Abstract
microRNA (mir)-365 exerts tumor suppressor function by targeting thyroid transcription factor-1 (TTF-1) in lung cancer cells. The purpose of the present study was to assess mir-365 and its target mRNA TTF-1 in lung cancer and their correlations with patients’ survival. Quantitative real-time PCR was used to examine the expression levels of mir-365 and TTF-1 in tumor tissue and its adjacent noncancerous tissue of 126 patients with non-small cell lung cancer (NSCLC). Our results showed that mir-365 was significantly decreased in tumor tissue than that in normal tissue (P=0.006), however, TTF-1 was significantly increased in tumor tissue than in normal tissue (P<0.001). Besides, significant correlations between decreased mir-365 and advanced tumor-node-metastasis (TNM) stage (P=0.001) and regional lymph node involvement (P=0.037) was observed. The similar result was also found between increased TTF-1 and TNM stage (P=0.003). Furthermore, mir-365 downregulation or TTF-1 upregulation were associated with poor outcome of patients than mir-365 upregulation or TTF-1 downregulation (for mir-365: P<0.001; for TTF-1: P=0.002). Of note, combination of decreased mir-365 and increased TTF-1 had worst overall survival (P<0.001). In conclusion, aberrant expression of mir-365/TTF-1 may be involved in the tumor development in patients with NSCLC. Moreover, mir-365 and TTF-1 could jointly predict the prognosis of patients and their combination may serve as a biomarker to predict risk of poor survival in NSCLC patients. Mir-365/TTF-1 might serve as a potential therapeutic target for clinical treatment of NSCLC.
Keywords: microRNA-365, NKX2-1, non-small cell lung cancer, prognostic, outcome
Introduction
Lung cancer is the leading cause of death worldwide in spite of therapeutic advances. Non-small cell lung cancer (NSCLC), as the most frequent type of lung cancer, consists of squamous cell carcinoma, large cell carcinomas and adenocarcinoma, accounts for over 80% of lung cancer cases [1]. Despite advances in early detection and personalized treatment, the 5-year overall survival rate is still low [2]. Additional prognostic assessments of patients are needed in order to choose appropriate therapeutic strategies for specific patients. The discovery of new robust predictive markers may help detect and select patients who will benefit most from treatment regimens [3].
MicroRNAs (miRNAs) are a large family of evolutionarily conserved small (~22 bp in length) endogenous, single-stranded noncoding RNAs. They regulate gene expression through pairing with the 3’-UTR of the target messenger RNAs, leading to translational suppression or degradation [4]. Growing evidence demonstrated that miRNAs regulate various cellular functions and play crucial roles in many biologic processes, including cell growth, proliferation, differentiation, cell metabolism, apoptosis and transformation [5]. The miRNAs are frequently located in cancer-associated genes or fragile sites, thus, deregulation of miRNAs may be implicated into carcinogenesis and cancer progression [6]. The miRNAs may exert functions as oncogenes or tumor suppressors by negatively modulate its targeted genes which are complementary to the miRNAs [7,8]. Since expression profiling of many miRNAs in various types of cancer have unique expression patterns, some of these miRNAs have been demonstrated as potential biomarkers for diagnosis, prognosis, and therapy in human cancers [9-11].
It has been demonstrated that mir-365 is implicated in cell proliferation, differentiation and apoptosis in colon cancer cells [12], lung cancer cells [13], breast cancer [14], vascular smooth muscle cells (VSMCs) [15] and endothelial cells [16]. Meanwhile, it has been indicated that mir-365 modulates thyroid transcription factor-1 (TTF-1) by interacting with its 3’-untranslated region, and the expression levels of mir-365 and TTF-1 were in an inverse correlation in human lung cancer [13]. In addition, TTF-1 overexpression could significantly increase cell proliferation by overcoming the inhibition effect of mir-365 [17]. TTF-1, also known as (NK2 homeobox 1), NKX2-1, is a 38-kDa homeobox-containing transcription factor. It is mainly expressed in adult thyroid and lung tissue. Several studies have investigated the association between TTF-1 expression and NSCLC prognosis with conflicting results [18,19]. All of the above evidence implies that mir-365/TTF-1 play the critical roles in NSCLC. However, the clinical significance of mir-365 in NSCLC remains to be elucidated. The aim of this study was to investigate: (1) the expression pattern of mir-365 in tumor tissue, (2) the association of mir-365 with clinical characteristics and survival of the patients, (3) whether mir-365 can be treated as a potential prognosis marker and therapeutic target in patients with NSCLC. These findings may provide a new interpretation of the functional and clinical application of mir-365/TTF-1 in NSCLC.
