Abstract
Endometrium modulated by estrogen (E) and progesterone (P) is important for implantation and pregnancy. The present study compared the expression of chemokine CXCL12 and chemokine receptor CXCR4 and CXCR7 between human cycling and early pregnant endometria by immunohistochemistry (IHC). Then the modulation of E and P on expression of CXCL12, CXCR4 and CXCR7 in human endometrial stromal cells (ESCs) was explored at both mRNA and protein level. The result of IHC showed that human ESCs of the menstrual period did not express CXCL12, CXCR4 or CXCR7 protein, however, the expression of CXCR4 and CXCR7 but not CXCL12 in ESCs increased in the proliferative and secretory phase, and the expression intensity for CXCR4 and CXCR7 in ESCs was the highest in the first trimester. Moreover, E and P were able to up-regulate the mRNA and protein expression of CXCR4 and protein expression of CXCR7 in ESCs (P<0.01). Thus, ESCs spatiotemporally co-express CXCR4 and CXCR7 rather than CXCL12, and E and P are able to regulate the expression of CXCR4 and CXCR7 in ESCs, suggesting the modulation of steroid hormones on chemokine receptor expression in ESCs.
Keywords: Endometrial stromal cells, chemokine/chemokine receptor, steroidal hormone
Introduction
As one of the most important medical events in the 20th century, the worldwide application of assisted reproductive technology (ART) in clinical practice has greatly changed the fate of many infertile couples. However, the implantation rate (IR) in fresh ART cycles always hovers at 30%, despite of the advancements in ovarian stimulation protocol and laboratory technology [6]. Especially, there are still a considerable proportion of refractory infertilities suffering from recurrent implantation failure (RIF) or recurrent miscarriage (RM), indicating implantation rather than fertilization is a rate-limiting factor in fertility [16,22].
Although successful implantation acquires the delicate co-operation between the embryo and mother, the initiation of a receptive endometrium is of maternal origin [2,22]. In normal reproductive cycle, the endometrium undergoes luteinization and develops towards a receptive milieu under the modulation of steroid hormones [16]. Normal endometrial stromal cells (ESCs) even function as embryo biosensors and selectors via recognizing the developmentally impaired embryos and inhibiting the secretion of some key implantation mediators [23]. Impaired ESCs disturbs the responses of endometrium to embryonic signals and causes RM [19]. Although many proteins and molecules have been identified in the endometrium [22,24,28], our knowledge of their function and modulation of steroid hormones on pregnancy-related molecules still remains poorly defined.
Chemokine (C-X-C motif) ligand 12 and chemokine (C-X-C motif) receptor 4 (CXCL12/CXCR4) have attracted much attention because of their distinctive functions in several crucial reproductive biology processes, including uterine Natural Killer cell recruitment, placentation, implantation and embryogenesis [5,8,17,27,29]. A recent study even found that CXCL12 is expressed in human mature oocytes and mediates the intracellular calcium influx and hyperactivation of spermatozoa [32]. Our previous researches also reveal the importance of CXCL12/CXCR4 axis in the interaction between trophoblasts (TCs) and decidualized ESCs during human first trimester pregnancy [17,29]. However, our knowledge regard to the spatiotemporal expression of CXCL12/CXCR4 in human cycling endometrium, especially in the implantation period, is still very limited. Moreover, the traditional viewpoint on the exclusive interaction of CXCL12 with CXCR4 has been challenged since the identification of CXCR7. CXCR7 can bind to CXCL12 with exceptionally high affinity and provide cells with a survival and adhesion advantage [3]. Two studies have reported the expression of CXCR7 in human TCs in different stages of pregnancy [21,25]. As a novel receptor for CXCL12, there are many puzzles on the expression pattern and function of CXCR7 at the materno-fetal interface.
In the present study, we compared the expression pattern of CXCL12, CXCR4 and CXCR7 between human cycling and early pregnant endometria. Then the modulation of steroid hormones, estrogen (E) and progesterone (P), on the expression of CXCL12, CXCR4 and CXCR7 in human ESCs was explored by reverse transcription-polymerase chain reactions (RT-PCR), in cell western, flow cytometry (FCM) and enzyme-linked immunosorbent assay (ELISA), respectively. We hope this investigation is beneficial to better understanding the modulation of steroid hormones on the expression of pregnancy related key molecules in endometrium, which will shed lights on the mechanisms underlying the preparation of endometrium for the arrival of the embryo and provide new insights for endometrium-related infertility.
Materials and methods
Tissue collection
Human endometrial and villous tissues were collected from patients who accepted total abdominal hysterectomy, total laparoscopic hysterectomy or artificial abortion at the Obstetrics and Gynecology Hospital of Fudan University or Beijing Chaoyang Hospital of Capital Medical University from 2011 to 2013. The recruitment criteria included a normal pregnancy history, regular menstrual cycle and without any hormone treatment within the last three months. The exclusion criteria were irregular menstrual cycle, endometrial diseases or incomplete data. The samples were collected into ice-cold DMEM/F12 (Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12; Gibco), then immediately transported to the laboratory and separated for paraffin embedding or ESCs isolation. The study was approved by the Human Research Ethics Committee of the Obstetrics and Gynecology Hospital and Beijing Chaoyang Hospital. All participants completed an informed consent to the collection of tissue samples.
Isolation and culture of human ESCs
ESCs were isolated according to our previous method [17,29]. With this method we obtained highly purified ESCs and the detailed information on the purity and characteristics of ESCs is displayed in our previous publication [29].
