Abstract
Lithium has been shown to inhibit apoptosis of neural progenitor cells (NPCs) and promote differentiation of NPCs. However, there was rare data to discuss the effects of lithium on neural differentiation of mesenchymal stem cells (MSCs). Here, we investigated the potential promotion of lithium to MSC proliferation and neural differentiation in vitro and after transplanted into the ventral horn of rat spinal cord in vivo. We found that lithium possesses the ability to promote proliferation of GFP-MSCs in a dose dependent manner as verified by growth curve and bromodeoxyuridine (BrdU) incorporation assays; While in neural induction medium, lithium (0.1 mM) promotes neural differentiation of GFP-MSCs as verified by immunostaining and quantitative analysis. After transplantation of GFP-MSCs into the rat spinal cord, lithium treatment enhanced cell survival and neural differentiation after transplantation as verified by immunohistochemistry. These data suggested that lithium could be a potential drug to augment the therapeutic efficiency of MSCs transplantation therapy in central nervous system (CNS) disorders.
Keywords: Mesenchymal stem cells, lithium, proliferation, neural differentiation, spinal cord
Introduction
Mesenchymal stem cells (MSCs) have the potential to self-renew and differentiate into multiple cell types [1-3], such as osteoblasts, adipocytes, muscle cells, cardiac cells, neural cells and other cell types. Recent evidence suggests that MSCs can be used to replace damaged neural tissue in brain and spinal cord [4-6]. Thus, MSCs have been evaluated as a renewable source of neural precursors for regenerative transplantation in various central nervous system (CNS) diseases, including Alzheimer’s disease, multiple sclerosis and ischemia/reperfusion injury [7-9]. In spite of the therapeutic potential of MSCs to repair and replace neural tissue, the adult CNS, especially the injured CNS, may provide a relatively non-permissive environment for transplanted MSCs and other stem cells [10,11]. The cell survival and differentiated ratio towards neural lineage were relatively low, and therefore limited their therapeutic efficacy in CNS disorders.
Lithium, used as antidepressant drug in clinical, was used in preclinical research for inhibition of GSK-3β which is one of the key components in apoptosis signaling [12,13]. It has been demonstrated that clinical dose of lithium treatment improves axon regeneration and promotes locomotor functional recovery in rats with thoracic spinal cord transaction of contusions injuries [14]. Also lithium has been reported to be capable of inhibiting apoptosis of neural progenitor cells [15], exerting neuroprotective effect against various insults [16-18], and promoting proliferation and differentiation of neural stem cells [19]. However, there was rare data to discuss the effects of lithium on proliferation and neural differentiation of MSCs.
Based on the known activities of lithium, we hypothesized that lithium might be a potential drug to upgrade the therapeutic efficiency of MSC therapy in CNS disorders. In this study, we firstly tested the influence of lithium on GFP-MSCs proliferation and neural differentiation potential in vitro. Then, we performed cell transplantation into the ventral horn of the rat spinal cord, and the rats were treated with lithium with MSC-application and continued throughout the study. After cell transplantation and lithium treatment, we examined the cell survival, proliferation and neural differentiation post transplantation. We found that lithium (0.1 mM) promotes proliferation and neural differentiation of GFP-MSCs in vitro. Further, lithium promotes cell survival and neural differentiation of GFP-MSCs after transplantation into the spinal cord in rats.
Materials and methods
Animals
Sprague-Dawley (SD) rats (8 weeks old, female) were used in the present study. The experimental protocol for this study was in compliance with the Standard Medical Laboratory Animals’ Care and Use Protocols (Ministry of Health PR China, 1998) and the Laboratory Animal Ethical Standards of China Medical University.
Cell culture and characterization of MSCs
Bone marrow mesencymal stem cells (MSCs), harvested from GFP-transgenic Sprague-Dawley rats, were purchased from Cyagen biosciences inc (Guangzhou, China). Cells were grown in L-Dulbecco’s modified Eagle’s medium (L-DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin (all from Gibco, New York, NY, USA) and maintained in an incubator with a humidified atmosphere containing 5% CO2 at 37°C. The medium was changed every 2 days. At around 80% confluence, cells were passaged at the ratio of 1: 3-4 after initial plating. These sub-cultured cells were designated as ‘first passage’ (P1). All experiments were carried out using P1-P3 MSCs in this study. These MSCs constitutively express green fluorescent protein (GFP). The phenotype of GFP-MSCs at P3 was analyzed by flow cytometry. Dissociated single MSCs were harvested and stained with fluorescence-labeled anti-CD11b, CD34, CD44, CD45, CD90 antibody for 30 min, then analyzed by FACScan flow cytometer (BD Biosciences, San Diego, CA, USA) [3].
Multipotent differentiation of GFP-MSCs
Multipotency was studied by measuring osteogenic differentiation and adipogenic properties. To assess osteogenic differentiation, MSCs (1 × 105/ml) were seeded onto 6 well plate and cultured in an osteogenic differentiation medium (10% FBS, 1 μM dexamethasone, 10 mM β-glycerol phosphate and 50 mg/mL ascorbic acid in high glucose DMEM) for 18 days [20]. Cells were fixed with 4% paraformaldehyde and stained with Alizarin red. To measure the adipogenic properties, MSCs (1 × 105/ml) were seeded onto 6 well plate and cultured in an adipogenic differentiation medium (10% FBS, 1 μM dexamethasone, 10 mg/mL insulin, 60 μM indomethacin and 0.5 mM isobutyl-methylxanthine in high glucose DMEM) for 21 days [21]. Cells then were fixed with 4% paraformaldehyde and stained for with oil red. The cells were then examined using a microscope.
