Abstract
The purpose of this study is to identify significant immune-system related for symptom of patients with prostatic inflammation in order to investigate the etiology of prostatic inflammation which may relate to potentially chronic prostatitis (CP). We investigated the expression of immune system-related biomarkers such as Interleukin (IL) -6 (humoral immunity), CD-3 (T-lymphocyte), and CD-163 (macrophage) in prostate biopsy (PBx) specimens from patients with prostatic inflammation (without cancer) which had been neither clinically diagnosed benign prostatic hyperplasia nor chronic prostatitis. We examined the correlation between these markers’ expressions and the symptom scores using the National Institutes of Health-Chronic Prostatitis Symptom Index (NIH-CPSI), International Prostate Symptom Score (IPSS)/quality of life (QOL) which are the index for lower urinary tract symptoms (LUTS). Our results showed CD-163 (macrophage) reflected pain or discomfort on NIH-CPSI scores (P = 0.0389 and r = 0.3307) in the patients with prostatic inflammation; however, the control patients had no significant correlation between symptom scores and those immune-related markers’ expression. These results suggest that pain or discomfort related to macrophages in the relationship between immune-system and the symptom of prostatic inflammation. In conclusion, CD-163, related to immune-system (macrophage), correlated with symptoms (pain or discomfort) of prostatic inflammation and might represent a significant immune-system related biomarker for pain or LUTS score in potentially CP.
Keywords: CD-163, CD-3, IL-6, prostatic inflammation, symptom scores
Introduction
Chronic prostatitis (CP) is a complex condition in terms of its etiology, patient symptoms and treatments [1]. Nickel identified 5 research emphases for CP: clinical syndrome, etiology/pathogenesis, NIH classification, lower urinary tract evaluation, and treatment [2]. CP is sometimes accompanied by psychiatric characteristics [3] and patient complaints are complicated, making the diagnosis even more difficult. Moreover, CP patients can easily relapse, partly because of the complexity of CP, which has numerous etiological, pathophysiological, endocrine, neurological, and infective factors [4].
The National Institutes of Health-Chronic Prostatitis Symptom Index (NIH-CPSI), developed to assess symptoms and quality of life (QOL) in CP patients [5], has demonstrated good reliability, validity, and responsiveness to change, and it has been used as the primary outcome variable in several large-scale treatments studies of CP [6-8].
Desireddi et al. suggested the possibility that biomarkers related to CP might include chemokines from monocytes or macrophages [9]. CD-3, a T lymphocyte marker, is expressed inflammatory disease along with moderate levels of IL-6 which relates to humoral immunity and a potentially important mediator of this process is CD-163, a macrophage-specific member of the scavenger receptor cysteine-rich family [10]. On the other hand, Macoska stated that specimens with benign prostatic hyperplasia (BPH)/lower urinary tract symptoms (LUTS) showed inflammatory infiltration contained 70% T-lymphocytes, 15% B-cells, and 15% both macrophages and mast cells; therefore, we chose the markers significant for LUTS as mentioned above [11].
In this study, we investigated to identify the correlation of immune-system markers with the symptom of prostatic inflammation in order to identify the occurring events in tissues of potentially CP using the markers, Interleukin (IL) -6 (humoral immunity), CD-3 (as a T-lymphocyte marker), and CD-163 (as a macrophage marker) with NIH-CPSI symptom scores and the International Prostate Symptom Score (IPSS)/QOL score in those patients [12].
Materials and methods
Patients
Data were analyzed from 40 consecutive patients diagnosed with prostate inflammation (without benign prostatic hyperplasia) after a finding of lymphocytic infiltration in prostate biopsy (PBx) specimens from patients with suspected prostate cancer between February 2009 and October 2011 in the Department of Urology, Kobe University Hospital. Ten control cases with no findings of inflammation or cancer in PBx results were also investigated. All these 50 patients had no diagnoses of benign prostatic hyperplasia or clinically chronic prostatitis. The median age was 69 (54-80) in the patients with prostate inflammation and was 69 (54-81) in the control cases (P > 0.05). Data were gathered and analyzed retrospectively. The study was approved by IRB at this institution.