Immunohistochemistry
IHC was performed according to our previous method [17,29]. The antibodies used in this experiments were rabbit anti-human CXCR4 (1:50), CXCR7 (1:25) or CXCL12 (1:50) (Abcam). Human first-trimester villi were used as positive control. The corresponding non-immune rabbit antibody was also used as negative control. The immunostaining intensity was observed and scored according to our previous research [11,12]. The experiments were performed with twenty-one endometrial and seven decidual samples.
Immunofluorescence cell staining
Indirect immunofluorescence was used to detect the expression and position of CXCR7 and CXCR4 in ESCs [17,29]. The isolated ESCs were seeded on 6-well plates which were pre-coated with cover glasses. At 80-90% confluence, the cells were fixed in 4% polyformalin and treated with 0.2% Triton X-100. Then they were blocked with 10% FCS at room temperature for 30 min and incubated overnight at 4°C with goat anti-human CXCR4 (1:60, 16.67 μg/L) or rabbit anti-human CXCR7 (1:60, 16.67 μg/L) antibody (Abcam). After being thoroughly rinsed, the cells were incubated with fluorescein isothiocyanate (FITC) conjugated donkey anti-goat immunoglobulin (Ig) G (1:100, 10 μg/L) or Texas Red conjugated donkey anti-rabbit IgG (1:100, 10 μg/L) (Rockland) at room temperature for 60 min in darkness. After counterstain with 4’, 6-diamidino-2-phenylindole (DAPI), the results were observed under fluorescence microscope. The experiments were repeated three times.
Reverse transcription-polymerase chain reactions
ESCs in mid-log growth were digested with 0.25% trypsin and seeded at a density of 1×106 cells/ml on 6-well plates. At 80-90% confluence, the cells were starved with DMEM/F12 without FCS for 12 h, then treated with vehicle (DMEM/F12 with 2% stripped FCS), E (10-11-10-6 M) or P (10-11-10-6 M) for 24 h. Total cellular RNA of the cells was extracted using RNAiso plus (Takara) based on the instruction by the manufacture. Then the first strand cDNA was synthesized with the usage of First Strand cDNA Synthesis Kit (Fermentas), and polymerase chain reactions (PCR) (TIANGEN) were performed in 5 min precycle at 94°C, then followed by 35 cycles of 30 sec at 94°C, 15 sec at 60°C, and 45 sec at 72°C. When the final cycle was over, samples were kept at 72°C for 10 min to complete the synthesis [29]. Primer pairs for cDNA amplification were as follows: 5’-ATT CAC ATC TAA CCT CAT CTT CTT CAC-3’ (sense) and 5’-TGA CTT ACT CAC ATA GCA CAT TG-3’ (antisense) for human CXCL12; 5’-GGT GGT ATG TTG GCG TCT G-3’ (sense) and 5’-ATA GCA GGA CAG GAT GAC AAT ACC-3’ (antisense) for human CXCR4; 5’-AGA AGA CAG CGA CAA TGG AGA AGG-3’ (sense) and 5’-TGC CTG ACA CCT ACT ACC TGA AG -3’ (antisense) for human CXCR7; 5’-GCT GTG CTA CGT CGC CCT G-3’ (sense) and 5’-GGA GGA GCT GGA AGC AGC C-3’ (antisense) for β-actin. The expected fragment lengths for CXCL12, CXCR4, CXCR7 and β-actin were 155, 194, 164 and 66 base pairs (bp), respectively. The PCR reaction products of 5 μl were electrophoresed on 2% agarose gels and ethidium bromide-stained bands were photographed, and analyzed by gel imaging systems (AIPhaEase software). The intensity for CXCL12, CXCR4 or CXCR7 mRNA was equal to the ratio of the absorbance of the target gene to that of the actin. The experiments were carried out in triplicate and repeated three times.
In-cell Western blot
ESCs in mid-log growth were digested with 0.25% trypsin and seeded at a density of 1×106 cells/ml on 6-well plates. At 80-90% confluence, the cells were starved with DMEM/F12 without FCS for 12 h, then treated with vehicle (DMEM/F12 with 2% stripped FCS), E (10-11-10-6 M) or P (10-11-10-6 M) for 48 h. According to our previous method [30], the in-cell Western blot was conducted to explore the effects of steroid hormones on CXCL12, CXCR4 and CXCR7 protein expression in ESCs. Briefly, the cells were fixed with 4% formaldehyde (diluted with PBS) for 20 min at room temperature. After being washed with 0.1% Triton, the cells were blocked with 10% FBS for 90 min at room temperature. Then they were added with rabbit anti-human CXCR4 (1:100, 10 μg/L), CXCR7 (1:100, 10 μg/L) or CXCL12 (1:100, 10 μg/L) antibody (Abcam). The housekeeping protein, mouse anti-human actin (1:500, 2 μg/L) antibody (Abcam) was also added to each well at the same time as control. After incubation overnight at 4°C, the wells were added with the corresponding second anti-rabbit (1:80, 12.5 μg/L) or anti-mouse (1:60, 16.7 μg/L) fluorescence antibody (Rockland, Inc, Gilbertsville, PA, USA). This procedure was operated in dark place avoiding the exposure to light. The images were obtained using the Odyssey Infrared Imaging System (LI-COR Biosciences GmbH). The intensity was calculated as the ratio of the intensity of CXCL12, CXCR4 or CXCR7 protein to that of actin. The experiments were carried out in triplicate and repeated three times.