Assay for proliferation of GFP-MSCs
Disassociated GFP-MSCs at P3 were plated in 96 well plates at a density of 1.0 × 105 cells per well. The cells were exposed with saline (1 mM) or lithium chloride at various concentrations (n = 6 wells for each) for 0, 1, 2, 3, 4 days. After various treatments, cell viability was determined by MTT assay [22]. Briefly, the cells were incubated with 0.5 mg/ml MTT (Invitrogen) at 37°C for 4 h, then medium was removed and 100 ml of DMSO was added to dissolve the formed formazan product. Absorbance at 490 nm was determined with a microplate reader (Thermo Fisher Scientific Inc., USA). The results were presented as optical density (OD) value. Growth curves of GFP-MSCs revealed by cell viability at each time point was developed.
The proliferation potential of GFP-MSCs was also tested by in vitro 5-bromo-2’-deoxyuridine (BrdU) incorporation [23]. Briefly, the cells were seeded on 6 well plates at a density of 1.0 × 105 cells/cm2 and incubated with or without lithium chloride (1 mM, n = 5 wells) or sodium chloride (1 mM, n = 5 wells) as a negative control for 48 h. BrdU (10 μM, Sigma, St. Louis, MO) was added to cultures 2 h before fixation of the cells. The BrdU treated cells were fixed with 4% paraformaldehyde and stained with anti-BrdU antibody (1:150, Sigma-Aldrich Co., St. Louis, MO) and Cy3-labeled species specific secondary antibody (1:200, Jackson ImmunoResearch Lab, West Grove, PA, USA). The number of BrdU positive cells was shown as a percentage of the total numbers of GFP-positive cells.
Differentiation of GFP-MSCs into neural lineages
To initiate neural differentiation, culture medium was replaced with neural induction medium containing DMEM, 20 ng/mL EGF, 20 ng/mL bFGF (Peprotec, Rocky Hill, NJ, USA), 2% B27 (Invitrogen, Carlsbad, CA), 5 μM Forskolin (Sigma, St. Louis, MO), 125 μM 3-isobutyl-1-methylxanthine (IBMX; Sigma) with 10 μM β-mercaptoethanol (β-ME) [19]. After 4 weeks of treatment, cells were used for immunostaining analysis.
In vitro immunocytochemistry
GFP-MSCs were seeded on poly-L-lysine-coated chamber slides in neural induction medium. For immune staining, cells were fixed with 4% paraformaldehyde and then were permeabilized with 0.5 ml of 0.1% Triton X-100. After blocking with 5% BSA plus 10% goat serum, slides were incubated with primary antibody at 4°C overnight. The following primary antibodies were used: rabbit anti-βIII-tubulin (1:100, Covance, Berkeley, CA), rabbit anti-GFAP (1:100, StemCell Technologies, Vancouver, BC, Canada), rabbit anti-Olig-2 (1:200, EMD Millipore, Billerica, MA). Primary antibodies were washed out with PBS three times after overnight incubation. Cells were then stained with Cy3-labeled anti-rabbit IgG conjugate (Jackson ImmunoResearch Lab, West Grove, PA at 1:200 dilution). Nuclei were stained with DAPI [24]. The stained slides were covered with mounting medium (Vector Laboratories, Burlingame, CA). Results were visualized by fluorescent microscopy (Olympus, Tokyo, Japan).
Transplantation procedures
Surgery procedures have been previously described [19,25]. Briefly, GFP-MSCs (1 × 105 cells/μl) were suspended in PBS. Cell viability was assessed with trypan blue and typically over 90% of the cells excluded the dye. The rats were anesthetized by inhalation of isoflurane (1-2%) administered at a flow rate of 2 L/min. Laminectomy was performed at the Th8-9 vertebrates to expose the spinal cord. A GFP-MSCs suspension in 1 μL PBS was slowly injected over 2 min into the ventral horn with a micropipette left over 5 min after injection. Following injection, the layered muscles and skin were sutured to close the lesion. Immediately after cell transplantation, rats were received gentamicin i.p. and kept for a while on a warming pad until they awakened from the anesthesia. The animals were randomly divided into two groups (18 animals in each group). One group received daily LiCl (85 mg/kg bodyweight, dissolved in distilled water) i.p. until killing. The other group received daily saline i.p. as the control. On the Day 3 or 7 or 14 after GFP-MSCs transplantation, rats (n = 6 in each group) treated with LiCl or saline were sacrificed and extensively perfused, and spinal cords were harvested.
For in vivo BrdU incorporation, BrdU (10 mg/ml; Sigma, St. Louis, MO) was administered i.p. (0.1 mg/g of body weight). Rats received 4 daily injections of BrdU on days 3-7 or 24-28 of the days post transplantation. The number of BrdU-labeled GFP-MSCs in the spinal cord was determined by immunohistochemical methods, as described below.
In vivo immunohistochemical study
Ten-micrometer frozen sections from GFP-MSC-treated rat spinal cord were fixed with 4% paraformaldyhyde plus 0.5% glutaraldehyde for 30 minutes, then washed three times with PBS. Sections were incubated with 10% goat serum in PBS for 60 minutes, after which primary antibodies were added and incubated at 4°C overnight. The following primary antibodies were used: mouse anti-βIII-tubulin (1:150; BD Bioscience, San Jose, CA), rabbit anti-GFAP (1:100; StemCell Technologies, Vancouver, BC, Canada), rabbit anti olig-2 (1 μg/ml; Cell Signaling Solutions, Temecula, CA), mouse anti-BrdU antibody (1:150, Sigma-Aldrich Co., St. Louis, MO). Primary antibodies were washed out with PBS three times after overnight incubation. Sections were then incubated with Cy3-conjugated species-specific secondary antibodies (all from Jackson ImmunoResearch Lab, West Grove, PA at 1:200 dilution) for 60 minutes at room temperature, followed by washing with PBS three times [26]. Immunofluorescence controls were routinely performed with incubations in which primary antibodies were not included. Slides were covered with mounting medium (Vector Laboratories, Burlingame, CA). Cells expressing GFP (green), neural specific markers (red), and 4’,6-diamidino-2-phenylindole (DAPI; blue) were identified as GFP-MSCs. Cell counter of ImageJ software (NIH ImageJ) was used to count cells, and mean numbers were used for analysis. Results were visualized by fluorescent microscopy. Quantification of positive cells was performed on 4 sections per rat, and six rats per group were analyzed.