Symptom scores
We evaluated the patients’ symptom as follows: 1) NIH-CPSI scores classified under each symptom such as pain or discomfort, urination, impact of symptom, QOL and total scores. 2) IPSS/QOL classified under each symptom category such as incomplete emptying, diurnal frequency, intermittency, urgency, weak flow, straining to void, frequency at night, subgroups (voided and storage symptoms), and total scores classified under each symptom category such as diurnal frequency, frequency at night, urgency, incontinence and total scores. NIH-CPSI and IPSS/QOL were questioned prior to PBx. The obtained core number of PBx was 12 and prostate inflammation was diagnosed as the fraction of tissue (i.e. grandular, perigrandular and stromal) involved by inflammatory cells as neutrophil and lymphocyte including macrophage [13].
Immunohistochemistry
The expression of potential biomarkers (IL-6, CD-3 and CD-163) was investigated by immunohistochemistry (IHC) using PBx specimens. IHC staining of PBx specimens was performed as previously described [14]. Briefly, formaldehyde-fixed, paraffin-embedded tissue sections cut at 3-5 µm from PBx specimens were deparaffinized by xylene and rehydrated in decreasing concentrations of ethanol. After blocking endogenous peroxidase with 3% hydrogen peroxidase in methanol, sections were boiled in 0.01 M citrate buffer for 10 minutes and incubated with 5% normal blocking serum in Tris-buffered saline for 20 mins. The sections were then incubated with anti-human IL-6 rabbit polyclonal antibody (Santa Cruz Biotechnology, Dallas, Texas, USA), anti-human CD-3 rabbit monoclonal antibody (Epitomics, Burlingame, California, USA), and anti-human CD-163 mouse monoclonal antibody (Abnova, Jhongli, Taiwan). The sections were then incubated with biotinylated goat anti-mouse or rabbit IgG (Vector Laboratories, Burlingame, California, USA). After incubation in avidin-biotin peroxidase complex for 30 mins, the samples were exposed to diaminobenzidine tetrahydrochloride solution and counterstained with methyl green (Vector Laboratories) or hematoxylin. Negative controls (absence of primary antibody) were included for each antibody.
IHC staining results were scored by two observers who were blinded to the data from the NIH-CPSI and IPSS/QOL. For all the potential biomarkers tested (IL-6, CD-3, and CD-163), cytoplasmic staining was considered as positive and if more than 10% of the cells were stained, gene expression was considered as positive. Staining was scored as follows: (0): one or little staining in < 10% of cells, (1+): faint, partial staining in > 10% of cells, (2+): weak to moderate, complete staining in > 10% of cells, (3+): strong, complete membrane staining in > 10% of cells [15] as shown in Figure 1.
Figure 1.
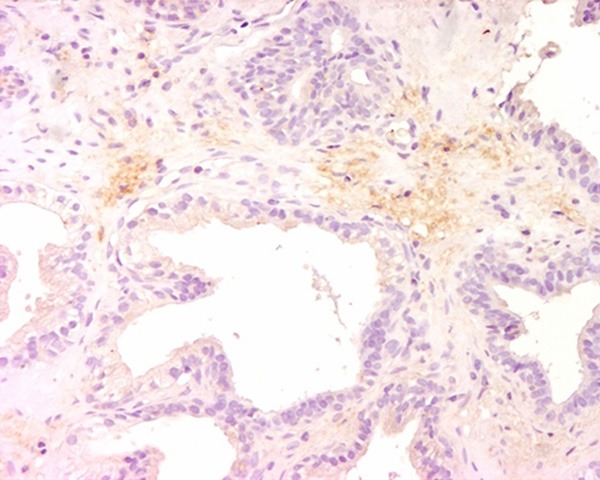
Typical outcomes of immunohistochemical (IHC) staining of prostatic inflammation specimens with IL-6 antibody were shown. Strong cytoplasmic expression of IL-6 was shown. The magnification power was 200 times.