Flow cytometry
ESCs in mid-log growth were digested with 0.25% trypsin and seeded at a density of 5×105 cells/mL on 24-well plates. At 80-90% confluence, the cells were starved with DMEM/F12 without FCS for 12 h, then added with vehicle (DMEM/F12 with 2% stripped FCS), E (10-9 M), P (10-10 M) or a combination of E (10-9 M) with P (10-10 M). After treatment for 48 h, the cells were collected for FCM analysis based on our previous method [17]. Briefly, to protect the membrane localization of chemokine receptor to the greatest extent possible, the cells were digested with 0.25% trypsin only for 30-50 s, then blown off gently and washed with phosphate-buffered saline (PBS). After blockade with 10% FBS, the recovered cells were mixed with mouse anti-human CXCR4-APC or CXCR7-PE antibody (Biolegend). The corresponding non-immune antibodies were also used as negative control. After incubation in darkness for 30 min at room temperature, the cells were analyzed immediately by FCM (FC500, Beckman Coulter, USA). The experiments were performed in triplicate and repeated three times.
Enzyme-linked immunosorbent assay
ESCs in mid-log growth were digested with 0.25% trypsin and seeded at a density of 2×105 cells/200 μl on 24-well plates. At 80-90% confluence, the cells were starved with DMEM/F12 without FCS for 12 h, then treated with vehicle (DMEM/F12 with 2% stripped FCS), E (10-9 M), P (10-10 M) or a combination of E (10-9 M) with P (10-10 M). The supernatants from each well were collected at 24, 48 and 72 h, respectively. Human CXCL12 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) was used to measure chemokine production in each supernatant according to our previous method [29]. The CXCL12 assay demonstrated a sensitivity of 47 pg/ml and an assay range from 156-10,000 pg/mL. The ELISA assay was carried out in duplicate in four separate experiments.
Statistics
The data were shown in the mean ± SE and assessed with the post hoc Dunnett’s t test or Dunnett T3 test, when appropriate. The differences were accepted as significant at P < 0.05.
Results
Expression of CXCL12, CXCR4 and CXCR7 in human endometrial tissues
In IHC analysis, human first-trimester villi were also used as positive control at the same time [21,25,29]. The results in Figure 1 demonstrated that human in situ ESCs did not express CXCL12 protein during different stages of the reproductive cycle or the first trimester. Moreover, no positive signals for CXCR4 or CXCR7 could be detected in human ESCs in the menstrual period. For CXCR4 protein, the moderate to strong positive cases of four were all from the decidual tissues, and the weak positive cases were from the proliferative phase (3 cases), secretory phase (3 cases) and first trimester (3 cases), respectively. Of the seven CXCR4-negative samples, the proliferative phase and menstrual period accounted for four and three cases respectively. For CXCR7 protein, six of eleven proliferative phase samples and four of seven secretory phase samples were weak-positive. Of the seven decidual samples, the expression intensity for CXCR7 was strong (2 cases), moderate (1 case), weak (2 cases) and negative (2 cases), respectively.
Figure 1.
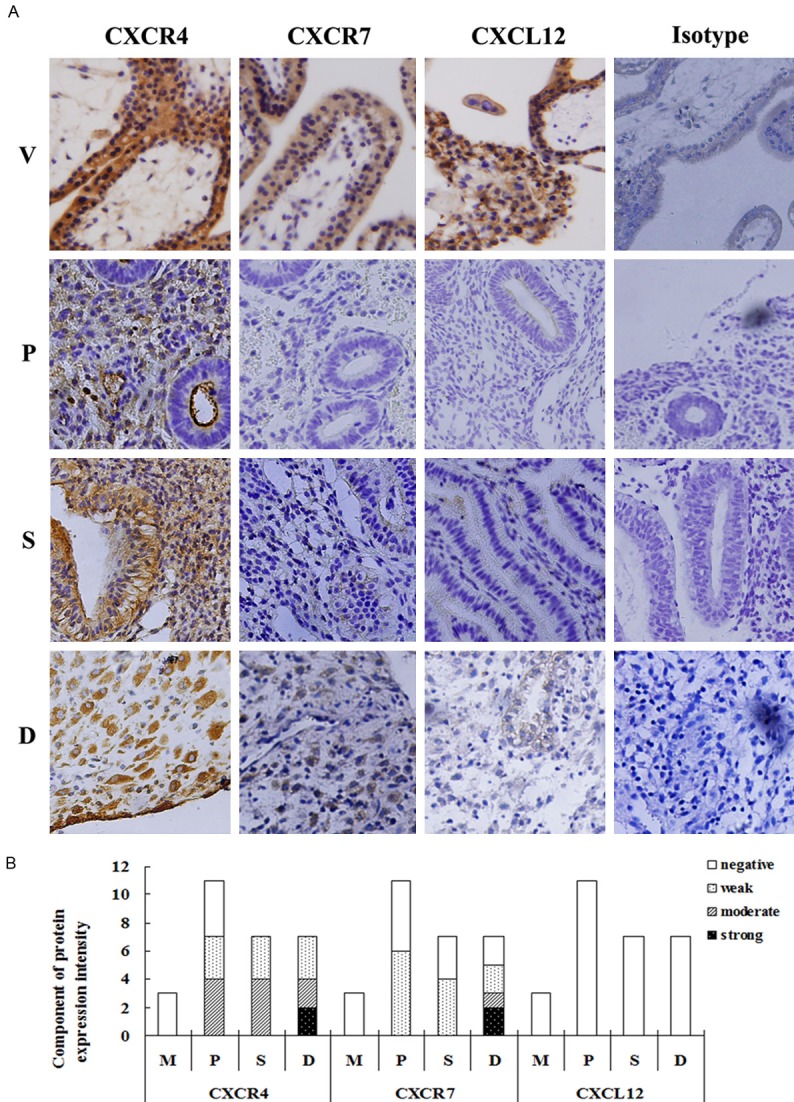
Expression of CXCL12, CXCR4 and CXCR7 protein in human endometrial and decidual tissues. Immunohistochemistry was performed to detect the protein expression of CXCL12, CXCR4 and CXCR7 in human endometrial and decidual tissues. The antibodies used in this experiment were rabbit anti-human CXCR4 (1:50), CXCR7 (1:25) and CXCL12 (1:50). A specific brown-coloured staining for CXCR4 or CXCR7 protein could be observed in the cytoplasm and membrane of human endometrial stromal cells in situ. There was no evidence for nonspecific staining with the control antibody. The pictures were the representative of the experiments. V: human first trimester villous tissues, as a positive control; M: endometrial tissues from the menstrual period; P: endometrial tissues from the proliferative phase; S: endometrial tissues from the secretory phase; D: decidual tissues from first trimester pregnancy. Magnification: ×200.
