Abstract
Constipation is frequently reported in Parkinson’s disease (PD). We evaluated the characteristics of patients with PD and constipation and explored the role of T cell subsets in PD-associated constipation. One hundred and two patients with PD treated at the First Affiliated Hospital of Bengbu Medical College were enrolled in this study between January 2012 and October 2013. All patients completed KESS questionnaires and constipation was rated. The proportions of peripheral blood Thl7 and Treg cells were assessed by flow cytometry in 45 patients. Colonoscopies were performed in six patients. Thirty-one patients with PD reported slow-transit constipation (STC), 15 rectal evacuation disorder (RED) and 33 mixed constipation (Mixed). STC most frequently occurred before onset of PD motor symptoms, while Mixed occurred before or after motor symptoms, and RED occurred most frequently after motor symptoms. CD4+ T cell infiltration in the colonic mucosa was observed in patients with PD and constipation. The frequency of Th17 and Treg cells in patients with PD and constipation was significantly higher than in those without constipation (P<0.001). Among patients with PD and constipation, the frequency of Th17 and Treg cells in STC was the highest. However, there was no difference in the ratio of Th17/Tregs between the patients with PD with and without constipation, or patients with PD and different types of constipations (P>0.05). Constipation reported before the onset of PD motor symptoms was most often STC or Mixed, and PD constipation may be associated with immune activation in the colonic mucosa.
Keywords: Parkinson’s disease, constipation, CD4+ T cells, Th17 cells, Treg cells
Introduction
Parkinson’s disease (PD) is caused by the accumulation of α-synuclein (αSYN) in dopaminergic neurons. αSYN aggregates to form Lewy bodies, leading to neuronal loss. When symptoms appear and PD is diagnosed, patients have already lost more than half of their dopaminergic neurons. Continuous neuronal loss has likely been occurring over the preceding five to six years [1] and αSYN abnormalities in the lower brain stem and peripheral neurons may have occurred even earlier [2,3].
Increasing focus on the early detection of PD has led to the recognition of PD-related non-motor symptoms (NMS). Indeed, constipation appears to be one of the earliest reported NMS and 50% of patients with PD suffer from constipation for up to 20 years before the onset of motor symptoms [4]. Motor symptoms of PD include bradykinesia, myotonia, and postural instability. Uncoordinated movement of the abdominal muscles, rectum, anal sphincter and pelvic floor muscles may lead to constipation. Constipation that occurs after the onset of motor symptoms can be explained in this way. However, it remains unclear what causes constipation before the emergence of motor symptoms.
The pathophysiological mechanisms responsible for constipation can be divided into three categories; slow-transit constipation (STC), rectal evacuation disorder (RED) and mixed constipation [5,6]. STC is characterized by prolonged colonic transit time and reduced number of high-amplitude colonic propulsions and contractions [7]. The impaired colonic function of STC has been attributed to the dysfunction of enteric neurons [8]. RED refers to the condition in which the abdominal muscles, rectum, anal sphincter and pelvic floor muscles cannot coordinate effectively during bowel movement, leading to insufficient rectal propulsion, decreased rectal sensation, and eventual impairment in emptying the rectum [9]. However, the category of constipation occurring in PD patients has not yet been reported.
PD is a neurodegenerative disease, but is also considered to be a neuroinflammatory disease, initiating from the gastrointestinal tract and involving multiple targets. The role of neuroinflammation in PD has recently received increasing attention [10], particularly the involvement of the systemic immune system in progressive degeneration of SN dopaminergic neurons in PD and glial cells has been well characterized [11-13]. Inflammatory and abnormal immune responses have been suggested to play a crucial role in PD [14-17]. Inflammation in the brain, and in the peripheral immune system is believed to contribute to the onset and progression of the neurodegenerative process seen in PD, due to serum αSYN-specific antibodies [12,18], human leukocyte antigen-DR induction [13,19] and lymphocyte infiltration into the brains of patients with PD [17]. However, the roles of systematic inflammatory in PD-associated constipation remain to be determined.