Statistical analysis
All experiments were done in at least triplicate. “n” represents the number of rats used for each experiment. Data were presented as the arithmetic mean ± standard deviation. Comparison between groups was analyzed using the one-way ANOVA test. P values of less than 0.05 were considered statistically significant.
Results
Characterization of GFP-MSCs in vitro
These GFP-MSCs constitutively express green fluorescent protein (GFP, Figure 1A). The phenotype of GFP-MSCs were characterized at P3. Flow cytometry was used to determine expression of cell surface markers that typically characterize MSCs. The majority of cells expressed the MSC surface markers CD90 and CD44, but only few cells expressed CD11b, CD34 and CD45 (Figure 1B), indicating that the cells were MSCs.
Figure 1.
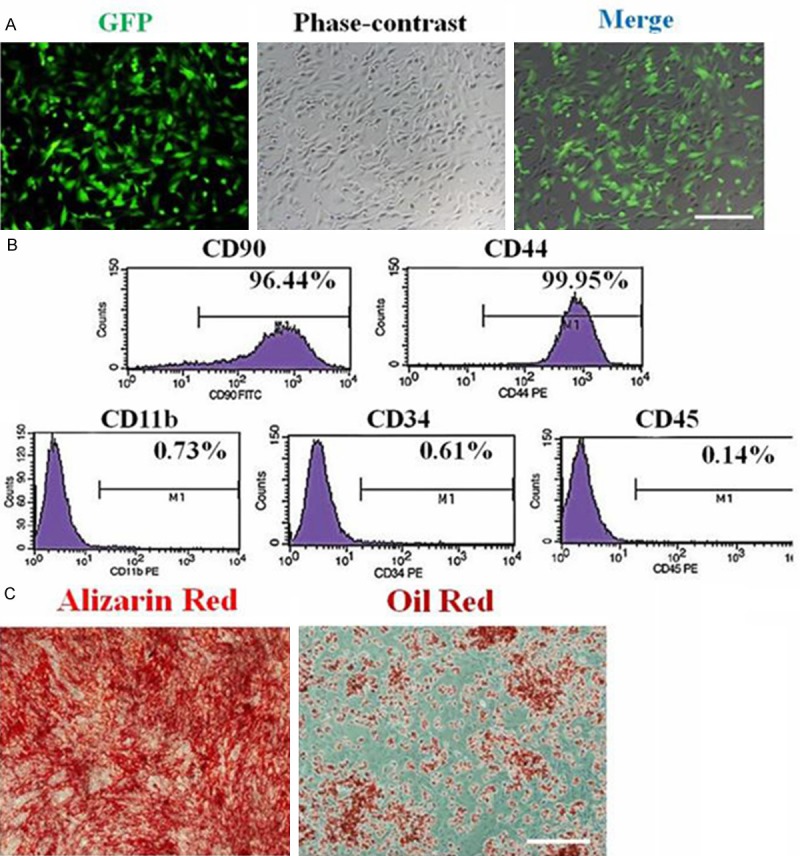
Characterization of rat GFP-MSCs in vitro. A. Morphological characteristics of GFP-MSCs at P3 under fluorescence and phase-contrast microscopy. Scale bar = 100 μm. B. Immunophenotype analysis of GFP-MSCs at P3 by flow cytometry. Adherent MSCs are positive for CD90 and CD44, but negative for CD11b, CD34 and CD45. C. Osteogenic differentiation as indicated by the formation of mineralized matrix shown by alizarin red staining. Adipocytic differentiation was noted by the presence of broadened morphology and formation of lipid vacuoles (positive oil-red O staining). Scale bars = 100 μm.
The multipotent differentiation of GFP-MSCs was analyzed at P3 to confirm characterization of MSCs. After osteogenic induction of GFP-MSCs for 18 days, mineralization nodules were observed with Alizarin red staining. After adipogenic induction of GFP-MSCs for 21 days, intracytoplasmic lipid vesicles were observed through oil red O staining (Figure 1C). All these results indicated that cells were MSCs and could be used in subsequent experiments.
Lithium enhanced self-renewal capacity of GFP-MSCs
To determine the effects of lithium on GFP-MSCs proliferation in vitro, GFP-MSCs at P3 were treated with increasing concentrations of lithium chloride (0.1, 1, 10 mM) or saline (1 mM) in cell culture medium. At day 0, 1, 2, 3, 4, cell viability was assayed by MTT. The curves displayed no significant differences in proliferation among lithium (0.1 mM), saline and control groups (P > 0.05; Figure 2A). Compared with saline and control groups, lithium (1 mM) treated cells exhibited a stronger proliferation capacity (P < 0.05 vs. control, P < 0.05 vs. saline). However, lithium at concentration of 10 mM decreased cell viability at day 3 and 4 (P < 0.05 vs. control), indicating that lithium exerted a cytoxity at high concentration.
Figure 2.