Materials and methods
Patients and tissue samples
This study was approved by the ethics committee of Xi’an Jiaotong University & Shaanxi Province Tumor Hospital, and the written informed consent was obtained from patients whose specimens were collected.
In the present study, 126 paired NSCLC and adjacent non-tumor tissues were obtained during surgery from Shaanxi Province Tumor Hospital. All the fresh tissues were then made as formalin-fixed, paraffin-embedded (FFPE) specimens by the department of pathology of Shaanxi Province Tumor Hospital. There were 92 (73.1%) male and 34 (26.9%) female patients with a median age of 61 years, range 34-86 years. None of the enrolled patients had received any preoperative chemotherapy or radiotherapy. All cases were diagnosed as NSCLC by two independent pathologists. Tumor stage and histological grade were evaluated based on TNM staging classification system and World Health Organization, respectively.
We collected all the clinical follow-up information on NSCLC patients from medical history and phone interview either by the patient or with a relative. Patients were followed-up from surgery through January 2010 for clinical outcome. The time of follow-up was calculated until death or last contact in June 2014. The median follow-up time for overall survival was 16.0 months (range 6.0-36.0 months) for patients who were survived until June 2014.
Quantitative real-time PCR (qRT-PCR) assay
Total RNA was isolated from FFPE tissues using E.Z.N.A FFPE RNA kit (Omega). RNA concentration and purity were measured by NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA, USA). The reverse transcription reaction was performed using a PrimeScript™ RT reagent Kit (TaKaRa) based on the manufacturer’s instruction. The cDNA specimens were amplified by quantitative real time PCR using SYBR Premix Ex Taq™ II (TaKaRa). The specific primers of mir-365, TTF-1, U6 and GAPDH (used as internal standard for normalization, respectively), are shown in Table 1 [12,20]. PCR amplification was carried out on the BIO-RAD IQ5 Optical System real-time PCR machine. All the experiments were conducted in triplicate. The comparative cycle threshold (Ct) method was calculated to determine the relative expression levels of mir-365 to U6 and TTF-1 mRNA to GAPDH mRNA using the 2-ΔΔCt.
Table 1.
Table 1 list of primers used in the present work
| Name | Primers | Sequences |
|---|---|---|
| mir-365 | Stem-loop RT primer | 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACATAAGG-3’ |
| Forward | 5’-CGTAATGCCCCTAAAAAT-3’ | |
| Reverse | 5’-GTGCAGGGTCCGAGGT-3’ | |
| U6 | Stem-loop RT primer | 5’-CGCTTCACGAATTTGCGTGTCAT-3’ |
| Forward | 5’-GCTTCGGCAGCACATATACTAAAAT-3’ | |
| Reverse | 5’-CGCTTCACGAATTTGCGTGTCAT-3’ | |
| TTF-1 | Forward | 5’-CGTTCTCAGTGTCTGACATCTTGA-3’ |
| Reverse | 5’-CCTCCATGCCCACTTTCTTG-3’ | |
| GAPDH | Forward | 5’-GGAGTCAACGGATTTGGTCGTA-3’ |
| Reverse | 5’-GGCAACAATATCCACTTTACCAGAGT-3’ |
Statistical analysis
All tests were performed using the software of SPSS version 17.0 for Windows (SPSS Inc, IL, USA). Data were expressed as mean ± standard deviation (SD). The associations between mir-365 and TTF-1 and the clinical features of the patients were determined by Chi-square test and Fishers exact test. The comparison of the different expression levels of mir-365 and TTF-1 between tumor tissues and adjacent normal tissues was conducted using paired sample T-test. The correlation between mir-365 and TTF-1 mRNA expression was assessed using Spearman’s test. The survival probabilities were estimated by log-rank test, and the survival curves were plotted based on Kaplan-Meier. All statistical tests were two-sided, P<0.05 was considered statistically significant.