In order to detect biomarkers for potentially CP, we investigated the protein expressions of IL-6, CD-3 and CD-163 in PBx specimens. We statistically correlated the expressions of these markers with symptoms using NIH-CPSI scores and IPSS/QOL as mentioned above especially in each symptom or each symptom group such as storage or voiding symptom.
Statistical analyses
Statistical analysis was conducted with the JSTAT-Java Virtual Machine Statistics Monitoring Tool (Sun Microsystems, Inc., Santa Clara, California, USA) using Student’s t-test and the Spearman rank-order correlation for the correlation of the potential biomarkers (IL-6, CD-3, and CD-163) with CP-related symptoms such as pain or LUTS. Statistical significance was established at P < 0.05.
Results
Symptom scores (NIH-CPSI and IPSS/QOL)
The patients with prostatic inflammation had NIH-CPSI scores of 4.15 ± 3.56 (control patients without prostatic inflammation: 0), 4.40 ± 2.59 (control: 3.00 ± 1.55), 3.05 ± 1.88 (control: 1.70 ± 0.64), 3.63 ± 1.56 (control: 2.80 ± 0.75) and 15.44 ± 8.12 (control: 7.50 ± 2.25) in pain or discomfort, urination, impact of symptom, QOL and total score, respectively (Tables 1 and 3).
Table 1.
Correlation (p-value) of significant immune-system related biomarkers for NIH-CPSI scores in the patients with prostate inflammation
| pain or discomfort | urination | Impact of symptom | QOL | total | |
|---|---|---|---|---|---|
|
|
|||||
| scores | 4.15 ± 3.56 | 4.40 ± 2.59 | 3.05 ± 1.88 | 3.63 ± 1.56 | 15.44 ± 8.12 |
| IL-6 | n.s.* | n.s. | n.s. | n.s. | n.s. |
| CD-3 | n.s. | n.s. | n.s. | n.s. | n.s. |
| CD-163 | 0.0389 ** (r = 0.3307) | n.s. | n.s. | n.s. | n.s. |
n.s.: not significant.
bold: statistically significant.
Table 3.
Correlation (p-value) of significant immune-system related biomarkers for NIH-CPSI scores in the control patients
| pain or discomfort | urination | Impact of symptom | QOL | total | |
|---|---|---|---|---|---|
| scores | 0 | 3.00 ± 1.55 | 1.70 ± 0.64 | 2.80 ± 0.75 | 7.50 ± 2.25 |
| IL-6 | n.s.* | n.s. | n.s. | n.s. | n.s. |
| CD-3 | n.s. | n.s. | n.s. | n.s. | n.s. |
| CD-163 | n.s. | n.s. | n.s. | n.s. | n.s. |
n.s.: not significant.
They had IPSS scores of 1.93 ± 1.53 (control: 1.40 ± 0.80), 2.60 ± 1.41 (control: 1.60 ± 1.20), 2.15 ± 1.55 (control: 0.80 ± 0.87), 2.20 ± 1.60 (control: 0.70 ± 0.64), 2.88 ± 1.34 (control: 1.40 ± 0.80), 1.68 ± 1.53 (control: 0.60 ± 0.66), 2.28 ± 1.11 (control: 1.40 ± 0.66), 6.70 ± 3.82 (control: 2.80 ± 2.04), 9.10 ± 4.50 (control: 5.00 ± 2.28) and 15.83 ± 7.90 (control: 7.80 ± 3.63) in incomplete emptying, diurnal frequency, intermittency, urgency, weak flow, straining to void, frequency at night, voiding symptoms, storage symptoms and total score, respectively, and 3.73 ± 1.52 (control: 2.80 ± 0.75) in QOL (Tables 2 and 4).
Table 2.