CD4+ helper T cells can be divided into four categories: Th1, Th2, Th17 and Treg cells. Th17 cells regulate the defense and resistance of host skin and mucus membranes against extracellular pathogens, and are distributed widely in the lamina propria of the gastrointestinal tract [20]. Th17 cells, cytokines and chemokines secreted by the gastrointestinal tract affect the gastrointestinal mucosal barrier [21-23], and the differentiation of Treg and Th17 cells depends upon TGF-β. Low concentrations of TGF-β and IL-6 synergistically promote differentiation into Th17 cells, while high concentrations of TGF-β promote the development of regulatory T cells (Tregs) and inhibit development of Th17 cells [24]. Th17 and Treg cells have been involved in mucosal inflammation [25]. During the inflammatory response, a large fraction of Treg cells in the peripheral blood and lymphoid tissues could be converted to Th17 cells in the mucous membranes [26].
In a CD3 antibody-induced animal tolerance model, the gastrointestinal tract was the site of Th17 cell control, highlighting the close relationship between the gastrointestinal tract and Th17 cells [21]. Since autopsy studies have demonstrated infiltration of CD4+ helper T cells into the nigrostriatum [12], the aim of the present study was to investigate the role of T helper cells in the colonic symptoms experienced by patients with PD.
Materials and methods
Subjects
One hundred and two patients with PD who met the diagnostic criteria for idiopathic PD established by the United Kingdom Parkinson’s Disease Society Brain Bank [19] and received treatment at the First Affiliated Hospital of Bengbu Medical College were enrolled between January 2012 and October 2013. All patients had no other diseases and did not take constipation-causing drugs, receiving only madopar (Hoffman-La Roche Pharmaceuticals, Bale, Switzerland) for PD. The Hoehn-Yahr staging of these patients ranged from 1 to 4. The mini-mental state examination (MMSE) and educational background were used to exclude patients with mental retardation. The Hamilton Depression Rating Scale (HAMD) was used to exclude patients with depression. Patients with diabetes or other autoimmune diseases, hematological diseases, endocrine disorders, or diseases of the heart, lung or liver were excluded. Patients with recent infection and intake of immunosuppressants were excluded. Women having their menstrual period or being pregnant were excluded. Patient history (including age of onset, course of disease, development of onset, past history, family history and medication history) was obtained. Neurologists with special training completed the relevant scoring, including the Unified Parkinson’s Disease Rating Scale-III (UPDS-III), Hoehn-Yahr staging, HAMD and MMSE questionnaires. General surgeons with special training conducted the diagnosis of constipation and Knowles-Eccersley-Scott Symptom (KESS) questionnaire were completed by the patients [5].
The mean patient age was 58.8 years (range: 40-74). There were 54 males and 48 females. Forty-five patients agreed to provide blood samples for T cell subset analysis (28 males and 27 females). The mean age of this subgroup of patients was 61.4 years (range: 40-74). An age- and gender-matched control group of 55 healthy subjects was recruited from the patients’ family and those subjects receiving medical examination at the First Affiliated Hospital of Bengbu Medical College. The mean age of the control group was 62 years (range 40-70), including 28 males and 27 females. Written informed consent was obtained from all participants and the study was conducted in accordance with the declaration of Helsinki after approval by the Ethics Committee of Bengbu Medical College.
Assessment of linear discriminant functions
The linear discriminant functions established for the KESS classification method [5] and for RED, STC and Mixed constipation were used. These functions were defined as: RED=-5.813+0.969* Duration of constipation -0.005* Laxative use -1.484* Frequency of bowel movement +1.899* Abdominal pain +2.003* Time taken; Mixed=-6.233+1.475* Duration of constipation +0.882* Laxative use -0.007* Frequency of bowel movement +1.035* Abdominal pain +1.169* Time taken; STC=-6.566+1.766* Duration of constipation +0.666* Laxative use +0.447* Frequency of bowel movement +1.039* Abdominal pain +0.339* Time taken.
The KESS score of each patient was substituted into the above three discriminant functions and the function with the largest value was considered to represent the constipation type of that patient. KESS scores <6 were considered to indicate no constipation and >6 points was considered to indicate constipation.
Collection of tissue and peripheral blood samples
Sterile quantitative blood collection tubes containing K2-EDTA were used to draw 2 ml of peripheral venous blood. Colonoscopy was performed in six patients with PD whose main symptom was severe constipation. The colonoscope (Pentax A12004, Ricoh, Tokyo, Japan) was sequentially passed through the rectum, ascending colon, transverse colon and descending colon. Biopsies were performed using standard biopsy forceps without needles. Colonic mucosa was collected at four points located >20 cm from the anus, and placed in 20% formalin. Specimens were embedded in paraffin for immunohistochemistry.