Results
Clinical and pathological features of all the patients
In total, 24 (19.0%) patients with stage I, 30 (23.8%) patients with stage II and 72 (57.1%) patients with stage III were enrolled in the present study. Among them, 52 (41.3%) patients were never smokers and 74 (58.7%) patients were current or ever smokers. 33 (26.2%) patients were both high or low expression of mir-365 and TTF-1, 30 (23.8%) patients were mir-365 high and TTF-1-low expression, and 63 (50.0%) patients were mir-365-low and TTF-1-high expression. Their clinical and pathological data are shown in Table 2.
Table 2.
The main clinical characteristics of the patients according to mir-365 and TTF-1 expression levels
| Characteristic | No. | mir-365 | P value | TTF-1 | P value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| High (n, %) | Low (n, %) | High (n, %) | Low (n, %) | ||||
| Age (range), y | |||||||
| ≤60 | 58 (46.0) | 21 (47.7) | 37 (45.1) | 0.852 | 37 (48.1) | 21 (42.9) | 0.568 |
| >60 | 68 (54.0) | 23 (52.3) | 45 (54.9) | 40 (52.0) | 28 (57.1) | ||
| Gender | |||||||
| Male | 92 (73.1) | 32 (72.7) | 60 (73.2) | 0.957 | 58 (75.3) | 34 (69.4) | 0.464 |
| Female | 34 (26.9) | 12 (27.3) | 22 (26.8) | 19 (24.7) | 15 (30.6) | ||
| Smoking status | |||||||
| Never | 52 (41.3) | 18 (40.9) | 34 (41.5) | 0.952 | 29 (37.7) | 23 (46.9) | 0.303 |
| Current or ever | 74 (58.7) | 26 (59.1) | 48 (58.5) | 48 (62.3) | 26 (53.1) | ||
| Pathological type | |||||||
| Squamous cell carcinoma | 50 (39.7) | 17 (38.6) | 33 (40.2) | 0.86 | 31 (40.3) | 19 (38.8) | 0.868 |
| Adenocarcinoma | 76 (60.3) | 27 (61.4) | 49 (59.8) | 46 (60.0) | 30 (61.2) | ||
| Tumor differentiation | |||||||
| Well | 11 (8.7) | 6 (13.6) | 5 (6.1) | 0.224 | 6 (7.8) | 5 (10.2) | 0.795 |
| Moderate | 53 (42.1) | 20 (45.5) | 33 (40.2) | 34 (44.2) | 19 (38.8) | ||
| Poor | 62 (49.2) | 18 (40.9) | 44 (53.7) | 37 (48.1) | 25 (51.0) | ||
| Stage | |||||||
| I | 24 (19.0) | 14 (31.8) | 10 (12.2) | 0.001 | 9 (11.7) | 15 (30.6) | 0.003 |
| II | 30 (23.8) | 15 (34.1) | 15 (18.3) | 15 (19.5) | 15 (30.6) | ||
| III | 72 (57.1) | 15 (34.1) | 57 (69.5) | 53 (68.8) | 19 (38.8) | ||
| Node | |||||||
| Negative | 76 (60.3) | 32 (72.7) | 44 (53.7) | 0.037 | 45 (58.4) | 31 (63.3) | 0.590 |
| Positive | 50 (39.7) | 12 (27.3) | 38 (46.3) | 32 (41.6) | 18 (36.7) | ||
mir-365 downregulation and TTF-1 upregulation in tumor tissue versus adjacent nontumorous tissue
The expression levels of mir-365 and TTF-1 in pairs of NSCLC and paracancerous tissues were investigated by qRT-PCR. As shown in Figure 1, mir-365 expression level was significantly decreased in NSCLC tissues than that in normal adjacent tissues (P=0.006), while TTF-1 mRNA expression level was significantly increased in NSCLC tissues compared to the corresponding noncancerous lung tissues (P<0.001, Figure 2). Of note, the relative expression levels of mir-365 in tumor tissue were negatively correlated with those of TTF-1 mRNA (Spearman correlation coefficient r=-0.409, P<0.001, Figure 3).