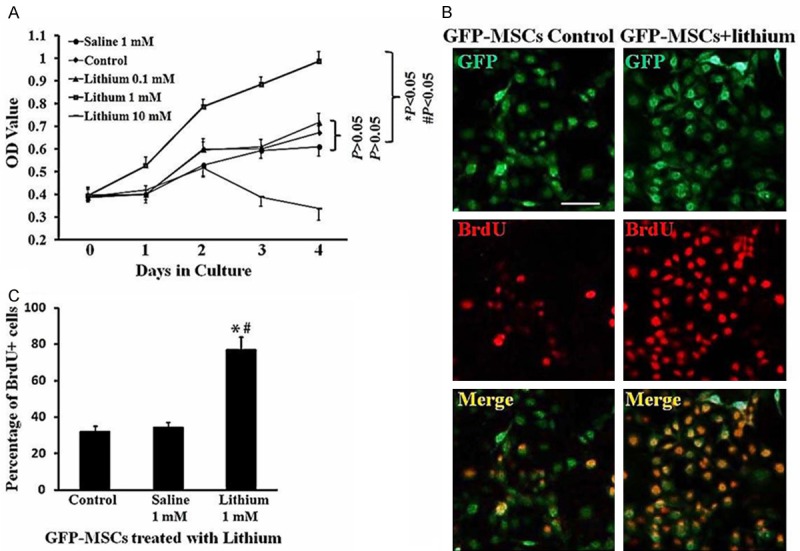
Promotion of lithium on GFP-MSCs proliferation in vitro. A. Growth curves. Disassociated GFP-MSCs at P3 were plated in 96 well plates. The cells were exposed with lithium chloride at various concentrations (n = 6 wells for each) for 0, 1, 2, 3, 4 days. After various treatments, cell viability was determined by MTT assay. B. BrdU labeling. GFP-MSCs were treated with lithium (1 mM, n = 5 wells) or saline (1 mM, n = 5 wells) for 48 h. BrdU (10 mM) were added to each groups of GFP-MSCs, and incubated for 2 h, followed by immunostaining with BrdU antibody. Green: GFP; red: BrdU+. Scale Bar = 100 μm. C. Quantitative analysis of BrdU incorporation. The number of BrdU positive cells was shown as a percentage of the total numbers of GFP-positive cells, *P < 0.05, comparison between control GFP-MSCs and lithium-treated GFP-MSCs. #P < 0.05, comparison between saline-treated GFP-MSCs and lithium-treated GFP-MSCs.
The proliferation of GFP-MSCs was also tested by in vitro BrdU incorporation. After incubation with lithium (1 mM) for 48 h, GFP-MSCs were treated with BrdU (10 μM) followed by immunostaining analysis. As shown in Figure 2B and 2C, significantly higher levels of BrdU-positive cells were found in lithium (1 mM) treated groups as compared with control (P < 0.05 vs. control, P < 0.05 vs. saline). All these results indicated that lithium (1 mM) promoted proliferation of GFP-MSCs in vitro.
Lithium promoted GFP-MSCs differentiated into neural lineage
To determine the effects of lithium on neural differentiation of GFP-MSCs in vitro, cell culture medium was replaced by neural induction medium with or without lithium chloride (1 mM). After 28 days, GFP-MSCs changed morphology and developed into neurons (βIII-tubulin+), astrocytes (GFAP+) and oligodendrocyte precursors (Olig-2+) (Figure 3A-C). Quantitive analysis showed that lithium promoted GFP-MSCs differentiated into greater numbers of neurons, astrocytes and oligodendrocytes (P < 0.05, P < 0.05, P < 0.05, Figure 3D) as compared with neural induction medium control group.
Figure 3.
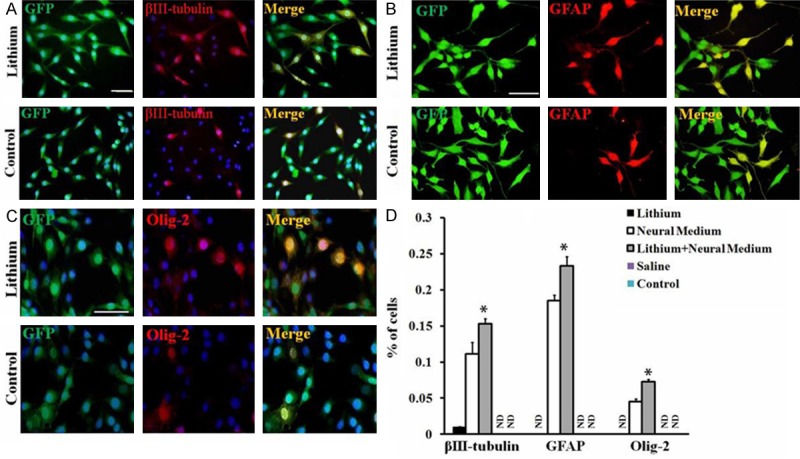
Promotion of lithium on GFP-MSCs neural differentiation in vitro. A-C. Neural differentiation of GFP-MSCs. To induce GFP-MSCs neural differentiation in vitro, dissociated single cells were cultured in neural induction medium with or without lithium (0.1 μM) for 4 weeks, followed by immunostaining. GFP-MSCs differentiated into (A) βIII-tubulin+ neurons, B. GFAP+ astrocytes and (C) olig-2+ oligodendrocytes. Nuclei were stained with DAPI. Scale Bar = 50 μm in A, B and C. One representative experiment of 3 is shown (n = 5 wells in each group). D. Quantitative analysis of percentages of GFP-MSCs differentiated into each type of neural cells among total numbers of GFP-positive cells. Data represent mean ± S.D. of three independent experiments. *P < 0.05, comparison between neural induction medium treated GFP-MSCs (Control) and lithium plus neural induction medium treated GFP-MSCs (Lithium).