Correlation (p-value) of significant immune-system-related for IPSS/QOL in the patients with prostate inflammation
| incomplete emptying | diurnal frequency | intermittency | urgency | weak flow | straining to void | |
|
|
||||||
| scores | 1.93 ± 1.53 | 2.60 ± 1.41 | 2.17 ± 1.55 | 2.20 ± 1.60 | 2.88 ± 1.34 | 1.68 ± 1.53 |
|
| ||||||
| IL-6 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| CD-3 | n.s. | 0.0387 ** (r = -0.3310) | n.s. | n.s. | n.s. | n.s. |
| CD-163 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
|
| ||||||
| frequency at night | voided symptoms*** | storage symptoms**** | Total | QOL | ||
|
|
||||||
| scores | 2.28 ± 1.11 | 6.70 ± 3.82 | 9.10 ± 4.50 | 15.83 ± 7.90 | 3.73 ± 1.52 | |
|
| ||||||
| IL-6 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CD-3 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CD-163 | n.s. | n.s. | n.s. | n.s. | n.s. | |
*n.s.: not significant.
bold: statistically significant.
voided symptoms consist of intermittency, weak flow, and straining to void.
storage symptoms consist of incomplete emptying, diurnal frequency, urgency, and nocturia.
Table 4.
Correlation (p-value) of significant immune-system-related for IPSS/QOL in the control patients
| incomplete emptying | diurnal frequency | intermittency | urgency | weak flow | straining to void | |
|
|
||||||
| score | 1.40 ± 0.80 | 1.60 ± 1.20 | 0.80 ± 0.87 | 0.70 ± 0.64 | 1.40 ± 0.80 | 0.60 ± 0.66 |
|
| ||||||
| IL-6 | n.s.* | n.s. | n.s. | n.s. | n.s. | n.s. |
| CD-3 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| CD-163 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
|
| ||||||
| frequency at night | voided symptom** | storage symtoms*** | Total | QOL | ||
|
|
||||||
| score | 1.40 ± 0.66 | 2.80 ± 2.04 | 5.00 ± 2.28 | 7.80 ± 3.63 | 2.80 ± 0.75 | |
|
| ||||||
| IL-6 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CD-3 | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CD-163 | n.s. | n.s. | n.s. | n.s. | n.s. | |
n.s.: not significant.
voided symptoms consist of intermittency, weak flow, and straining to void.
storage symptoms consist of incomplete emptying, diurnal frequency, urgency, and nocturia.
Correlation between potential biomarker expression and symptom scores
Our statistical data showed that in our tested immune-related markers, CD-163 significantly reflected pain or discomfort on NIH-CPSI scores (P = 0.0389 and r = 0.3307) and CD-3 had a negatively significant impact on diurnal frequency on IPSS scores (P = 0.0387 and r = -0.3310) in the patients with prostatic inflammation. There was no significant correlation between any of these 3 markers’ expressions and NIH-CPSI and IPSS/QOL in the 10 control patients with no inflammation (P > 0.05) (Tables 1, 2, 3 and 4, Figures 1, 2, 3, 4, 5 and 6).
Figure 2.
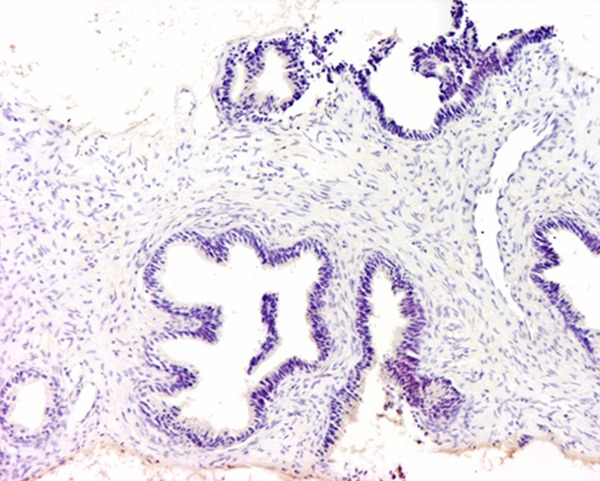
Weak expression of IL-6 was demonstrated. The magnification power was 100 times.
Figure 3.