Figure 1.
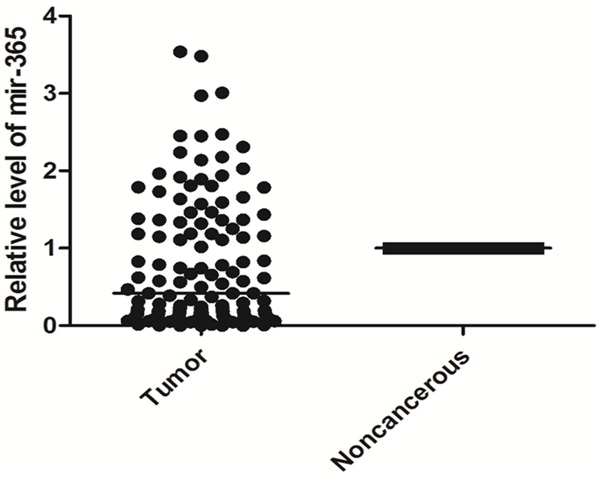
Relative expression level of mir-365 in formalin-fixed paraffin-embedded samples of NSCLC patients.
Figure 2.
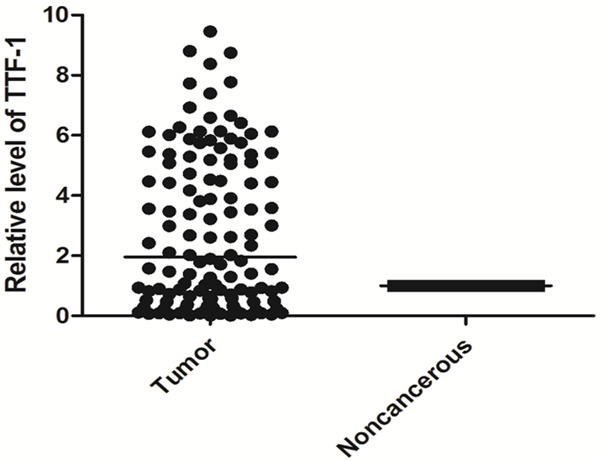
Relative expression level of TTF-1 in formalin-fixed paraffin-embedded samples of NSCLC patients.
Figure 3.
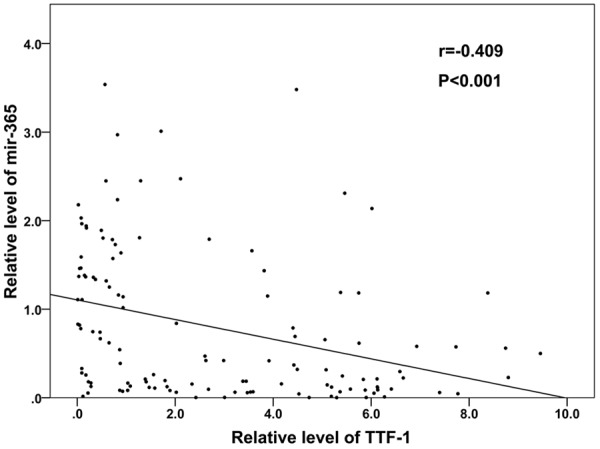
Spearman correlation between mir-365 and TTF-1 mRNA expression levels in NSCLC patients.