Lithium enhanced grafted cell survival but not proliferation in long term
As transplanted GFP-MSCs stayed in the spinal cord for 3 days, 1 week and 4 weeks, rats were sacrificed and frozen sections of spinal cord were prepared for examination of survival and proliferation of grafted cells (Figure 4A). Quantitative analysis was performed to determine the number of GFP-positive cells that survive in the spinal cord after the transplantation. There was no significant difference between lithium treatment and control groups on day 3 post transplantation (Figure 4B). However, the number of GFP-positive cells in the lithium treatment group was significantly higher than in the animals with only GFP-MSCs at 1 week (36954 ± 1209 cells/rat vs. 17842 ± 1609 cells/rat, P < 0.05, Figure 4B) and 4 weeks (14893 ± 1434 cells/rat vs. 5434 ± 1398 cells/rat, P < 0.05, Figure 4B) after transplantation, indicating that lithium promoted grafted MSCs survival after transplantation in rat spinal cord.
Figure 4.
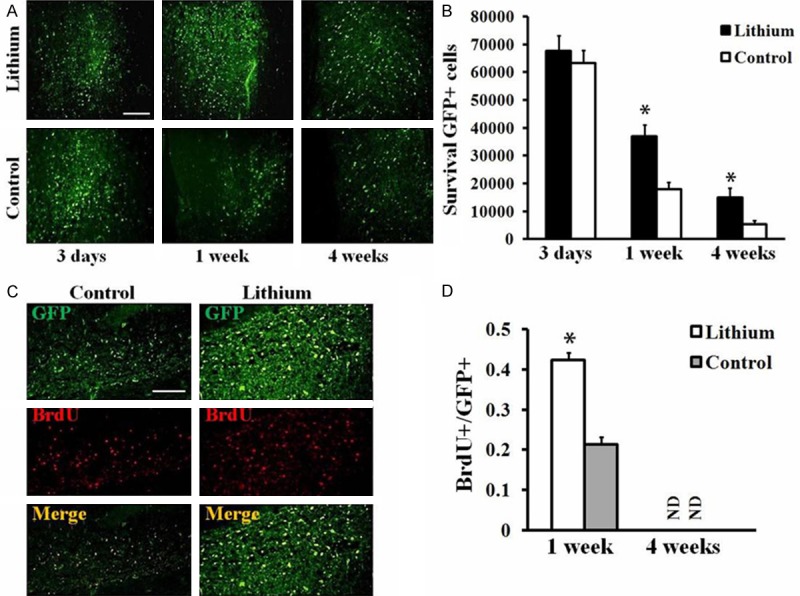
Effects of lithium on GFP-MSCs survival and proliferation after transplantation into the spinal cord. A. Grafted GFP-MSCs in the spinal cord of lithium or saline (Control) treated animals. Spinal cord of rats in each group was harvested at day 3, 7 and 28 and sections were prepared. Transplanted GFP-MSCs were found in sections of spinal cord based on the expression of GFP (green). Scale Bar = 100 μm. B. Quantitative analysis of GFP-positive cells in sections of spinal cord. Data represent mean ± S.D., 5 sections per rat, n = 6 rats in each group, *P < 0.05, comparison between GFP-MSCs treated control and lithium plus GFP-MSCs treated animals. C. BrdU labeling. Spinal cord of rats in each group were harvested at 1 and 4 weeks post transplantation, and sections were prepared and immunostained with BrdU antibody. Increased BrdU+ (red)/GFP+ (green) cells were found in the spinal cord of rats treated with lithium by compared with control animals at 1 week after cell transplantation. D. Quantitative analysis of BrdU+/GFP+ cells of the sections from rat spinal cord at 1 week or 4 weeks post cell transplantation. Data represent mean ± S.D., 4 sections per rat, n = 6 rats in each group, *P < 0.05, comparison between GFP-MSCs treated control and lithium plus GFP-MSCs treated animals.
Further, the frozen sections were staining with BrdU antibody to exam the proliferation potential of GFP-MSCs with lithium treatment. As shown in Figure 4C and 4D, lithium treatment group exhibited higher BrdU+/GFP+ in the sections than that in control group at 1 week after cell transplantation (0.423 ± 0.019 vs. 0.214 ± 0.017, P < 0.05, Figure 4D). Whereas, the BrdU positive cells could be observed in neither lithium treatment nor control groups at 4 weeks post cell transplantation (Figure 4D). These results indicated that lithium treatment can not promote proliferation of grafted MSCs in long term.
Lithium promoted neural differentiation of GFP-MSCs in the spinal cord
To exam the influence of lithium on neural differentiation of grafted GFP-MSCs in vivo, animals (n = 8) were sacrificed after 4 weeks post cell transplantation. Frozen sections of spinal cord were stained with neural marker βIII-tubulin (neuron), GFAP (astrocyte) and olig-2 (oligodendrocyte) and the results presented as βIII-tubulin+/GFP+, GFAP+/GFP+ and olig-2+/GFP+ (Figure 5A-C). Quantitive analysis showed that lithium promoted differentiation of GFP-MSCs into greater numbers of neurons, astrocytes and oligodendrocytes (βIII-tubulin+/GFP+ 0.123 ± 0.007, GFAP+/GFP+ 0.192 ± 0.011, olig-2+/GFP+ 0.083 ± 0.005 in lithium treatment group vs. βIII-tubulin+/GFP+ 0.074 ± 0.006, GFAP+/GFP+ 0.145 ± 0.006, olig-2+/GFP+ 0.058 ± 0.003, P < 0.05, P < 0.05 and P < 0.05, respectively, Figure 5D), indicating that lithium promoted neural differentiation of grafted GFP-MSCs in the spinal cord.
Figure 5.