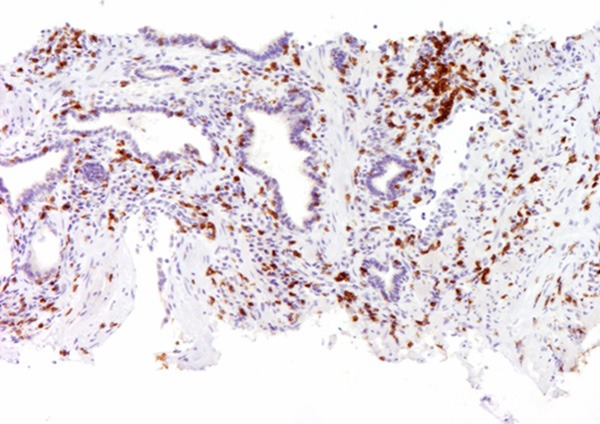
Typical outcomes of IHC staining of prostatic inflammation specimens with CD-3 antibody were shown. Strong cytoplasmic expression of CD-3 was shown. The magnification power was 100 times.
Figure 4.
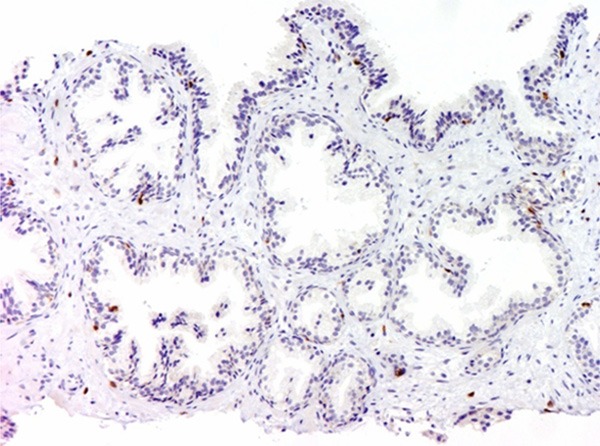
Weak expression of CD-3 was revealed. The magnification power was 100 times.
Figure 5.
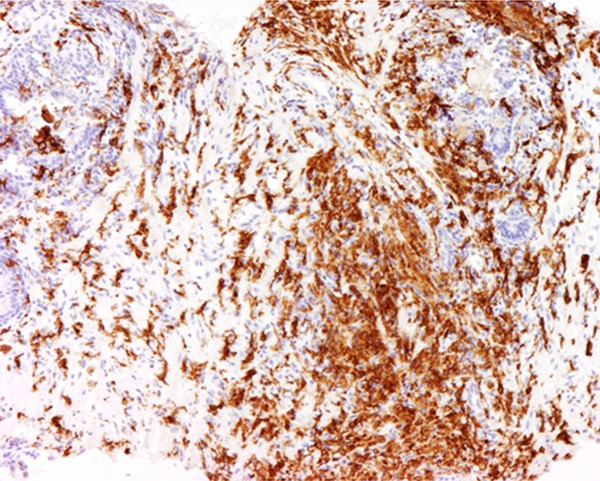
Typical outcomes of IHC staining of prostatic inflammation specimens with CD-163 antibody were shown. Strong cytoplasmic expressions of CD-163 were shown. The magnification power was 200 times.
Figure 6.
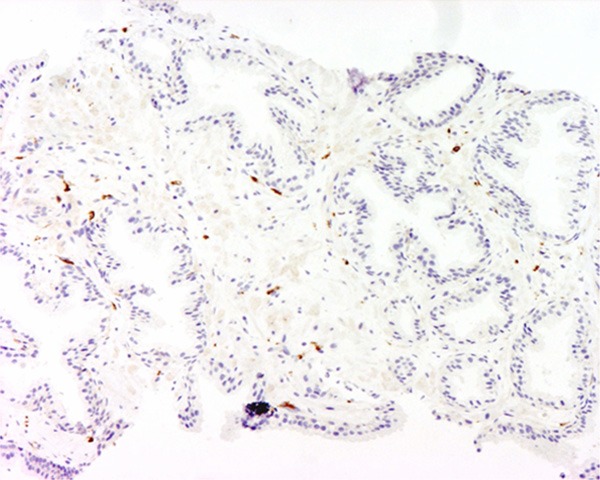
Weak expression of CD-163 was demonstrated. The magnification power was 100 times.
