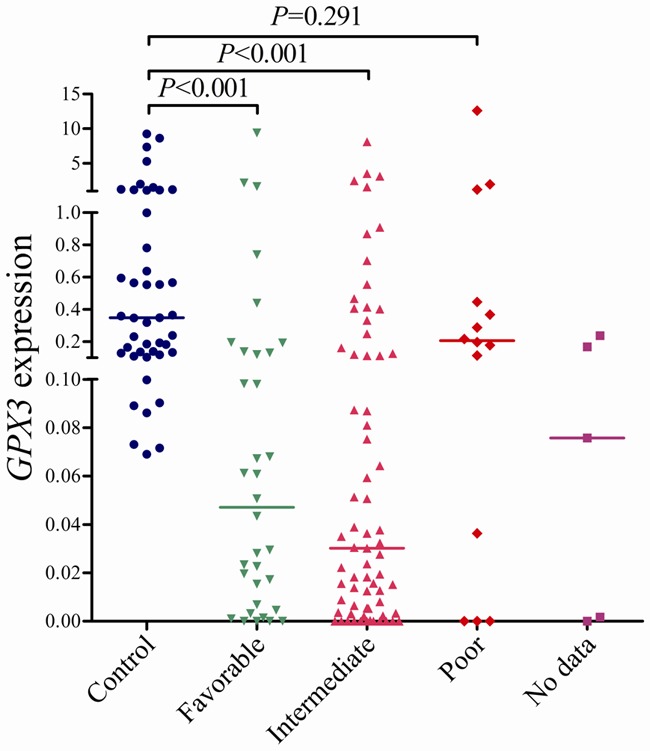
Relative levels of GPX3 expression in AML according to karyotypic risking.
Figure 5.
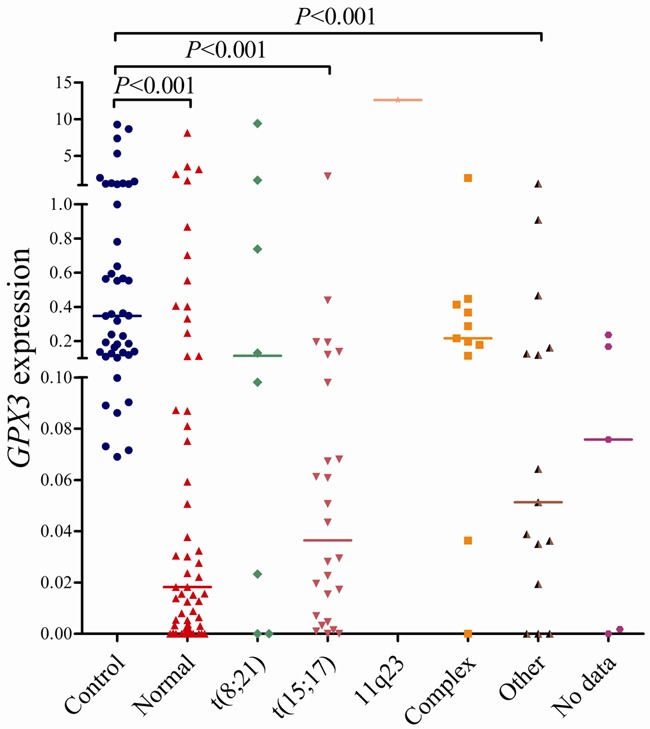
Relative levels of GPX3 expression in AML with different karyotypes.
Association between GPX3 expression and prognosis in AML patients
110 patients with available follow-up data were included in the complete remission (CR) analysis. GPX3 low patients had higher CR rate than GPX3 high patients in whole AML (52% vs. 43%, P = 0.339, Table 1). Moreover, GPX3 low and GPX3 high patients showed similar CR rate in both non-M3 and CN-AML [44% (22/50) vs. 39% (16/41) and 51% (18/35) vs. 47% (9/19); P = 0.674 and 1.000]. Survival analyses were performed in 107 patients with survival data ranging from 1 to 92 months with a median of 10 months. GPX3 low patients had a little longer overall survival (OS) than GPX3 high patients (median 11 vs. 9 months, P = 0.256, Figure 6). Among non-M3 and CN-AML patients, there were also no significant difference in OS between the GPX3 low and GPX3 high groups (median 6 vs. 7 months and 12 vs. 5 months; P = 0.774 and 0.555). Furthermore, multivariate analysis also failed to disclose the prognostic impact of GPX3 expression in both whole AML and non-M3 as well as CM-AML patients (data not shown).
Figure 6.