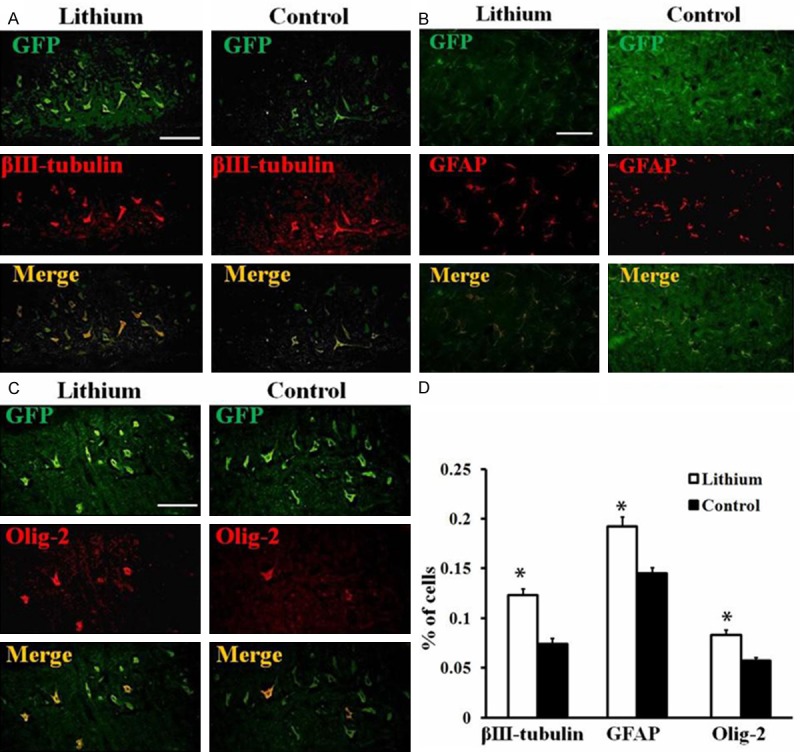
Promotion of lithium on GFP-MSCs neural differentiation after transplantation into rat spinal cord. (A-C) Neural differentiation of grafted GFP-MSCs in rat spinal cord. Spinal cord of rats in each group was harvested at 4 weeks post transplantation and sections were prepared. Transplanted GFP-MSCs differentiated into (A) βIII-tubulin+ neurons, (B) GFAP+ astrocytes and (C) olig-2+ oligodendrocytes after 4 weeks post transplantation in the spinal cord. Scale Bar = 50 μm; (D) Quantitative analysis of percentages of GFP-MSCs differentiated into each type of neural cells among total numbers of GFP-positive cells. Data represent mean ± S.D., 4 sections per rat, n = 6 rats in each group, *P < 0.05, comparison between GFP-MSCs treated control and lithium plus GFP-MSCs treated animals.
Discussion
MSCs have distinct advantages for cell transplantation, such as they are easy to harvest and to culture. MSCs can be harvested from a wide variety of tissue types, including bone marrow, adipose tissue, skeletal muscle, synovium, umbilical cord blood, as well as other tissues [27,28]. Bone marrow is a rich source of MSCs, therefore it is a tissue often used for harvesting MSCs. As a stem cell type, MSCs exert self-renew ability and multipotent differentiation potential. Particularly, MSCs can be induced into neurosphere and neural lineage by neural stem cell culture medium in vitro [26,29,30], and differentiated into neural lineage after transplanted into spinal cord and CNS directly or indirectly vie tail vein injection [14,26,29]. However, a very small proportion of transplanted MSCs survived and few cells differentiated into mature neural phenotypes, therefore pharmacologic strategies targeted at promotion of cell survival, proliferation and neural differentiation may enhance their therapeutic efficacy in CNS disorders. Lithium, a GSK-3β inhibitor, has been reported to exert neuroprotective effect and have the ability to promote neural regeneration [16-19], may act on MSC activities. In the present study, we first cultured and identified the GFP-MSCs, then investigated the influence of lithium on proliferation and neural differentiation of the cells in vitro. We found that lithium (0.1 mM) promoted cell viability and BrdU-positive cells of GFP-MSCs, whereas lithium at concentration of 10 mM decreased cell viability, indicating that lithium promoted proliferation of MSCs at a specific concentration (0.1 mM) and exerted a cytoxity at high concentration (10 mM).
To date, the trans-differentiation potential of bone marrow stem cells into neural cell types has been controversial. Neurons or glia can be derived by bone marrow cells in vivo [31,32] and in vitro [33,34], though some groups did not find this phenomenon [35,36]. In this study, cultured in neural induction medium for 4 weeks, GFP-MSCs developed into βIII-tubulin+ neurons, GFAP+ astrocytes and olig-2+ oligodendrocytes. Further, lithium (0.1 mM) promoted GFP-MSCs differentiated into greater numbers of neurons, astrocytes and oligodendrocytes. The results indicated that lithium may be a potential drug for enhancing MSCs neural differentiation and facilitating structural and functional reconstruction of damaged neural tissues.
Cell transplantation for the regeneration of an injured spinal cord would be one of the promising regenerative trials. In previous studies, MSCs have been demonstrated to have an ability to home into the injured site and trans-differentiate into neural lineage cells [37-39], thus the survival, proliferation and differentiation potential of the engrafted MSCs are crucial for cell based therapy. In our current study, GFP-MSCs were transplanted directly into the ventral horn of rat spinal cord by a micropipette injection with or without lithium treatment. After 1 week post transplantation, we found that lithium significantly increased the number of GFP-positive cells at 1 week and 4 weeks, indicating lithium treatment enhanced grafted cell survival. Further, we found that lithium treatment increased BrdU+/GFP+ at 1 week but not at 4 weeks after cell transplantation, indicating lithium enhanced cell proliferation in a short term. Also, in vivo assay, we found that lithium increased the ratio of βIII-tubulin+/GFP+ (neurons), GFAP+/GFP+ (astrocytes) and olig-2+/GFP+ (oligodendrocytes) by compared with control animals, indicating that lithium promotes neural differentiation of grafted GFP-MSCs in the spinal cord. The mechanism underlying lithium enhanced neural differentiation of MSCs is presently not clear, but may possibly involve induction of neurotrophins such as BDNF [19], which is an important neurotrophic factor that possessed ability to promote neural precursor proliferation, survival, and differentiation in a variety of conditions, such as CNS injury [40,41].