Associations between mir-365 downregulation and TTF-1 upregulation with clinical characteristics of NSCLC
We next investigated associations between the altered mir-365 and TTF-1 expression levels with the clinicopathological characteristics of human NSCLC (Table 2). There were strong correlations between mir-365 downregulation and advanced TNM stage (P=0.001) and regional lymph node involvement (P=0.037). A similar association between TTF-1 upregulation and advanced TNM stage was also observed (P=0.003). No other significant associations between mir-365 or TTF-1 expression and clinicopathological features including age, gender, histological type, smoking history was observed (P>0.05, Table 2).
Association of mir-365 and TTF-1 mRNA expression with overall survival in NSCLC patients
Because the expression levels of mir-365 and TTF-1 were altered in NSCLC tissue and noncancerous tissue, we further assessed whether their alteration could predict prognosis in NSCLC patients. The median survival time of the patients was 16.0 months. During the follow-up period, 52 (41.3%) of the 126 patients died of disease progression. The Kaplan-Meier plot showed that NSCLC patients with decreased mir-365 or increased TTF-1 mRNA expression was obviously associated with worse overall survival than those with increased mir-365 expression or decreased TTF-1 mRNA expression (for mir-365: P<0.001; for TTF-1: P=0.002. Figures 4 and 5). More importantly, there was a trend toward the worst overall survival in the patient group with combined low mir-365 expression and high TTF-1 expression (P<0.001, Figure 6).
Figure 4.
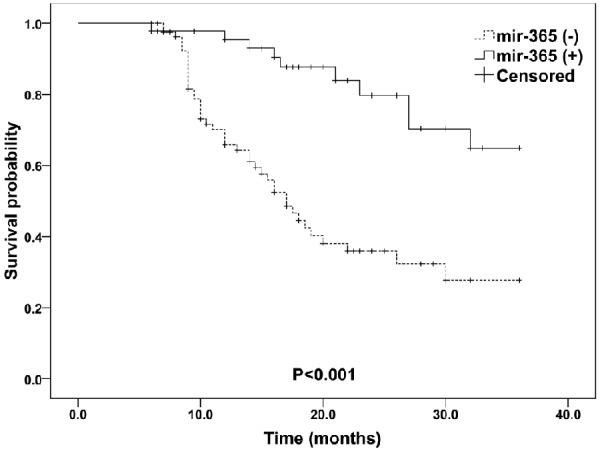
Kaplan-Meier curves for overall survival according to mir-365 expression levels in patients with NSCLC.
Figure 5.
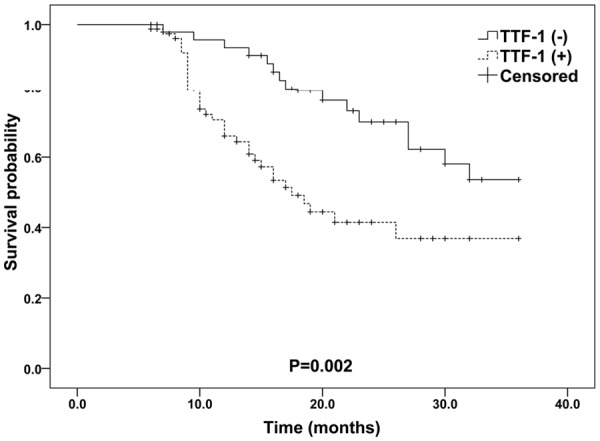
Kaplan-Meier curves for overall survival according to TTF-1 mRNA expression levels in patients with NSCLC.
Figure 6.
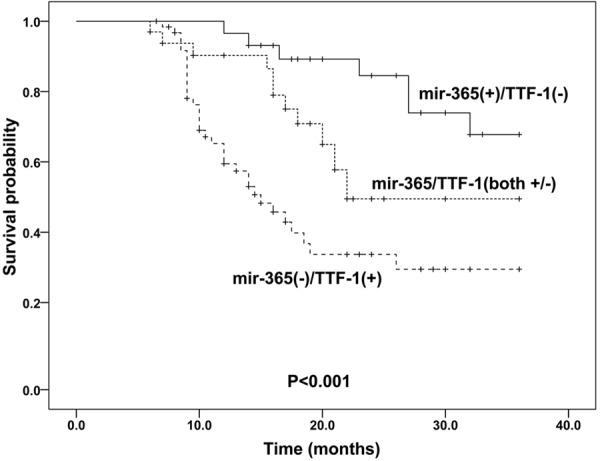
Kaplan-Meier curves for overall survival according to mir-365 and TTF-1 mRNA expression levels in patients with NSCLC.