CXCR7 and CXCR4 protein were co-expressed in human cultured ESCs
The immunofluorescence results in Figure 2 showed that CXCR7 and CXCR4 protein were co-expressed in human in vitro cultured ESCs. Both the red fluorescence (CXCR7) and green fluorescence (CXCR4) was clearly recognized in the same area of the cells, including the cytoplasm and cytomembrane. Moreover, the expression pattern of CXCR7 (red fluorescence) in ESCs was highly consistent with that of CXCR4 protein (green fluorescence).
Figure 2.
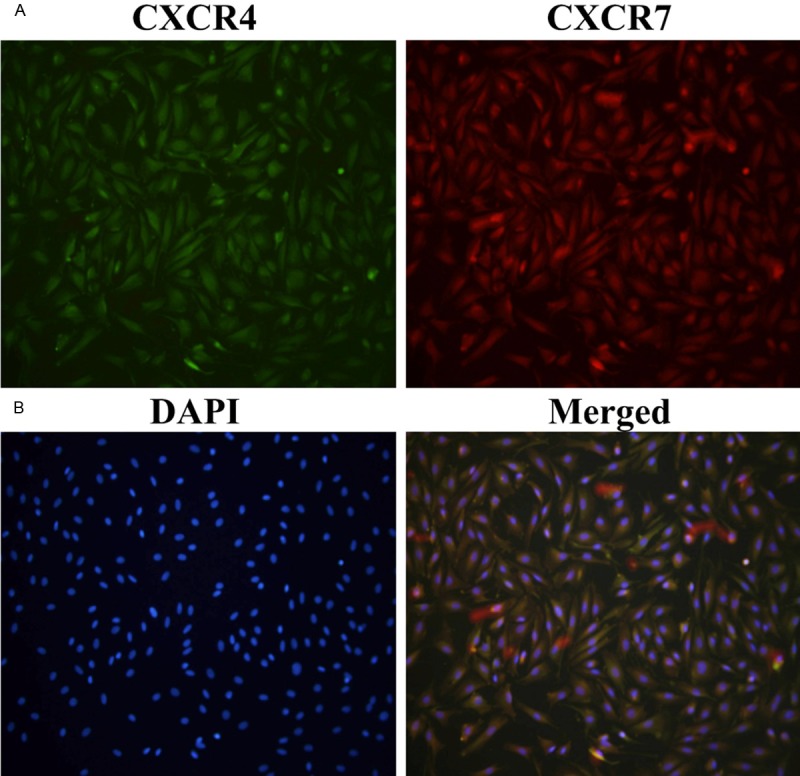
CXCR7 and CXCR4 were co-expressed in human cultured endometrial stromal cells. Indirect immunofluorescence was used to detect the expression and position of CXCR7 and CXCR4 in human endometrial stromal cells (ESCs). Both CXCR4 (green fluorescence) and CXCR7 (red fluorescence) protein were co-expressed in the cytoplasm and cytomembrane of ESCs. A: Expression of CXCR4 (green fluorescence) in ESCs; B: Expression of CXCR7 (red fluorescence) in ESCs; C: DAPI staining in ESCs; D: The merged picture. Magnification: ×400.
Modulation of estrogen and progesterone on CXCL12, CXCR4 and CXCR7 mRNA expression in human ESCs
The results of RT-PCR in Figure 3 showed that after the treatment of different concentration of E or P, the mRNA level of CXCL12, CXCR4 and CXCR7 in ESCs manifested different changes. Both E and P were able to up-regulate the mRNA level of CXCR4 in human ESCs in vitro. The effective concentration was from 10-11 M to 10-7 M and 10-10 M to 10-6 M for E and P, respectively. However either E or P showed no effects on CXCR7 mRNA expression in ESCs. Moreover, contrary to P which failed to stimulate the transcription of CXCL12 in ESCs, E at a concentration of 10-11 M to 10-8 M significantly increased the mRNA expression of CXCL12 (compared with the corresponding control group, P < 0.01).
Figure 3.