Immunohistochemistry
Colon sections were dewaxed, subjected to an ethanol gradient, and incubated in 3% H2O2 at room temperature for 10 minutes to eliminate endogenous peroxidase activity. Sections were washed, blocked with normal goat serum (SP-9002 kit, Beijing Zhongshan Inc., Beijing, China), and incubated for 10 minutes at room temperature. After goat serum was removed, PE-conjugated mouse anti-human CD4 monoclonal antibody (Abcam, Cambridge, UK) was added and incubated at 37°C for 1-2 hours. After rinsing, biotin-labeled secondary antibody (Fuzhou Maixin Biotech., Fuzhou, China) was added at 37°C for 30 minutes. After rinsing, alkaline phosphatase-labeled streptavidin was added at 37°C for 30 minutes. Sections were rinsed, and the Diaminobenzidine Detection Kit (Fuzhou MaiXin Biotechnology Development Co., Fuzhou, China) was added for 10 minutes. Hematoxylin counterstaining was performed after rinsing. A coverglass was then applied. The slides were examined by a senior pathologist.
Th17 cell detection
Fresh sterile blood samples (2 ml) were mixed with 100 μl of heparin RPMI 1640 medium (MultiScience LiankeBiotech Co., Ltd., Hangzhou, China), 5 μl of 2 μg/ml Phorbol ester, 5 μl of 50 μg/ml ionomycin and 4 μl of 5 mg/ml brefeldin (MultiScience LiankeBiotech Co., Ltd., Hangzhou, China). Cells were incubated at 37°C in a 5% CO2 incubator for 5 h. Fixative was added and the mixture was incubated in the dark at room temperature for 15 min. Cells were then washed, centrifuged, and the supernatant was discarded. A solubilizing agent (BD Biosciences, Franklin Lake, NJ, USA) was added to disrupt the cell membrane. Cells were then stained with APC-conjugated mouse anti-human CD3 (BD Biosciences), PERCP-labeled mouse anti-human CD4 (BD Biosciences) and PE-labeled IL-17A monoclonal antibodies (Abcam, Cambridge, UK) or PE-labeled isotype control at 35°C in the dark for 15 min. After rinsing and centrifugation, the supernatant was discarded. PBS was added, and the samples were examined in a FACSCalibur flow cytometer (BD Biosciences) to determine the proportions of CD3+, CD4+, and IL-17A+ cells.
Tregs detection
Tregs were defined as CD3+ CD4+ CD25high CD127dim as it has been shown these cells represent the large majority of FoxP3+ Tregs [27,28]. We chose this definition instead of the commonly accepted conception (CD3+ CD4+ CD25high FoxP3+) to avoid excessive cell loss from blood samples during the fixation and washing steps required for FoxP3 staining. Fresh sterile blood (100 μl) containing heparin was incubated with APC-conjugated mouse anti-human CD3 (BD Biosciences), FITC-labeled mouse anti-human CD25 (BD Biosciences), PERCP-labeled mouse anti-human CD4 and PE-conjugated mouse anti-human CD127 (BD Biosciences) monoclonal antibodies and the appropriate isotype controls at 35°C in the dark for 15 min. FACS lysing solution was added and both tubes were incubated for 5 min, centrifuged, washed and fixed with 0.5 mL of 1% paraformaldehyde. Multicolor flow cytometric analysis was performed using a FACSCalibur flow cytometer (BD Biosciences).
Statistical analysis
SPSS 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data are expressed as means ± standard deviation. To compare characteristics between the patients with PD with and without constipation, categorical data were analyzed using the X2 test and quantitative data were analyzed using t-test. To compare clinical symptoms and T cell subsets among various groups, analysis of variance (ANOVA) was performed for between-group comparisons with the Student-Newman-Keuls post hoc test. For KESS score analysis, independent sample t test was used. P-values <0.05 were considered statistical significant.
Results
Characteristics of the patients
One hundred and two patients with PD treated at the First Affiliated Hospital of Bengbu Medical College were enrolled in this study between January 2012 and October 2013. There was no significant difference in age between the PD with constipation and PD without constipation groups (Table 1).
Table 1.