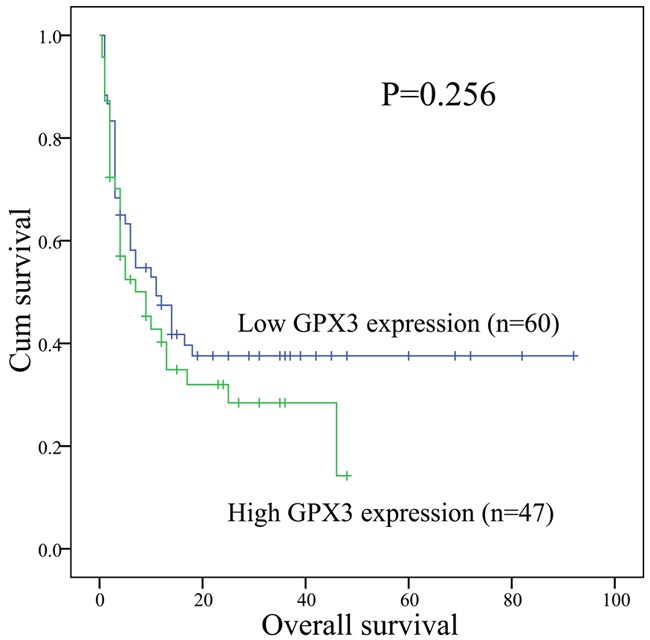
Overall survival of whole AML patients.
Discussion
GPX3, the most studied GPX family member, accounts for almost all of the GPX activity in plasma [7]. Recently, tumor suppressive roles of the GPX3 have been observed in numerous solid cancers including prostate cancer, colitis-associated carcinoma and hepatocellular carcinoma [27-29]. Interestingly, a few studies revealed the up-regulated status of GPX3 expression in clear cell epithelial ovarian carcinoma tissues as well as in ovarian serous carcinoma effusions [30,31]. These results suggest that GPX3 expression may be tumor-specific in the process of tumorigenesis. However, GPX3 expression pattern is rarely studied in hematological malignancies. A previous study has revealed the role of GPX3 in LSCs as well as normal hematopoietic stem cells (HSCs) and indicated that GPX3 is critical for the competitiveness of LSCs and self-renewal activity of HSCs [32]. Moreover, Kaiser et al. [33] disclosed the hypermethylation status of GPX3 promoter is associated with shorter OS in multiple myeloma. These results suggested that GPX3 also played a vital role in hematological malignancies.
In the current study, we investigated the GPX3 expression pattern and further analyzed the clinical significance of GPX3 expression in de novo AML patients. Our study revealed that GPX3 was down-regulated in both leukemic cell lines and de novo AML patients. Moreover, our investigation by ROC curve analysis disclosed that GPX3 expression could be a promising biomarker for discriminating AML as well as CN-AML from normal controls.
When analyzed the GPX3 expression status in different karyotypes and karyotypic classifications, our data demonstrated that GPX3 expression was significantly decreased in patients with favorable/intermediate karyotypes especially in t(15;17) and normal karyotypes but not in those with poor karyotypes. Similarly, the previous study also observed the increased level of GPX3 expression with the rising risk of karyotype [32]. These results suggested that the role of GPX3 in the process of leukemogenesis may be dependent on the context of different cytogenetics.
Accumulating studies have revealed the prognostic value of GPX3 expression in several solid cancers. In gastric carcinomas, decreased GPX3 expression was associated with lymph node metastasis [12]. Zhang et al. [10] indicated that down-regulation of GPX3 was a predictive marker not only for the lymph node metastasis but also for the poor prognosis in cervical cancer. Yang et al. [11] demonstrated that positive ALDH1A3 and negative GPX3 expressions were associated with adverse prognosis in gallbladder cancer. Moreover, the negative impact of low GPX3 expression on prognosis has also been revealed in hepatocellular carcinoma [29]. However, our investigation by both Kaplan-Meier analysis and multivariate analysis failed to indicate that GPX3 expression could be a potential biomarker for the prognosis of AML.
The underlying mechanism regulating GPX3 expression has been abundantly investigated. The association between GPX3 expression and its promoter methylation has been found in various cancers [9,12,13,15-18,33]. Moreover, hypermethylation of GPX3 promoter has been identified to be helpful not only for the early diagnosis but also for the prognosis in a few cancers [15,33-35]. A recent study demonstrated the epigenetic mechanism regulating GPX3 expression in multiple myeloma [33]. Further studies are needed to explore GPX3 promoter methylation pattern in AML patients and determine its role in regulating GPX3 expression.
In conclusion, our study indicates that decreased GPX3 expression is associated with favorable/intermediate karyotypes. Moreover, downregulation of GPX3 expression has no impact on the clinical outcome of de novo AML patients.
Acknowledgements
This study was supported by National Natural Science foundation of China (81270630, 81172592), Science and Technology Special Project in Clinical Medicine of Jiangsu Province (BL2012056), 333 Project of Jiangsu Province (BRA2013136), Science and Technology Infrastructure Program of Zhenjiang (SS2012003), Medical Key Talent Project of Zhenjiang, Social Development Foundation of Zhenjiang (SH2013042, SH2013082, SH2014044, SH2014086), and Jiangsu Government Scholarship for Overseas Studies.