Discussion
Biological prognostic factors are very helpful to guide decision-making and personalized treatment for NSCLC patients. Although some serologic biomarkers such as carcino-embryonic antigen (CEA), alpha fetoprotein (AFP), CA125 could provide some clinical instructions for physicians. Effective tissue-based biomarkers which is more intuitive to reflect the real condition in vivo is more appealing. The identification of biomarkers which is involved in the initiation and progression of NSCLC is very important to improve the outcome of the patients. The aim of the present study was to clarify the clinical significance of mir-365 and its target gene TTF-1. Our results showed there was a close correlation between the ectopic expressions of mir-365 and TTF-1 and TNM stage and lymph node metastasis. Moreover, aberrant expression levels of mir-365 and TTF-1 may be predictive markers for NSCLC survival. In detail, downregulation of mir-365 and or upregulation of TTF-1 may be used as prognosis factors which promote cancer progression. Our current study is the first study to explore the predictive role of mir-365 and TTF-1 in NSCLC patients.
Mir-365 is broadly conserved among vertebrates. It modulates several biological processes, such as cell proliferation, differentiation, cell cycle and apoptosis. The role of mir-365 in the tumor progression is debating. Mir-365 exerts oncogenic functions in some cancers such as cutaneous squamous cell carcinoma (CSCC) [21], pancreatic cancer [22] and breast cancer . However, mir-365 considered as a tumor suppressor in some other cancers. In both colon and gastric cancers, decreased mir-365 could prohibit cell cycle progression and induce apoptosis by target Cyclin D1 and Bcl-2 [12,23]. In lung cancer cells, it was demonstrated that mir-365 was decreased while TTF-1 was increased, it was showed that mir-365 expression could downregulate TTF-1 [13,17]. These findings suggest that deregulation of mir-365, which is involved in tumor’s progression and apoptosis, may play a role in cancer cell growth and affect patients’ survival. In our current study, mir-365 was observed to be decreased in tumor tissue and was significantly correlated with poor survival in NSCLC patients.
Thyroid transcription factor 1 (TTF-1), encoded by NKX2 homeobox 1 (NKX2-1) gene which is located on chromosome 14q13. TTF-1, belongs to the NKX2 family, is a 38 kDa nuclear protein with 371 amino acids [24]. TTF-1 has a unique expression pattern, which is specifically restricted to the thyroid and lung tissue during embryogenesis. TTF-1 activates the promoters of the lung-specific gene which encode for surfactant and Clara cell secretory proteins, thus plays a crucial role in modulating lung development, surfactant homeostasis and morphogenesis [25]. TTF-1 could modulate the activity of the proliferating cells and formation of new vessels, and enhance the proliferation rate, therefore, it was considered to be involved in lung cancerization and progression. Till now, most findings supported the oncogenic role of TTF-1, however, some findings showed that TTF-1 also exerted biological and clinical functions as a tumor suppressor. Several studies have evaluated TTF-1 as a potential predictive marker in NSCLC with contradictory. Our present study showed that TTF-1 overexpression in mRNA level was significantly associated with an unfavorable prognostic factor for survival in patients with lung cancer.