Characteristics of the patients with PD
| PD with constipation | PD without constipation | ||
|---|---|---|---|
|
|
|||
| Occurrence before PD | Occurrence after PD | ||
| Patient number | 52 | 27 | 23 |
| Gender | |||
| Male | 23 | 17 | 14 |
| Female | 29 | 10 | 9 |
| Age | 59.46±8.23 | 60.81±6.63 | 59.39±11.04 |
| UPDRS part III | 28.08±6.04 | 30.93±5.98 | 19±7.93* |
| Disease duration (months) | 55.15±37.14 | 71.56±33.03 | 33.91±16.84* |
| Hoehn & Yahr Stage | 2.25±0.59 | 2.52±0.7 | 1.65±0.57* |
P<0.05 vs. both PD with constipation groups.
Constipation category and clinical symptoms
All patients completed the KESS questionnaire and constipation was rated. Twenty-three patients had no constipation and 79 (77.5%) had constipation (Figure 1). Among the 79 PD patients with constipation, 52 were diagnosed before the occurrence of PD and 27 were diagnosed after the occurrence of PD symptoms. Over 80% of the patients with PD and constipation had slow colonic transit. In the remaining 20% of patients with PD and constipation, colonic transit was not involved, and there was only uncoordinated anorectal movement. Linear discriminant analysis of the 79 patients with constipation revealed that this patient group included 31 patients with STC, 15 with RED and 33 with mixed constipation.
Figure 1.
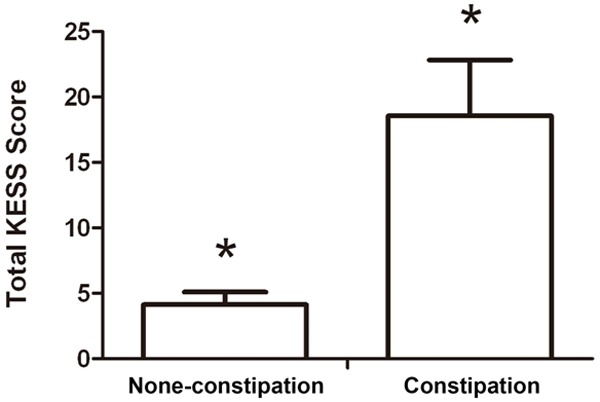
KESS score in patients with PD and constipation and in those without constipation. Total KESS score of patients with PD and constipation (n=79) and PD without constipation (n=23). *P<0.001.
There were no significant differences in age between the PD patients with RED, STC and mixed constipation groups. STC constipation most frequently occurred before onset of PD motor symptoms, mixed constipation occurred before or after motor symptoms, and RED occurred most frequently after motor symptoms. Patients with mixed constipation had a significant longer disease course compared with RED or STC patients. Patients with RED, mixed constipation and STC constipation had a significantly increasingly duration of constipation (Table 2).
Table 2.
Clinical symptoms in patient with PD and different types of constipation
| Types of constipation in PD patients | |||
|---|---|---|---|
|
| |||
| RED (n=15) | Mixed (n=33) | STC (n=31) | |
| Age (years) | 62.2±6.1 | 60.5±8.0 | 58.20±7.9 |
| Disease course (months) | 59.2±33.8 | 70.9±23.7* | 50.7±45.8 |
| Constipation duration (months) | 34.4±14.2# | 78.9±37.0# | 213.0±122.2# |
P<0.05 vs. RED and STC.
P<0.05 between the three groups.
Colonic mucosal pathological observation
The fact that 39.2% (31/79) of the patients with PD and constipation were diagnosed with STC implied the presence of colonic lesions. To test this hypothesis, colonoscopy was performed in six patients with PD whose main symptom was severe constipation. Five had varying degrees of colonic mucosal congestion, edema and obscured texture of the vessels. Figure 2 shows the colonoscopic observation of colon mucosa in a typical patient with hemorrhoids, but without PD and constipation (Figure 2A) and a typical patient with PD and constipation (Figure 2B). Congestion in colonic mucous, edema, and vascular lake was not clear (Figure 2B).
Figure 2.