Disclosure of conflict of interest
None.
References
- 1.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Smith M, Barnett M, Bassan R, Gatta G, Tondini C, Kern W. Adult acute myeloid leukaemia. Crit Rev Oncol Hematol. 2004;50:197–222. doi: 10.1016/j.critrevonc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Haferlach T. Molecular genetic pathways as therapeutic targets in acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2008:400–11. doi: 10.1182/asheducation-2008.1.400. [DOI] [PubMed] [Google Scholar]
- 4.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–64. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 5.Krug U, Ganser A, Koeffler HP. Tumor suppressor genes in normal and malignant hematopoiesis. Oncogene. 2002;21:3475–95. doi: 10.1038/sj.onc.1205322. [DOI] [PubMed] [Google Scholar]
- 6.Mrózek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: Are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–48. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289–303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Brigelius-Flohé R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790:1555–68. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed MM, Sabet S, Peng DF, Nouh MA, El-Shinawi M, El-Rifai W. Promoter hypermethylation and suppression of glutathione peroxidase 3 are associated with inflammatory breast carcinogenesis. Oxid Med Cell Longev. 2014;2014:787195. doi: 10.1155/2014/787195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Zheng Z, Yingji S, Kim H, Jin R, Renshu L, Lee DY, Roh MR, Yang S. Downregulation of glutathione peroxidase 3 is associated with lymph node metastasis and prognosis in cervical cancer. Oncol Rep. 2014;31:2587–92. doi: 10.3892/or.2014.3152. [DOI] [PubMed] [Google Scholar]
- 11.Yang ZL, Yang L, Zou Q, Yuan Y, Li J, Liang L, Zeng G, Chen S. Positive ALDH1A3 and negative GPX3 expressions are biomarkers for poor prognosis of gallbladder cancer. Dis Markers. 2013;35:163–72. doi: 10.1155/2013/187043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng DF, Hu TL, Schneider BG, Chen Z, Xu ZK, El-Rifai W. Silencing of glutathione peroxidase 3 through DNA hypermethylation is associated with lymph node metastasis in gastric carcinomas. PLoS One. 2012;7:e46214. doi: 10.1371/journal.pone.0046214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min SY, Kim HS, Jung EJ, Jung EJ, Jee CD, Kim WH. Prognostic significance of glutathione peroxidase 1 (GPX1) down-regulation and correlation with aberrant promoter methylation in human gastric cancer. Anticancer Res. 2012;32:3169–75. [PubMed] [Google Scholar]
- 14.Agnani D, Camacho-Vanegas O, Camacho C, Lele S, Odunsi K, Cohen S, Dottino P, Martignetti JA. Decreased levels of serum glutathione peroxidase 3 are associated with papillary serous ovarian cancer and disease progression. J Ovarian Res. 2011;4:18. doi: 10.1186/1757-2215-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen B, Rao X, House MG, Nephew KP, Cullen KJ, Guo Z. GPX3 promoter hypermethylation is a frequent event in human cancer and is associated with tumorigenesis and chemotherapy response. Cancer Lett. 2011;309:37–45. doi: 10.1016/j.canlet.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Falck E, Karlsson S, Carlsson J, Helenius G, Karlsson M, Klinga-Levan K. Loss of glutathione peroxidase 3 expression is correlated with epigenetic mechanisms in endometrial adenocarcinoma. Cancer Cell Int. 2010;10:46. doi: 10.1186/1475-2867-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Wang Y, Li P, Zhu S, Wang J, Zhang S. Identification of GPX3 epigenetically silenced by CpG methylation in human esophageal squamous cell carcinoma. Dig Dis Sci. 2011;56:681–8. doi: 10.1007/s10620-010-1369-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee OJ, Schneider-Stock R, McChesney PA, Kuester D, Roessner A, Vieth M, Moskaluk CA, El-Rifai W. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett’s tumorigenesis. Neoplasia. 2005;7:854–61. doi: 10.1593/neo.05328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. France: IARC Press; 2008. [Google Scholar]