In conclusion, in the present study, we found that lithium (0.1 mM) promotes proliferation and neural differentiation of GFP-MSCs in vitro. Moreover, lithium enhances cell survival and promotes transplanted GFP-MSCs to differentiate into more oligodendrocytes, astrocytes and neurons, thus promoting neural regeneration in rat spinal cord. Therefore, our study provides a promising approach to pave the way to a highly effective therapy for MSC-based therapy in CNS disorders.
Acknowledgements
We thank Katherine Page for editorial assistance.
Disclosure of conflict of interest
None.
References
- 1.Sensebe L, Krampera M, Schrezenmeier H, Bourin P, Giordano R. Mesenchymal Stem Cells for clinical application. Vox Sang. 2010;98:93–107. doi: 10.1111/j.1423-0410.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 2.Griffin M, Iqbal SA, Bayat A. Exploring the application of mesenchymal stem cells in bone repair and regeneration. J Bone Joint Surg Br. 2011;93:427–434. doi: 10.1302/0301-620X.93B4.25249. [DOI] [PubMed] [Google Scholar]
- 3.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Aizman I, McGrogan M, Case CC. Quantitative microplate assay for studying mesenchymal stromal cell-induced neuropoiesis. Stem Cells Transl Med. 2013;2:223–232. doi: 10.5966/sctm.2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexanian AR, Fehlings MG, Zhang Z, Maiman DJ. Transplanted neurally modified bone marrow-derived mesenchymal stem cells promote tissue protection and locomotor recovery in spinal cord injured rats. Neurorehabil Neural Repair. 2011;25:873–880. doi: 10.1177/1545968311416823. [DOI] [PubMed] [Google Scholar]
- 6.Kang ES, Ha KY, Kim YH. Fate of transplanted bone marrow derived mesenchymal stem cells following spinal cord injury in rats by transplantation routes. J Korean Med Sci. 2012;27:586–593. doi: 10.3346/jkms.2012.27.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salem AM, Ahmed HH, Atta HM, Ghazy MA, Aglan HA. Potential of bone marrow mesenchymal stem cells in management of Alzheimer’s disease in female rats. Cell Biol Int. 2014;38:1367–1383. doi: 10.1002/cbin.10331. [DOI] [PubMed] [Google Scholar]
- 8.Harris VK, Faroqui R, Vyshkina T, Sadiq SA. Characterization of autologous mesenchymal stem cell-derived neural progenitors as a feasible source of stem cells for central nervous system applications in multiple sclerosis. Stem Cells Transl Med. 2012;1:536–547. doi: 10.5966/sctm.2012-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Fu W, Zhang S, He X, Liu Z, Gao D, Xu T. CXCR-7 receptor promotes SDF-1alpha-induced migration of bone marrow mesenchymal stem cells in the transient cerebral ischemia/reperfusion rat hippocampus. Brain Res. 2014;1575:78–86. doi: 10.1016/j.brainres.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Yan Y, Ma CG, Kang T, Zhang N, Gran B, Xu H, Li K, Ciric B, Zangaladze A, Curtis M, Rostami A, Zhang GX. Accelerated and enhanced effect of CCR5-transduced bone marrow neural stem cells on autoimmune encephalomyelitis. Acta Neuropathol. 2012;124:491–503. doi: 10.1007/s00401-012-0989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Yan Y, Xia Y, Kang T, Li X, Ciric B, Xu H, Rostami A, Zhang GX. Neurotrophin 3 transduction augments remyelinating and immunomodulatory capacity of neural stem cells. Mol Ther. 2014;22:440–450. doi: 10.1038/mt.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Zhang L, Shu R, Wang C, Wang H, Li N, Wang G. Hydrogen-rich saline controls remifentanil-induced hypernociception and NMDA receptor NR1 subunit membrane trafficking through GSK-3beta in the DRG in rats. Brain Res Bull. 2014;106:47–55. doi: 10.1016/j.brainresbull.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SW, Kim JE, Park KS, Choi WJ, Hong YH, Kim SM, Kim SH, Lee KW, Sung JJ. The neuroprotective effect of the GSK-3beta inhibitor and influence on the extrinsic apoptosis in the ALS transgenic mice. J Neurol Sci. 2012;320:1–5. doi: 10.1016/j.jns.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Su H, Yuan Q, Qin D, Yang X, Wong WM, So KF, Wu W. Lithium enhances axonal regeneration in peripheral nerve by inhibiting glycogen synthase kinase 3beta activation. Biomed Res Int. 2014;2014:658753. doi: 10.1155/2014/658753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabrera O, Dougherty J, Singh S, Swiney BS, Farber NB, Noguchi KK. Lithium protects against glucocorticoid induced neural progenitor cell apoptosis in the developing cerebellum. Brain Res. 2014;1545:54–63. doi: 10.1016/j.brainres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngok-Ngam P, Watcharasit P, Thiantanawat A, Satayavivad J. Pharmacological inhibition of GSK3 attenuates DNA damage-induced apoptosis via reduction of p53 mitochondrial translocation and Bax oligomerization in neuroblastoma SH-SY5Y cells. Cell Mol Biol Lett. 2013;18:58–74. doi: 10.2478/s11658-012-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Straiko MM, Young C, Cattano D, Creeley CE, Wang H, Smith DJ, Johnson SA, Li ES, Olney JW. Lithium protects against anesthesia-induced developmental neuroapoptosis. Anesthesiology. 2009;110:862–868. doi: 10.1097/ALN.0b013e31819b5eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan M, Song C, Wang T, Li L, Dong Y, Jin W, Lu P. Protective effects of lithium chloride treatment on repeated cerebral ischemia-reperfusion injury in mice. Neurol Sci. 2015;36:315–21. doi: 10.1007/s10072-014-1943-x. [DOI] [PubMed] [Google Scholar]