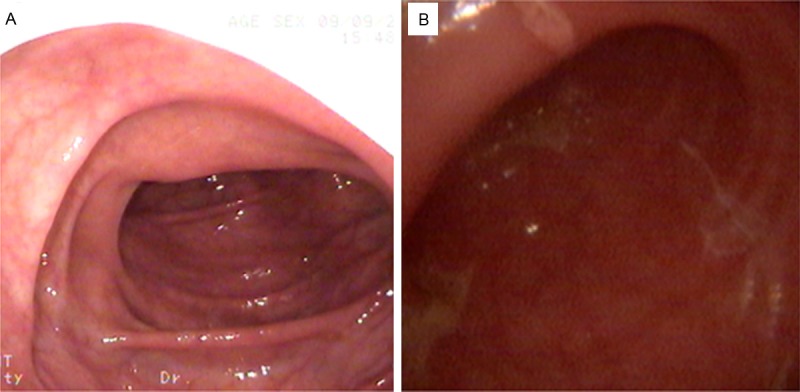
Colonic mucous observation. Colonoscopic observation of colon mucosa in a typical patient with hemorrhoids, but without PD and constipation (A) and a typical patient with PD and constipation (B). Congestion in colonic mucous, edema, and vascular lake was not clear.
Immunohistochemistry of colonic mucosal biopsies was performed to identify CD4+ cells and CD8+ cells in the colonic mucosa of healthy subjects and of those with PD and constipation. The results indicated that CD4-positive cells were increased (up to 50-60% CD4+ cells) while CD8-positive cells were not present in the colonic mucosa of the patients with PD-associated constipation (illustrated in Table 3; Figure 3).
Table 3.
Colonoscopic observation in six patients with PD and constipation
| No. | Gender | Age (years) | Duration of PD (years) | Duration of constipation (years) | Constipation type | Mucosal appearance | CD4+ cell | CD8+ cell |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 65 | 8 | >30 | Mixed | Chronic inflammation | 50-60% | Negative |
| 2 | Female | 59 | 6 | >10 | Mixed | Colon polyps | 20-30% | Negative |
| 3 | Female | 54 | 3 | >40 | STC | Chronic inflammation | 50-60% | Negative |
| 4 | Female | 67 | 7 | >30 | STC | Chronic inflammation | 50-60% | Negative |
| 5 | Male | 70 | 1 | >20 | STC | Chronic inflammation | 30-40% | Negative |
| 6 | Male | 69 | 8 | >15 | Mixed | Chronic inflammation | 30-40% | Negative |
Figure 3.
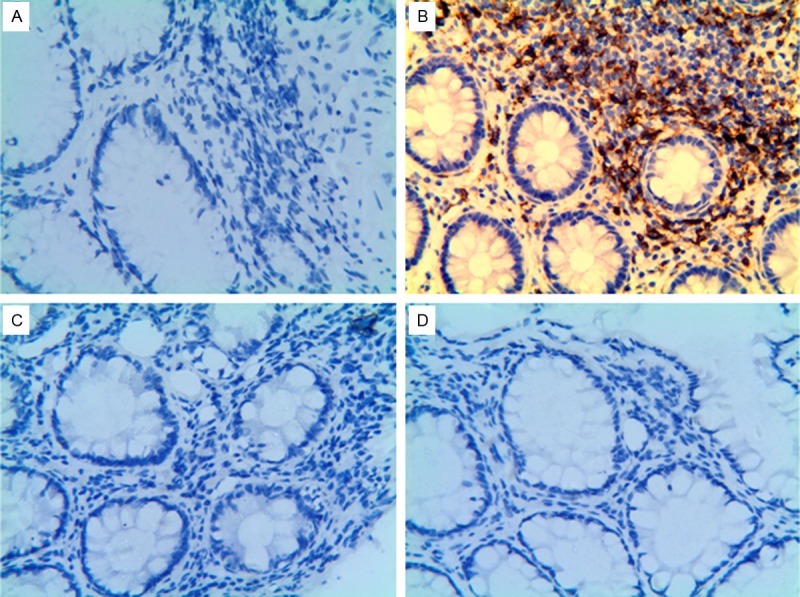
CD4+ cell infiltration into colonic muscle in PD patients with constipation. Colonic mucosal CD4+ cells and CD8+ in a normal healthy subject and a patient with PD and constipation by immunohistochemistry. Magnification 40×. A. CD4+ cells in a normal healthy subject. B. CD4+ cells in PD constipation patient. C. CD8+ cells in normal healthy subject. D. CD8+ cells in patient with PD and constipation.