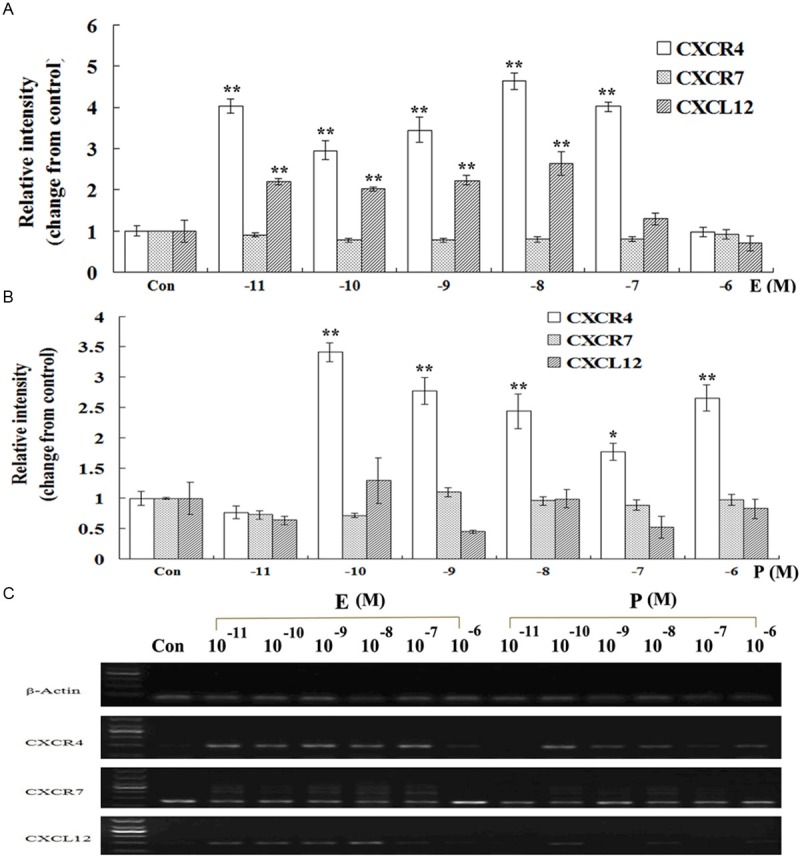
Effects of estrogen and progesterone on CXCL12, CXCR4 and CXCR7 mRNA expression in human ESCs. ESCs in mid-log growth were starved with DMEM/F12 for 12 h, then treated with vehicle (DMEM/F12 with 2% stripped FCS), estrogen (10-11-10-6 M) or progesterone (10-11-10-6 M) for 24 h. Reverse transcription-polymerase chain reactions (RT-PCR) were performed to detect the effects of different concentration of steroid hormones on mRNA expression of CXCL12 (155 bp), CXCR4 (194 bp) and CXCR7 (164 bp) genes. The intensity of CXCL12, CXCR4 or CXCR7 gene was equal to the ratio of the absorbance of the target gene to that of the actin. Then the relative expression level of the target gene was changed from the corresponding control. A: Modulation of estrogen on CXCL12, CXCR4 and CXCR7 mRNA expression in ESCs. B: Modulation of progesterone on CXCL12, CXCR4 and CXCR7 mRNA expression in ESCs. C: One of the representative pictures for RT-PCR. Error bars depict the standard error of the mean. Compared with the control, *P < 0.01; **P < 0.05.
Effects of estrogen and progesterone on protein expression of CXCR4, CXCR7 and CXCL12 in human ESCs
It was demonstrated in Figure 4 that after being treated with E or P, the protein level of CXCR4 in ESCs significantly increased (compared with the control P < 0.01). But there was a difference in the effective concentration between E (10-9, 10-8 M) and P (10-10 to 10-7 M). Different from the effects of steroidal hormone on mRNA expression of CXCR7, both E (10-10 to 10-7 M) and P (10-9 to 10-7 M) were also able to up-regulate the protein expression of CXCR7 in ESCs. However, at the same detection level, no positive signals for CXCL12 protein could be observed in ESCs in each treatment group.
Figure 4.
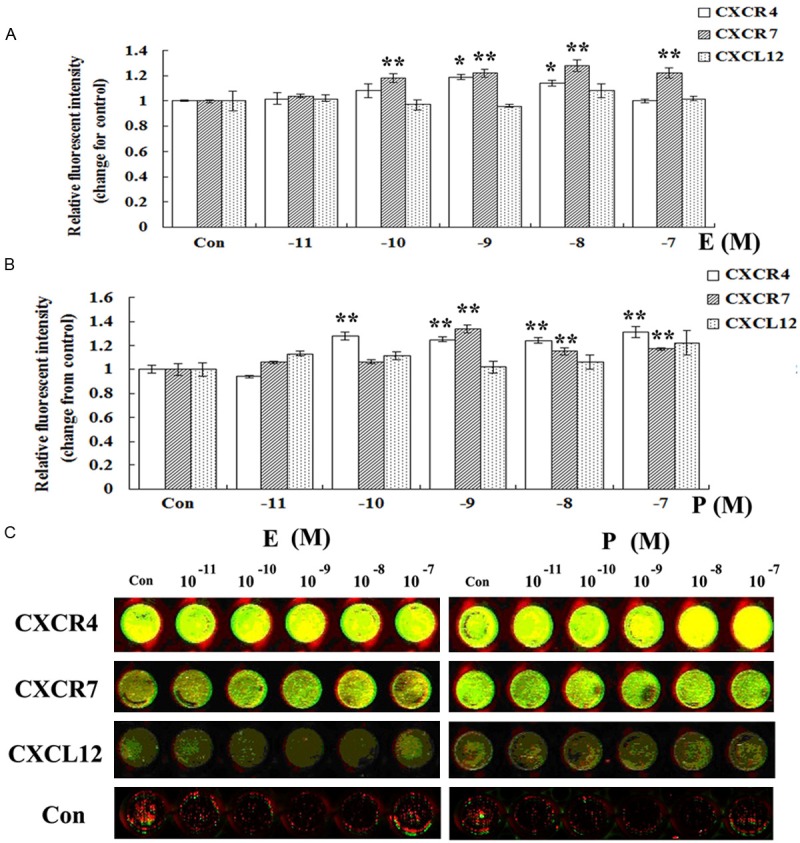
Modulation of estrogen and progesterone on CXCR4 and CXCR7 protein expression in human ESCs by in-cell Western analysis. ESCs in mid-log growth were starved with DMEM/F12 for 12 h, then treated with vehicle (DMEM/F12 with 2% stripped FCS), estrogen (10-11-10-7 M) or progesterone (10-11-10-7 M) for 48 h. In cell western was conducted to explore the influence of exogenous steroid hormones on protein expression of CXCR4 and CXCR7 in human ESCs. The green fluorescence represents actin, and the red fluorescence represents CXCR4 or CXCR7. The intensity was calculated as the ratio of the intensity of CXCR4 or CXCR7 protein to that of actin. Then the relative protein level of the target protein was changed from the corresponding control. A: Modulation of estrogen on CXCR4 and CXCR7 protein expression in ESCs. B: Modulation of progesterone on CXCR4 and CXCR7 protein expression in ESCs. C: One of the representative pictures for in-cell western. Error bars depict the standard error of the mean. Compared with the control, *P < 0.01; **P < 0.05.