Discussion
CP is notorious for its complex symptoms and etiology and its pathogenesis is not fully understood [16,17]. A possible explanation for the deferred pain in CP is that the pathogenesis of CP involves inflammation initiator stimulus with allodynia and hyperalgesia [18]. CP may have inflammatory or non-inflammatory etiologies [4,19-21]. We therefore planned the histological evaluation of potentially CP patients using the markers relating to immune-systems for the following reasons: 1) The difficulty in CP diagnosis may result from heterogeneous etiologies and symptoms [22]; 2) Immunologic factors may play a role in the development and maintenance of symptoms from prostatic inflammation [23]; 3) The histological changes in CP or potentially CP are relatively well unknown or undetermined. True et al. reported that some patients with prostatic inflammation had more than one pattern of inflammation involving glandular, periglandular and multifocal or diffuse distribution of leukocytes in the stroma on prostate histopathology. They stated the need to reevaluate the current concepts about the pathophysiology of CP. As the study of Shimoura et al. showed that some prostate tissues in PBx revealed various inflammatory findings [24], we used the specimens taken during PBx to assess histopathological changes, not the values for instance which can be detected by semen or expressed prostatic secretion depending on the patient’s general condition or technical problem for taking samples.
Regarding symptom scores in CP, Anothaisintawee et al. reviewed the studies using the NIH-CPSI and IPSS/QOL as an outcome assessment tool [4,7,8]. We accordingly used the symptom scores from the NIH-CPSI and IPSS/QOL for the detailed evaluation of patients’ symptoms, assessed for each subgroup and each item from these symptom score sheets including pain, urination, impact of symptoms, and QOL in the NIH-CPSI and voiding and storage symptoms in the IPSS. Such detailed category and symptom evaluations, when correlated with potentially significant markers, may be informative for understanding and evaluating which biomarker relates to prostate inflammation-related symptoms.
Several studies have searched for significant markers for CP or prostatic inflammation. Khadra et al. showed that IL-8 level was significantly correlated with pain score, urinary symptom score, and QOL impact score in CP patients [1]. Miller et al. revealed that cytokines regulating inflammation (IL-6 and IL-10) may play a role in the pain symptoms experienced by patients with CP [25]. Other significant markers for prostatic inflammation in BPH patients were reported by Robert et al. who stated that T-lymphocyte markers such as CD-8 or CD-3 were highly expressed, while CD-20, a marker of B-lymphocytes, was expressed in about half of their patients [26]. John et al. performed IHC detection of CD-3 and CD-20 expressing cells in 71 biopsy cylinders from CP patients [27]. The IPSS score was higher (21 versus 12; P = 0.02) in the high grade inflammation group compared to the low grade group. Our data, in the viewpoints of immune-system and prostatic inflammation, showed that only CD-163 significantly correlated with pain or discomfort in the NIH-CPSI, CD-3 negatively correlated with diurnal frequency in the IPSS/QOL but IL-6 had no positive impact on symptom scoring both NIH-CPSI and IPSS/QOL. The control patients without histological finding of prostatic inflammation had no correlation between these 3 immune system-related markers and these symptom scores. Maake et al stated that CD-4 became predominant over CD-3 in prostatic low inflammation [28]. These findings suggest that macrophage has potential influence on CP-related symptoms and could have a possibility to be a therapeutic target especially in the intractable CP cases.
We would like to emphasize the limitations of this study. First, the number of patients is not large enough to draw definite conclusions. Secondly, we tested only 3 potential significant immune-system related markers and the IHC staining method was not quantitative. Third, we did not examine the relationship between these markers and therapeutic effects. These limitations will be addressed in our future work.