- 19.Su H, Zhang W, Guo J, Guo A, Yuan Q, Wu W. Lithium enhances the neuronal differentiation of neural progenitor cells in vitro and after transplantation into the avulsed ventral horn of adult rats through the secretion of brain-derived neurotrophic factor. J Neurochem. 2009;108:1385–1398. doi: 10.1111/j.1471-4159.2009.05902.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Guan XX, Zhu ZL, Guo J, Huang YC, Hou WW, Yu HY. Caffeine inhibits the viability and osteogenic differentiation of rat bone marrow-derived mesenchymal stromal cells. Br J Pharmacol. 2010;161:1542–1552. doi: 10.1111/j.1476-5381.2010.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadzadeh A, Pourfathollah AA, Shahrokhi S, Hashemi SM, Moradi SL, Soleimani M. Immunomodulatory effects of adipose-derived mesenchymal stem cells on the gene expression of major transcription factors of T cell subsets. Int Immunopharmacol. 2014;20:316–321. doi: 10.1016/j.intimp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Chen C, Ye D, Guan D, Ye L, Jin J, Zhao H, Chen Y, Wang Z, Wang X, Xu Y. Diammonium glycyrrhizinate upregulates PGC-1alpha and protects against Abeta1-42-induced neurotoxicity. PLoS One. 2012;7:e35823. doi: 10.1371/journal.pone.0035823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng H, Ying H, Wiedemeyer R, Yan H, Quayle SN, Ivanova EV, Paik JH, Zhang H, Xiao Y, Perry SR, Hu J, Vinjamoori A, Gan B, Sahin E, Chheda MG, Brennan C, Wang YA, Hahn WC, Chin L, DePinho RA. PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas. Cancer Cell. 2010;17:497–509. doi: 10.1016/j.ccr.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang N, Kang T, Xia Y, Wen Q, Zhang X, Li H, Hu Y, Hao H, Zhao D, Sun D, Yan Y, Zhang GX, Yang J. Effects of salvianolic acid B on survival, self-renewal and neuronal differentiation of bone marrow derived neural stem cells. Eur J Pharmacol. 2012;697:32–39. doi: 10.1016/j.ejphar.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Nakano N, Nakai Y, Seo TB, Homma T, Yamada Y, Ohta M, Suzuki Y, Nakatani T, Fukushima M, Hayashibe M, Ide C. Effects of bone marrow stromal cell transplantation through CSF on the subacute and chronic spinal cord injury in rats. PLoS One. 2013;8:e73494. doi: 10.1371/journal.pone.0073494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Yan Y, Ciric B, Yu S, Guan Y, Xu H, Rostami A, Zhang GX. Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. Am J Pathol. 2010;177:1989–2001. doi: 10.2353/ajpath.2010.091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Hayes M, Curley G, Laffey JG. Mesenchymal stem cells - a promising therapy for Acute Respiratory Distress Syndrome. F1000 Med Rep. 2012;4:2. doi: 10.3410/M4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Jiang Z, Fitzgerald DC, Ma C, Yu S, Li H, Zhao Z, Li Y, Ciric B, Curtis M, Rostami A, Zhang G. X. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest. 2009;119:3678–3691. doi: 10.1172/JCI37914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foudah D, Monfrini M, Donzelli E, Niada S, Brini AT, Orciani M, Tredici G, Miloso M. Expression of neural markers by undifferentiated mesenchymal-like stem cells from different sources. J Immunol Res. 2014;2014:987678. doi: 10.1155/2014/987678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonilla S, Silva A, Valdes L, Geijo E, Garcia-Verdugo JM, Martinez S. Functional neural stem cells derived from adult bone marrow. Neuroscience. 2005;133:85–95. doi: 10.1016/j.neuroscience.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 33.Kabos P, Ehtesham M, Kabosova A, Black KL, Yu JS. Generation of neural progenitor cells from whole adult bone marrow. Exp Neurol. 2002;178:288–293. doi: 10.1006/exnr.2002.8039. [DOI] [PubMed] [Google Scholar]
- 34.Tropel P, Platet N, Platel JC, Noel D, Albrieux M, Benabid AL, Berger F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–2876. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- 35.Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- 36.Roybon L, Ma Z, Asztely F, Fosum A, Jacobsen SE, Brundin P, Li JY. Failure of transdifferentiation of adult hematopoietic stem cells into neurons. Stem Cells. 2006;24:1594–1604. doi: 10.1634/stemcells.2005-0548. [DOI] [PubMed] [Google Scholar]
- 37.Kamada T, Koda M, Dezawa M, Anahara R, Toyama Y, Yoshinaga K, Hashimoto M, Koshizuka S, Nishio Y, Mannoji C, Okawa A, Yamazaki M. Transplantation of human bone marrow stromal cell-derived Schwann cells reduces cystic cavity and promotes functional recovery after contusion injury of adult rat spinal cord. Neuropathology. 2011;31:48–58. doi: 10.1111/j.1440-1789.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- 38.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 39.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 40.Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci U S A. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