Previous studies by Qi and Kang demonstrated that TTF-1 is a direct target gene of mir-365 in lung cancer cells and the expression levels of mir-365 and TTF-1 were in a negative correlation in human lung cancer [13,17]. Moreover, aberrant mir-365 expression suppressed NKX2-1 expression and the inhibitory effect of mir-365 on cell proliferation could be significantly reversed by overexpression of TTF-1 in lung cancer cells. Based on the above findings, we made a hypothesis that mir-365 and TTF-1 may be associated with the progression of lung cancer, and the predictive value of combination of mir-365 and TTF-1 (mir-365/TTF-1) would be better than mir-365 or TTF-1 alone. The aim of the present study was to validate the hypothesis, associations between expression levels of mir-365/TTF-1 combination, mir-365 and TTF-1 and clinical parameters and survival of patients with NSCLC were investigated. Expression patterns of mir-365/TTF-1 combination, mir-365 and TTF-1 were strong prognostic indicators for NSCLC patients. Of note, it seemed that downregulation of mir-365 and upregulation of TTF-1 might be a strongest prognostic marker in NSCLC patients, which indicated that comprehensive assessment of both mir-365 and TTF-1 should be applied in personalized treatment decision. Additional studies are required to verify our current findings in a large-scale and independent cohort. Besides, the functions and roles of mir-365/TTF-1 in the progression of NSCLC are needed to further studied.
Taken together, our data suggests the aberrant expression of mir-365/TTF-1 may be involved in the tumor development and associated with survival in patients with NSCLC. Moreover, mir-365 and TTF-1 could synergistically predict the prognosis of patients and their combination may serve as a biomarker to predict risk of poor survival in NSCLC patients. Mir-365/TTF-1 might serve as a promising therapeutic target for clinical treatment of NSCLC.
Acknowledgements
This work was partly supported by Postdoctoral Science Foundation of China (No.2014M552461). We would like to thank all the participants and staff of Shaanxi Tumor Hospital, for their valuable contributions on collecting specimens and sorting out clinical data.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Zhao E, Li C, Huang L, Xiao L, Cheng L, Huang X, Song Y, Xu D. TRIM28, a new molecular marker predicting metastasis and survival in early-stage non-small cell lung cancer. Cancer Epidemiol. 2013;37:71–78. doi: 10.1016/j.canep.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Ni S, Xu L, Huang J, Feng J, Zhu H, Wang G, Wang X. Increased ZO-1 expression predicts valuable prognosis in non-small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2887–2895. [PMC free article] [PubMed] [Google Scholar]
- 4.Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 5.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Zhao JJ, Chen PJ, Duan RQ, Li KJ, Wang YZ, Li Y. Up-regulation of miR-630 in clear cell renal cell carcinoma is associated with lower overall survival. Int J Clin Exp Pathol. 2014;7:3318–3323. [PMC free article] [PubMed] [Google Scholar]
- 7.Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. microRNAs in cancer management. Lancet Oncol. 2012;13:e249–258. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z, Liu J, Wang C, Wang Y, Jiang Y, Guo M. MicroRNA-25 regulates small cell lung cancer cell development and cell cycle through cyclin E2. Int J Clin Exp Pathol. 2014;7:7726–7734. [PMC free article] [PubMed] [Google Scholar]
- 9.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 10.Yu SL, Chen HY, Chang GC, Chen CY, Chen HW, Singh S, Cheng CL, Yu CJ, Lee YC, Chen HS, Su TJ, Chiang CC, Li HN, Hong QS, Su HY, Chen CC, Chen WJ, Liu CC, Chan WK, Li KC, Chen JJ, Yang PC. MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell. 2008;13:48–57. doi: 10.1016/j.ccr.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Zhong KZ, Chen WW, Hu XY, Jiang AL, Zhao J. Clinicopathological and prognostic significance of microRNA-107 in human non small cell lung cancer. Int J Clin Exp Pathol. 2014;7:4545–4551. [PMC free article] [PubMed] [Google Scholar]