In conclusions, this study showed that CD-163 (macrophage) expressions were significantly related to the symptom (pain or discomfort) in NIH-CPSI score in the patients with prostatic inflammation who may be considered to have potentially CP. These results contribute to the research to understand the etiology of CP histologically and suggest the possibility of diagnosing CP by the use of these immune-system related markers.
Disclosure of conflict of interest
None.
References
- 1.Khadra A, Fletcher P, Luzzi G, Shattock R, Hay P. Interleukin-8 levels in seminal plasma in chronic prostatitis/chronic pelvic pain syndrome and nonspecific urethritis. BJU Int. 2006;97:1043–6. doi: 10.1111/j.1464-410X.2006.06133.x. [DOI] [PubMed] [Google Scholar]
- 2.Nickel CJ. Prostatitis syndromes: an update for urologic practice. Can J Urol. 2000;7:1091–1098. [PubMed] [Google Scholar]
- 3.Fishbain DA, Cole B, Cutler RB, Lewis J, Rosomoff HL, Rosomoff RS. Chronic pain and the measurement of personality: do states influence traits? Pain Med. 2006;7:509–529. doi: 10.1111/j.1526-4637.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 4.Anothaisintawee T, Attia J, Nickel JC, Thammakraisorn S, Numthavaj P, McEvoy M, Thakkinstian A. Management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis. JAMA. 2011;305:78–86. doi: 10.1001/jama.2010.1913. [DOI] [PubMed] [Google Scholar]
- 5.Litwin MS, McNaughton-Collins M, Fowler FJ Jr, Nickel JC, Calhoun EA, Pontari MA, Alexander RB, Farrar JT, O’Leary MP. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. J Urol. 1999;162:369–375. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 6.Propert KJ, Litwin MS, Wang Y, Alexander RB, Calhoun E, Nickel JC, O’Leary MP, Pontari M, McNaughton-Collins M Chronic Prostatitis Collaborative Research Network (CPCRN) Responsiveness of the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) Qual Life Res. 2006;15:299–305. doi: 10.1007/s11136-005-1317-1. [DOI] [PubMed] [Google Scholar]
- 7.Alexander RB, Propert KJ, Schaeffer AJ, Landis JR, Nickel JC, O’Leary MP, Pontari MA, McNaughton-Collins M, Shoskes DA, Comiter CV, Datta NS, Fowler JE Jr, Nadler RB, Zeitlin SI, Knauss JS, Wang Y, Kusek JW, Nyberg LM Jr, Litwin MS Chronic Prostatitis Collaborative Research Network. Ciprofloxacin or tamsulosin Figure 6. Weak expression of CD-163 was demonstrated. The magnification power was 100 times. in men with chronic prostatitis/chronic pelvic pain syndrome: a randomized, double-blind trial. Ann Intern Med. 2004;141:581–589. doi: 10.7326/0003-4819-141-8-200410190-00005. [DOI] [PubMed] [Google Scholar]
- 8.Nickel JC, Krieger JN, McNaughton-Collins M, Anderson RU, Pontari M, Shoskes DA, Litwin MS, Alexander RB, White PC, Berger R, Nadler R, O’Leary M, Liong ML, Zeitlin S, Chuai S, Landis JR, Kusek JW, Nyberg LM, Schaeffer AJ Chronic Prostatitis Collaborative Research Network. Alfuzosin and symptoms of chronic prostatitis-chronic pelvic pain syndrome. N Engl J Med. 2008;359:2663–2673. doi: 10.1056/NEJMoa0803240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desireddi NV, Campbell PL, Stern JA, Sobkoviak R, Chuai S, Shahrara S, Thumbikat P, Pope RM, Landis JR, Koch AE, Schaeffer AJ. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008;179:1857–1861. doi: 10.1016/j.juro.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macoska JA. Chemokines and BPH/LUTS. Differentiation. 2011;82:253–260. doi: 10.1016/j.diff.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CS, Chang PJ, Lin WY, Huang YC, Ho DR. Evidences of the inflammasome pathway in chronic prostatitis and chronic pelvic pain syndrome in an animal model. Prostate. 2013;73:391–397. doi: 10.1002/pros.22580. [DOI] [PubMed] [Google Scholar]