Effects of estrogen and progesterone on membrane expression of CXCR4 and CXCR7 in human ESCs
As shown in Figure 5, the proportion of CXCR4-positive ESCs remarkably increased after being treated with E (10-9 M) or E (10-9 M) combined with P (10-10 M), however, P of 10-10 M had no effects on membrane expression of CXCR4 in ESCs. Compared with CXCR4, there was almost no membrane expression of CXCR7 in ESCs in the control group. However, the treatment of E (10-9 M), P (10-10 M) or E (10-9 M) combined with P (10-10 M) was able to significantly increase the membrane protein level of CXCR7 in ESCs.
Figure 5.
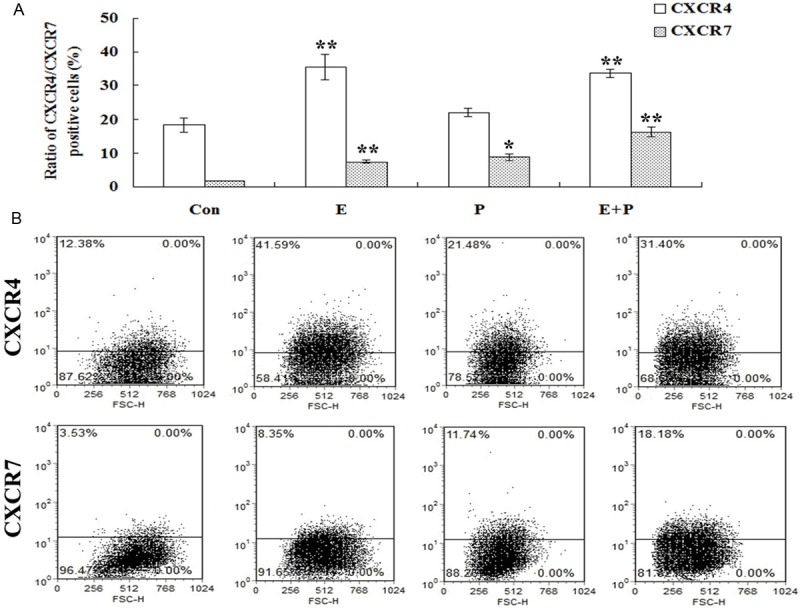
Effects of estrogen and progesterone on membrane expression of CXCR4 and CXCR7 in human ESCs. ESCs in mid-log growth were starved with DMEM/F12 for 12 h, then added with vehicle (DMEM/F12 with 2% stripped FCS), estrogen (10-9 M), progesterone (10-10 M) or a combination of estrogen (10-9 M) and progesterone (10-10 M) for 48 h. The membrane expression pattern of CXCR4 and CXCR7 by human ESCs was determined by FCM. A: Modulation of estrogen and progesterone on CXCR4 and CXCR7 protein expression in ESCs detected by FCM. B: One of the representative pictures for FCM. Error bars depict the standard error of the mean. Compared with the control, *P < 0.01; **P < 0.05.
Effects of estrogen and progesterone on CXCL12 secretion by human ESCs
ELISA assay was used to observe the production of CXCL12 by ESCs. Human first-trimester TCs were used as positive control [29] and at present detection level TCs secreted CXCL12 spontaneously. Consistent with our expectation, although ESCs were in a good growth state in in vitro culture, they did not secret CXCL12 under natural state. Moreover, either E or P showed no detectable effects on the secretion of CXCL12 by ESCs.
Discussion
The present study using IHC confirmed the spatiotemporal expression of CXCR4 and CXCR7 in human in situ ESCs, and the expression intensity for CXCR4 and CXCR7 was the highest in the first trimester. However, no positive signals for CXCL12 protein could be detected in the cycling or early pregnant endometria. As the ligand for CXCR4 and CXCR7, CXCL12 has been confirmed to be expressed in human oocytes, early embryos and even fetal-derived TCs [4,8,27,29,32], suggesting the role of CXCL12/CXCR4/CXCR7 axis in mediating the crosstalk between the fetus and endometrium during implantation and early pregnancy. Moreover, this unique expression pattern of CXCR4 and CXCR7 in ESCs also indicates the function of ESCs to “sense” the signals released from the embryo, which is consistent with Teklenburg group’s research in which ESCs function as embryo biosensors and selectors to recognize the developmental embryos [17,19,23].