- 12.Nie J, Liu L, Zheng W, Chen L, Wu X, Xu Y, Du X, Han W. microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting Cyclin D1 and Bcl-2. Carcinogenesis. 2012;33:220–225. doi: 10.1093/carcin/bgr245. [DOI] [PubMed] [Google Scholar]
- 13.Qi J, Rice SJ, Salzberg AC, Runkle EA, Liao J, Zander DS, Mu D. MiR-365 regulates lung cancer and developmental gene thyroid transcription factor 1. Cell Cycle. 2012;11:177–186. doi: 10.4161/cc.11.1.18576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kodahl AR, Lyng MB, Binder H, Cold S, Gravgaard K, Knoop AS, Ditzel HJ. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer: a case control study. Mol Oncol. 2014;8:874–883. doi: 10.1016/j.molonc.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MH, Ham O, Lee SY, Choi E, Lee CY, Park JH, Lee J, Seo HH, Seung M, Min PK, Hwang KC. MicroRNA-365 inhibits the proliferation of vascular smooth muscle cells by targeting cyclin D1. J Cell Biochem. 2014;115:1752–1761. doi: 10.1002/jcb.24841. [DOI] [PubMed] [Google Scholar]
- 16.Qin B, Xiao B, Liang D, Xia J, Li Y, Yang H. MicroRNAs expression in ox-LDL treated HUVECs: MiR-365 modulates apoptosis and Bcl-2 expression. Biochem Biophys Res Commun. 2011;410:127–133. doi: 10.1016/j.bbrc.2011.05.118. [DOI] [PubMed] [Google Scholar]
- 17.Kang SM, Lee HJ, Cho JY. MicroRNA-365 regulates NKX2-1, a key mediator of lung cancer. Cancer Lett. 2013;335:487–494. doi: 10.1016/j.canlet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Berghmans T, Paesmans M, Mascaux C, Martin B, Meert AP, Haller A, Lafitte JJ, Sculier JP. Thyroid transcription factor 1--a new prognostic factor in lung cancer: a meta-analysis. Ann Oncol. 2006;17:1673–1676. doi: 10.1093/annonc/mdl287. [DOI] [PubMed] [Google Scholar]
- 19.Tang X, Kadara H, Behrens C, Liu DD, Xiao Y, Rice D, Gazdar AF, Fujimoto J, Moran C, Varella-Garcia M, Lee JJ, Hong WK, Wistuba II. Abnormalities of the TITF-1 lineage-specific oncogene in NSCLC: implications in lung cancer pathogenesis and prognosis. Clin Cancer Res. 2011;17:2434–2443. doi: 10.1158/1078-0432.CCR-10-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice SJ, Lai SC, Wood LW, Helsley KR, Runkle EA, Winslow MM, Mu D. MicroRNA-33a mediates the regulation of high mobility group AT-hook 2 gene (HMGA2) by thyroid transcription factor 1 (TTF-1/NKX2-1) J Biol Chem. 2013;288:16348–16360. doi: 10.1074/jbc.M113.474643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou M, Zhou L, Zheng L, Guo L, Wang Y, Liu H, Ou C, Ding Z. miR-365 promotes cutaneous squamous cell carcinoma (CSCC) through targeting nuclear factor I/B (NFIB) PLoS One. 2014;9:e100620. doi: 10.1371/journal.pone.0100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamada S, Masamune A, Miura S, Satoh K, Shimosegawa T. MiR-365 induces gemcitabine resistance in pancreatic cancer cells by targeting the adaptor protein SHC1 and pro-apoptotic regulator BAX. Cell Signal. 2014;26:179–185. doi: 10.1016/j.cellsig.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Guo SL, Ye H, Teng Y, Wang YL, Yang G, Li XB, Zhang C, Yang X, Yang ZZ. Akt-p53-miR-365-cyclin D1/cdc25A axis contributes to gastric tumorigenesis induced by PTEN deficiency. Nat Commun. 2013;4:2544. doi: 10.1038/ncomms3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi T, Hosono Y, Yanagisawa K, Takahashi T. NKX2-1/TTF-1: an enigmatic oncogene that functions as a double-edged sword for cancer cell survival and progression. Cancer Cell. 2013;23:718–723. doi: 10.1016/j.ccr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Mu D. The complexity of thyroid transcription factor 1 with both pro- and anti-oncogenic activities. J Biol Chem. 2013;288:24992–25000. doi: 10.1074/jbc.R113.491647. [DOI] [PMC free article] [PubMed] [Google Scholar]