- 20.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposed revised criteria for the classification of acute myeloid leukaemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Lin J, Yang J, Qian J, Qian W, Yao DM, Deng ZQ, Liu Q, Chen XX, Xie D, An C, Tang CY. Overexpressed let-7a-3 is associated with poor outcome in acute myeloid leukemia. Leuk Res. 2013;37:1642–7. doi: 10.1016/j.leukres.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Yao DM, Qian J, Chen Q, Qian W, Li Y, Yang J, Wang CZ, Chai HY, Qian Z, Xiao GF, Xu WR. Recurrent DNMT3A R882 muta-tions in Chinese patients with acute myeloid leukemia and myelodysplastic syndrome. PLoS One. 2011;6:e26906. doi: 10.1371/journal.pone.0026906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Yao DM, Qian J, Chen Q, Qian W, Li Y, Yang J, Wang CZ, Chai HY, Qian Z, Xiao GF, Xu WR. IDH1 and IDH2 mutation analysis in Chinese patients with acute myeloid leukemia and myelodysplastic syndrome. Ann Hematol. 2012;91:519–25. doi: 10.1007/s00277-011-1352-7. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Qian J, Sun A, Lin J, Xiao G, Yin J, Chen S, Wu D. RAS mutation analysis in alarge cohort of Chinese patients with acute myeloid leukemia. Clin Biochem. 2013;46:579–83. doi: 10.1016/j.clinbiochem.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Qian J, Yao DM, Lin J, Qian W, Wang CZ, Chai HY, Yang J, Li Y, Deng ZQ, Ma JC, Chen XX. U2AF1 Mutations in Chinese patients with acute myeloid leukemia and myelodysplastic syndrome. PLoS One. 2012;7:e45760. doi: 10.1371/journal.pone.0045760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen XM, Lin J, Yang J, Yao DM, Deng ZQ, Tang CY, Xiao GF, Yang L, Ma JC, Hu JB, Qian W, Qian J. Double CEBPA mutations are prognostically favorable in non-M3 acute myeloid leukemia patients with wild-type NPM1 and FLT3-ITD. Int J Clin Exp Pathol. 2014;7:6832–40. [PMC free article] [PubMed] [Google Scholar]
- 27.Yu YP, Yu G, Tseng G, Cieply K, Nelson J, Defrances M, Zarnegar R, Michalopoulos G, Luo JH. Glutathione peroxidase 3, deleted or methylated in prostate cancer, suppresses prostate cancer growth and metastasis. Cancer Res. 2007;67:8043–50. doi: 10.1158/0008-5472.CAN-07-0648. [DOI] [PubMed] [Google Scholar]
- 28.Barrett CW, Ning W, Chen X, Smith JJ, Washington MK, Hill KE, Coburn LA, Peek RM, Chaturvedi R, Wilson KT, Burk RF, Williams CS. Tumor suppressor function of the plasma glutathione peroxidase GPX3 in colitis-associated carcinoma. Cancer Res. 2013;73:1245–55. doi: 10.1158/0008-5472.CAN-12-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi X, Ng KT, Lian QZ, Liu XB, Li CX, Geng W, Ling CC, Ma YY, Yeung WH, Tu WW, Fan ST, Lo CM, Man K. Clinical significance and therapeutic value of glutathione peroxidase 3 (GPx3) in hepatocellular carcinoma. Oncotarget. 2014;5:11103–11120. doi: 10.18632/oncotarget.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saga Y, Ohwada M, Suzuki M, Konno R, Kigawa J, Ueno S, Mano H. Glutathione peroxidase 3 is a candidate mechanism of anticancer drug resistance of ovarian clear cell adenocarcinoma. Oncol Rep. 2008;20:1299–303. [PubMed] [Google Scholar]
- 31.Tuft Stavnes H, Nymoen DA, Hetland Falkenthal TE, Kærn J, Tropé CG, Davidson B. APOA1 mRNA expression in ovarian serous carcinoma effusions is a marker of longer survival. Am J Clin Pathol. 2014;142:51–7. doi: 10.1309/AJCPD8NBSHXRXQL7. [DOI] [PubMed] [Google Scholar]
- 32.Herault O, Hope KJ, Deneault E, Mayotte N, Chagraoui J, Wilhelm BT, Cellot S, Sauvageau M, Andrade-Navarro MA, Hébert J, Sauvageau G. A role for GPX3 in activity of normal and leukemia stem cells. J Exp Med. 2012;209:895–901. doi: 10.1084/jem.20102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser MF, Johnson DC, Wu P, Walker BA, Brioli A, Mirabella F, Wardell CP, Melchor L, Davies FE, Morgan GJ. Global methylation analysis identifies prognostically important epigenetically inactivated tumor suppressor genes in multiple myeloma. Blood. 2013;122:219–26. doi: 10.1182/blood-2013-03-487884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Zhou F, Jiang C, Wang Y, Lu Y, Yang F, Wang N, Yang H, Zheng Y, Zhang J. Identification of a DNA methylome profile of esophageal squamous cell carcinoma and potential plasma epigenetic biomarkers for early diagnosis. PLoS One. 2014;9:e103162. doi: 10.1371/journal.pone.0103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobosy JR, Roberts JL, Fu VX, Jarrard DF. The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol. 2007;177:822–31. doi: 10.1016/j.juro.2006.10.063. [DOI] [PubMed] [Google Scholar]