Peripheral T cell subsets in PD patients with constipation
The frequency of Th17 cells in the peripheral blood of patients with PD and constipation and in those without constipation (0.892±0.195% and 0.764±0.151%, respectively) was significantly higher than that in the control group (0.516±0.157%) (both P<0.001, Figure 4A). The frequency of Treg cells in the peripheral blood of patients with PD and constipation and in those without constipation (4.258±0.941% and 3.671±0.640%, respectively) was significantly lower than that in the control group (5.914±0.419%) (both P<0.001, Figure 4B).
Figure 4.
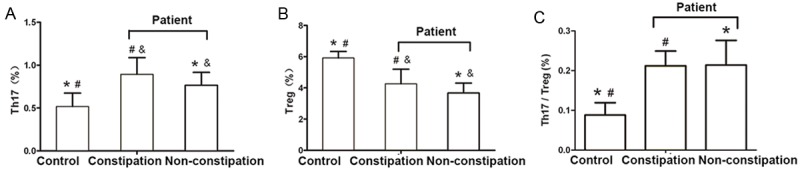
T cell subsets in the peripheral blood of patients with PD and constipation, in PD without constipation and in healthy controls. A. The proportion of Th17 cells in the peripheral blood of patients with PD and constipation, PD without constipation and healthy controls; B. The proportion of Treg cells in the peripheral blood of patients with PD and constipation, PD without constipation and healthy controls; C. The Th17/Treg balance in patients with PD and constipation, PD without constipation and healthy controls. *P<0.001, comparison between the healthy control group and the PD without constipation group; #P<0.001, comparison between the healthy control group and the PD constipation group; &P<0.05, comparison between the PD without constipation group and the PD constipation group. Control group (n=40), PD without constipation group (n=11), PD constipation group (n=34).
The frequency of Th17 and Treg cells in patients with PD and constipation (0.892±0.195% and 4.258±0.941%, respectively) was higher than that in those with PD without constipation (0.764±0.151% and 3.671±0.640%, respectively) (both P<0.001, Figure 4A, 4B).
The ratio of Th17/Treg in patients with PD and constipation (0.212±0.037) and those without constipation (0.214±0.062) was significantly higher than in the healthy control group (0.088±0.031) (both P<0.001). However, no significant difference was observed between patients PD and with or without constipation (P>0.05) (Figure 4C).
Peripheral T cell subsets in PD patient with different types of constipation
There was a significant difference in Th17 and Treg proportions between patients with PD and different constipation types (P<0.01, Figure 5A, 5B). The proportions of Th17 and Treg cells in patients with PD and STC (1.082±0.191% and 5.404±0.784%, respectively) were higher than in patients with PD and RED (0.723±0.180% and 3.317±0.372%, respectively) or mixed constipation (0.842±0.106% and 3.936±0.396%, respectively) or patients with PD but without constipation (0.764±0.151% and 3.671±0.640%, respectively) (all P<0.001, Figure 5A, 5B). In addition, the Treg proportion in PD with mixed constipation (3.936±0.396%) was significantly higher than in PD with RED (3.317±0.372%) (P=0.025, Figure 5B). However, no significant difference in the ratio of Th17/Tregs was observed between the patients with PD and different types of constipation (P=0.804, Figure 5C).
Figure 5.
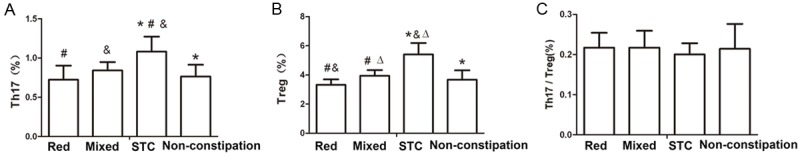
T cell subsets in the peripheral blood of patients with PD but without constipation, and PD with RED, STC or mixed constipation. A. The proportion of Th17 cells in the peripheral blood of patients with PD and constipation; B. The proportion of Treg cells in the peripheral blood of patients with PD and constipation; C. The Th17/Treg balance in the peripheral blood of patients with PD and constipation. *P<0.05, comparison between the PD non-constipation group and the STC group; #P<0.01, comparison between the RED and Mixed group; &P<0.01, comparison between the RED and STC group; DP<0.01, comparison between the STC and Mixed group. Constipation group (n=79) including RED (n=15), STC (n=31) and mixed (n=33). Non-constipation group (n=23).