Moreover, except for the menstrual period, the positive-expression rate and expression intensity for CXCR7 in ESCs was always relatively lower than that of CXCR4. As the co-receptor for CXCL12, CXCR7 displays more complicated biological characteristics than CXCR4 [1,9,13-15,20,26]. It can function as not only the activator of CXCL12 in a CXCR4 independent manner to stimulate proliferation, apoptosis, adhesion and invasion of cells, but also the scavenger receptor to internalize the chemokine to ensure the correct migration of CXCR4-positive cells toward the target position [1,9,13-15,26]. Thus, the possible reasons for this unique expression pattern of CXCR4 and CXCR7 in ESCs are inferred as follows: 1) A small number of CXCR7 receptor is enough to execute its function because of the higher affinity to CXCL12 compare with CXCR4 [3]; 2) To insure the smooth transduction of CXCR4 mediated signals, CXCR7 may mainly function as a modulator of CXCL12 gradient rather than activator; 3) The mixed-expression also abide by the principles of excessive biological signals and self-protection in organisms. When one of the signals is blocked, an alternative signal can be activated to maintain the normal biological functions.
The advancement of ART provides us a deeper insight to the importance of endometrium in normal pregnancy [7,16,18]. E and P are the only hormones necessary to prepare the endometrium for implantation or pregnancy [16]. Thus, in the present study, we also observed the modulation of E and P on CXCR4 and CXCR7 expression in ESCs. It was shown that both E and P were able to up-regulate the mRNA and protein expression of CXCR4 in ESCs, suggesting steroid hormones had a direct regulatory effect on CXCR4 expression. However, E and P mainly increased CXCR7 protein rather than mRNA expression. The inconsistence between gene and protein suggests the complexity of post-transcription regulatory network in ESCs. Moreover, there is also a complicated interaction between CXCR7 and CXCR4 [1,9,13-15,20,26]. Thus, we could not exclude the possibility that the increase of CXCR7 expression is concomitant with the increase of CXCR4. The up-regulation of CXCR4 expression induced by hormones activates some common signals, which in turn promotes the post-transcription regulation of CXCR7 expression. In addition, we observed CXCL12 mRNA expression in ESCs, but no positive signals for CXCL12 protein could be detected in present detective level which further suggests the importance of post-transcription regulation. The detailed mechanisms deserve further investigation.
Both our previous and other group’s studies have revealed that the embryo and embryo-derived TCs are the main source of CXCL12 and CXCL12 can mediate the cooperation between the embryo and CXCR4-positive endometrium during normal pregnancy [2,4,8,10,17,27], which suggests the leading role of fetal-derived signals at the matero-fetal interface. However, it was demonstrated in the present study that before encounter with the embryo or TCs, E and P alone were enough to regulate both CXCR4 and CXCR7 expression in ESCs. Thus in our opinion, the fact that ESCs are able to self-regulate the expression of chemokine receptors under the treatment with E and/or P, indicates the ability of ESCs to “actively” self-prepare for “sensing” the forthcoming of the embryo. Moreover, it was also demonstrated in the present study that there was a difference in the effective concentration between E and P, suggesting the refine and complicated role of steroid hormones in endometrium. Of course, the effective concentration suggested by in vitro experiments can not be fully equivalent to the optimal concentration in vivo. The detailed mechanisms underlying the regulation of hormones on endometrium still deserve further investigation.
In conclusion, the present study confirmed the spatiotemporal expression of CXCR4 and CXCR7 rather than CXCL12 in human primary ESCs, and E and P are able to regulate the expression of CXCR4 and CXCR7 in ESCs. This research will contribute to our better understanding of the changes of pregnancy-related key molecules under the modulation of steroid hormones, which sheds intellectual lights on the involved mechanisms of the interaction between the endometrium and embryo, and provides new insights for endometrium-related infertility.
Acknowledgements
The authors are very grateful to Prof. Da-Jin Li (Hospital and Institute of Obstetrics and Gynecology of Fudan University, Shanghai, China) and Ph.D. Xiao-Xi Huang (Beijing Chaoyang Hospital, Capital Medical University, Beijing, China) for their kind help during the performance of the experiments. This work is supported by National Natural Science Foundation of China (81270753, 81471511, 31171437, 81370770), Personnel Training Plan of The Health Care System of Beijing (2013-3-021), the Key Project of Shanghai Municipal Education Commission (14ZZ013) and Cooperation Project of Basic Research and Clinical Application of Capital Medical University (13JL11).
Disclosure of conflict of interest
None.