- 13.Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 14.Kurahashi T, Miyake H, Hara I, Fujisawa M. Expression of major heat shock proteins in prostate cancer: correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. J Urol. 2007;177:757–761. doi: 10.1016/j.juro.2006.09.073. [DOI] [PubMed] [Google Scholar]
- 15.Huang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, Berteloot P, Amant F, Vergote I, Christiaens MR. Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancer. J Clin Pathol. 2005;58:611–616. doi: 10.1136/jcp.2004.022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M, Adorini L. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51:524–533. doi: 10.1016/j.eururo.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 17.McNaughton Collins M, MacDonald R, Wilt TJ. Diagnosis and treatment of chronic abacterial prostatitis: a systematic review. Ann Intern Med. 2000;133:367–381. doi: 10.7326/0003-4819-133-5-200009050-00013. [DOI] [PubMed] [Google Scholar]
- 18.Pontari MA, Ruggieri MR. Mechanisms in prostatitis/chronic pelvic pain syndrome. J Urol. 2004;172:839–845. doi: 10.1097/01.ju.0000136002.76898.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hetrick DC, Ciol MA, Rothman I, Turner JA, Frest M, Berger RE. Musculoskeletal dysfunction in men with chronic pelvic pain syndrome type III: a case-control study. J Urol. 2003;170:828–31. doi: 10.1097/01.ju.0000080513.13968.56. [DOI] [PubMed] [Google Scholar]
- 20.Hochreiter WW, Nadler RB, Koch AE, Campbell PL, Ludwig M, Weidner W, Schaeffer AJ. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology. 2000;56:1025–1029. doi: 10.1016/s0090-4295(00)00844-x. [DOI] [PubMed] [Google Scholar]
- 21.Shoskes DA, Nickel JC, Rackley RR, Pontari MA. Clinical phenotyping in chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis: a management strategy for urologic chronic pelvic pain syndromes. Prostate Cancer Prostatic Dis. 2009;12:177–183. doi: 10.1038/pcan.2008.42. [DOI] [PubMed] [Google Scholar]
- 22.Pontari MA. Etiologic theories of chronic prostatitis/chronic pelvic pain syndrome. Curr Urol Rep. 2007;8:307–312. doi: 10.1007/s11934-007-0077-6. [DOI] [PubMed] [Google Scholar]
- 23.True LD, Berger RE, Rothman I, Ross SO, Krieger JN. Prostate histopathology and the chronic prostatitis/chronic pelvic pain syndrome: a prospective biopsy study. J Urol. 1999;162:2014–2018. doi: 10.1016/S0022-5347(05)68090-1. [DOI] [PubMed] [Google Scholar]
- 24.Shimomura T, Kiyota H, Takahashi H, Madarame J, Kimura T, Onodera S. Prostate histopathology of NIH category IV prostatitis detected by sextant prostate needle biopsy from the patients with high prostatic specific antigen. Kansenshogaku Zasshi. 2003;77:611–617. doi: 10.11150/kansenshogakuzasshi1970.77.611. [DOI] [PubMed] [Google Scholar]
- 25.Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, Kreutzer DL. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002;59:603–608. doi: 10.1016/s0090-4295(01)01597-7. [DOI] [PubMed] [Google Scholar]
- 26.Robert G, Descazeaud A, Nicolaïew N, Terry S, Sirab N, Vacherot F, Maillé P, Allory Y, de la Taille A. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate. 2009;69:1774–1780. doi: 10.1002/pros.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John H, Maake C, Barghorn A, Zbinden R, Hauri D, Joller-Jemelka HI. Immunological alterations in the ejaculate of chronic prostatitis patients: clues for autoimmunity. Andrologia. 2003;35:294–299. [PubMed] [Google Scholar]
- 28.Maake C, John H. Prostatitis versus pelvic pain syndrome: immunologic studies. Curr Urol Rep. 2003;4:327–334. doi: 10.1007/s11934-003-0094-z. [DOI] [PubMed] [Google Scholar]