Discussion
In order to evaluate the characteristics of constipation in patients with PD and to investigate the role of T helper cells in the gastrointestinal symptoms, a questionnaire on constipation was distributed to 102 patients with PD. In more than 60% of the patients, constipation was reported before the occurrence of motor symptoms. These findings suggest that PD involves the colon, either before or after the brain is affected. STC occurred most frequently in patients with PD and a short disease duration and constipation occurring before motor symptoms. Mixed constipation occurred most frequently in patients with PD and a long disease duration and constipation occurring either before or after motor symptoms. RED occurred most frequently in patients with PD and a short disease duration and constipation occurring after motor symptoms. Although constipation category has been reported, PD-associated constipation has not been classified yet. Moreover, it was found that the manifestation of constipation varied with time, most of constipation (STC) occurring before onset of motor symptoms, mainly exhibiting colonic transmission impairment, and a portion of constipation after motor symptoms occurrence, mainly showing import and export dysfunctions. This finding has not been reported previously. This study would be helpful for the exploration of the disease pathogenesis and potential treatments.
Colonoscopy was performed in six PD patients in which constipation had persisted for 10-20 years before the onset of PD motor symptoms. Chronic colitis was seen in five of the six patients and the remaining patient had colonic polyps. Immunohistochemistry revealed substantial CD4+ cell infiltration of the colonic mucosa. This further suggests that PD involves the colon. The observation that PD patients had inflammatory changes in their colon supports the hypothesis that immune inflammation was involved in the development and progression of PD-associated constipation.
Autopsy studies have demonstrated that CD4+ helper T cells infiltrate the nigrostriatum [12]. In the present study, it was found that CD4+ helper T cells also infiltrated the colonic mucosal tissues. We hypothesized that changes in Th17 cells, Treg cells or the balance between the two might occur in PD-associated constipation. Hence, we determined the frequency of Th17 cells and Treg cells in the peripheral blood of 45 patients with PD and in the peripheral blood of age- and gender-matched healthy control subjects. Patients with PD and constipation had a significantly increased proportion of circulating Th17 and Treg cells compared with those without constipation. Among patients with PD and constipation, the proportion of Th17 and Treg cells in STC was the highest. However, no significant difference in the ratio of Th17/Tregs was observed between patients with PD and with or without constipation, or patients with PD and different types of constipation. The involvement of Th17 and Treg cells in the pathogenesis of PD-associated constipation needs to be further investigated.
PD associated with constipation was previously considered to be caused by neurological disorders. However, in the present study, we observed inflammation in the colon and increased CD4 T cell infiltration into the colonic mucosae in PD associated with constipation. We also observed that peripheral Th17 and Treg cell proportions were positively correlated with the severity of colonic involvement, which has not been previously reported. These finding would provide a new basis for the mechanistic research and treatment of PD-associated constipation.
This study has some limitations. Constipation was only classified using KESS questionnaires and related analyses. We pursued no confirmatory physiological tests such as anorectal manometry, anorectal sensory function testing, evacuation proctography, or assessment of colonic transit by radiopaque markers. This study was also carried out at a single-center, and the number of patients who underwent colonic biopsy was not large. Future studies with a larger number of patients are needed to confirm our findings. In addition, we did not enroll a control group of patients with constipation without PD because the causes of constipation in the general population are too numerous and diverse, and thus the number of confounding factors were too numerous to properly select control subjects with constipation but without PD.
In summary, PD is likely a multi-centric neurodegenerative disease. Inflammation and other pathological processes may involve not only the brain, but also the colon. Such inflammation likely involves CD4+ helper T cells. PD-related affects on both the colon and the brain may be involved in the development of constipation in patients with PD, the former mainly via colonic transit disorder, and the latter mainly via uncoordinated bowel-related muscle movement.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81001457) and the Key Projects of Anhui Provincial Department of Education (KJ2014A163).
Disclosure of conflict of interest
None.