References
- 1.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 2.Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, Brosens IA. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol. 2009;200:e1–e6. doi: 10.1016/j.ajog.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 3.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng JW, Sadeghi Z, Levine AD, Penn MS, von Recum HA, Caplan AI, Hijaz A. The role of CXCL12 and CCL7 chemokines in immune regulation, embryonic development, and tissue regeneration. Cytokine. 2007;69:277–283. doi: 10.1016/j.cyto.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Dominguez F, Pellicer A, Simon C. The chemokine connection: hormonal and embryonic regulation at the human maternal-embryonic interface-a review. Placenta. 2003;24(Suppl B):S48–55. doi: 10.1016/s0143-4004(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 6.Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, de Mouzon J, Castilla JA, Erb K, Korsak V, Nyboe Andersen A European IVF-monitoring (EIM); Consortium, for The European Society of Human Reproduction and Embryology (ESHRE) Assisted reproductive technology in Europe 2009: results generated from European registers by ESHRE. Hum Reprod. 2013;28:2318–2331. doi: 10.1093/humrep/det278. [DOI] [PubMed] [Google Scholar]
- 7.Hamdine O, Macklon NS, Eijkemans MJ, Laven JS, Cohlen BJ, Verhoeff A, van Dop PA, Bernardus RE, Lambalk CB, Oosterhuis GJ, Holleboom CA, van den Dool-Maasland GC, Verburg HJ, van der Heijden PF, Blankhart A, Fauser BC, Broekmans FJ CETRO trial study group. Elevated early follicular progesterone levels and in vitro fertilization outcomes: a prospective intervention study and meta-analysis. Fertil Steril. 2014;102:448–454. doi: 10.1016/j.fertnstert.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, Peled A, Mandelboim O. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16-human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 9.Hattermann K, Mentlein R. An infernal trio: the chemokine CXCL12 and its receptor CXCR4 and CXCR7 in tumor biology. Ann Anat. 2013;195:103–110. doi: 10.1016/j.aanat.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- 11.Hu W, Liu Y, Zhou W, Si L, Ren L. CXCL16 and CXCR6 are coexpressed in human lung cancer in vivo and mediate the invasion of lung cancer cell lines in vitro. Plos one. 2014;9:e99056. doi: 10.1371/journal.pone.0099056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W, Zhen X, Xiong B, Wang B, Zhang W, Zhou W. CXCR6 is expressed in human prostate cancer in vivo and is involved in the in vitro invasion of PC3 and LNCap cells. Cancer Sci. 2008;99:1362–1369. doi: 10.1111/j.1349-7006.2008.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luker KE, Lewin SA, Mihalko LA, Schmidt BT, Winkler JS, Coggins NL, Thomas DG, Luker GD. CXCR7 mediated Gialpha independent activation of ERK and Akt promotes cell survival and chemotaxis in T cells. Oncogene. 2012;31:4750–4758. doi: 10.1038/onc.2011.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD, Howard MC, Schall TJ. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ödemis V, Boosmann K, Heinen A, Küry P, Engele J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. J Cell Sci. 2010;123:1081–1088. doi: 10.1242/jcs.062810. [DOI] [PubMed] [Google Scholar]
- 16.Paulson RJ. Hormonal induction of endometrial receptivity. Fertil Steril. 2011;96:530–535. doi: 10.1016/j.fertnstert.2011.07.1097. [DOI] [PubMed] [Google Scholar]
- 17.Ren L, Liu YQ, Zhou WH, Zhang YZ. Trophoblasts derived chemokine CXCL12 promotes CXCR4 expression and invasion of human first-trimester decidual stromal cells. Hum Reprod. 2012;27:366–374. doi: 10.1093/humrep/der395. [DOI] [PubMed] [Google Scholar]
- 18.Revel A. Defective endometrial receptivity. Fertil Steril. 2012;97:1028–1032. doi: 10.1016/j.fertnstert.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 19.Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Macklon NS, Brosens JJ. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5:e10287. doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmaggi A, Maderna E, Calatozzolo C, Gaviani P, Canazza A, Milanesi I, Silvani A, DiMeco F, Carbone A, Pollo B. CXCL12, CXCR4 and CXCR7 expression in brain metastases. Cancer Biol Ther. 2009;8:1608–1614. doi: 10.4161/cbt.8.17.9202. [DOI] [PubMed] [Google Scholar]
- 21.Schanz A, Baston-Bust D, Krussel JS, Heiss C, Janni W, Hess AP. CXCR7 and syndecan-4 are potential receptors for CXCL12 in human cytotrophoblasts. J Reprod Immunol. 2011;89:18–25. doi: 10.1016/j.jri.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Strowitzki T, Germeyer A, Popovici R, von Wolff M. The human endometrium as a fertility-determining factor. Hum Reprod Update. 2006;12:617–630. doi: 10.1093/humupd/dml033. [DOI] [PubMed] [Google Scholar]
- 23.Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Brosens JJ, Macklon NS. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One. 2010;5:e10258. doi: 10.1371/journal.pone.0010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toth B, Würfel W, Germeyer A, Hirv K, Makrigiannakis A, Strowitzki T. Disorders of implantation--are there diagnostic and therapeutic options? Reprod. Immunol. 2011;90:117–123. doi: 10.1016/j.jri.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Tripathi V, Verma R, Dinda A, Malhotra N, Kaur J, Luthra K. Differential expression of RDC1/CXCR7 in the human placenta. J Clin Immunol. 2009;29:379–386. doi: 10.1007/s10875-008-9258-4. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16-NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol. 2005;175:61–68. doi: 10.4049/jimmunol.175.1.61. [DOI] [PubMed] [Google Scholar]
- 28.Yoshinaga K. Research on blastocyst implantation essential factors (BIEFs) Am J Reprod Immunol. 2010;63:413–424. doi: 10.1111/j.1600-0897.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou WH, Du MR, Dong L, Yu J, Li DJ. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum Reprod. 2008;23:2669–2679. doi: 10.1093/humrep/den308. [DOI] [PubMed] [Google Scholar]
- 30.Zhou WH, Du MR, Dong L, Zhu XY, Yang JY, He YY, Li DJ. Cyclosporin A increases expression of matrix metalloproteinase 9 and 2 and invasiveness in vitro of the first-trimester human trophoblast cells via the mitogen-activated protein kinase pathway. Hum Reprod. 2007;10:2743–2750. doi: 10.1093/humrep/dem097. [DOI] [PubMed] [Google Scholar]
- 31.Zhou WH, Ren L, Liu Y, Zhang YZ. Expression of chemokine receptor CXCR7 in human first-trimester trophoblasts and decidual stromal cells. Chin J Clinicians (Electronic Edition) 2010;7:1436–1440. [Google Scholar]
- 32.Zuccarello D, Ferlin A, Garolla A, Menegazzo M, Perilli L, Ambrosini G, Foresta C. How the human spermatozoa sense the oocyte: a new role of SDF1-CXCR4 signalling. Int J Androl. 2011;34:e554–65. doi: 10.1111/j.1365-2605.2011.01158.x. [DOI] [PubMed] [Google Scholar]