References
- 1.Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, Farinas I, Obeso JA, Bezard E, Vila M. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- 3.Marek K, Jennings D. Can we image premotor Parkinson disease? Neurology. 2009;72:S21–26. doi: 10.1212/WNL.0b013e318198df97. [DOI] [PubMed] [Google Scholar]
- 4.Savica R, Carlin JM, Grossardt BR, Bower JH, Ahlskog JE, Maraganore DM, Bharucha AE, Rocca WA. Medical records documentation of constipation preceding Parkinson disease: A case-control study. Neurology. 2009;73:1752–1758. doi: 10.1212/WNL.0b013e3181c34af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowles CH, Eccersley AJ, Scott SM, Walker SM, Reeves B, Lunniss PJ. Linear discriminant analysis of symptoms in patients with chronic constipation: validation of a new scoring system (KESS) Dis Colon Rectum. 2000;43:1419–1426. doi: 10.1007/BF02236639. [DOI] [PubMed] [Google Scholar]
- 6.Shahid S, Ramzan Z, Maurer AH, Parkman HP, Fisher RS. Chronic idiopathic constipation: more than a simple colonic transit disorder. J Clin Gastroenterol. 2012;46:150–154. doi: 10.1097/MCG.0b013e318231fc64. [DOI] [PubMed] [Google Scholar]
- 7.Dinning PG, Zarate N, Hunt LM, Fuentealba SE, Mohammed SD, Szczesniak MM, Lubowski DZ, Preston SL, Fairclough PD, Lunniss PJ, Scott SM, Cook IJ. Pancolonic spatiotemporal mapping reveals regional deficiencies in, and disorganization of colonic propagating pressure waves in severe constipation. Neurogastroenterol Motil. 2010;22:e340–349. doi: 10.1111/j.1365-2982.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- 8.Bassotti G, Villanacci V. Can “functional” constipation be considered as a form of enteric neuro-gliopathy? Glia. 2011;59:345–350. doi: 10.1002/glia.21115. [DOI] [PubMed] [Google Scholar]
- 9.Lunniss PJ, Gladman MA, Benninga MA, Rao SS. Pathophysiology of evacuation disorders. Neurogastroenterol Motil. 2009;21(Suppl 2):31–40. doi: 10.1111/j.1365-2982.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- 10.Lema Tome CM, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P. Inflammation and alpha-synuclein’s prion-like behavior in Parkinson’s disease--is there a link? Mol Neurobiol. 2013;47:561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens CH, Rowe D, Morel-Kopp MC, Orr C, Russell T, Ranola M, Ward C, Halliday GM. Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol. 2012;252:95–99. doi: 10.1016/j.jneuroim.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 12.González H, Contreras F, Prado C, Elgueta D, Franz D, Bernales S, Pacheco R. Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson’s disease. J Immunol. 2013;190:5048–5056. doi: 10.4049/jimmunol.1203121. [DOI] [PubMed] [Google Scholar]
- 13.Melief J, Koning N, Schuurman KG, Van De Garde MD, Smolders J, Hoek RM, Van Eijk M, Hamann J, Huitinga I. Phenotyping primary human microglia: tight regulation of LPS responsiveness. Glia. 2012;60:1506–1517. doi: 10.1002/glia.22370. [DOI] [PubMed] [Google Scholar]
- 14.Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N’Guyen JM, Chaumette T, Tasselli M, Paillusson S, Flamand M, Galmiche JP, Damier P, Derkinderen P. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One. 2010;5:e12728. doi: 10.1371/journal.pone.0012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umemura A, Oeda T, Tomita S, Hayashi R, Kohsaka M, Park K, Sugiyama H, Sawada H. Delirium and high Fever are associated with subacute motor deterioration in Parkinson disease: a nested case-control study. PLoS One. 2014;9:e94944. doi: 10.1371/journal.pone.0094944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noelker C, Morel L, Osterloh A, Alvarez-Fischer D, Lescot T, Breloer M, Gold M, Oertel WH, Henze C, Michel PP, Dodel RC, Lu L, Hirsch EC, Hunot S, Hartmann A. Heat shock protein 60: an endogenous inducer of dopaminergic cell death in Parkinson disease. J Neuroinflammation. 2014;11:86. doi: 10.1186/1742-2094-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.More SV, Kumar H, Kim IS, Song SY, Choi DK. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators Inflamm. 2013;2013:952375. doi: 10.1155/2013/952375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maetzler W, Apel A, Langkamp M, Deuschle C, Dilger SS, Stirnkorb JG, Schulte C, Schleicher E, Gasser T, Berg D. Comparable autoantibody serum levels against amyloid- and inflammation-associated proteins in Parkinson’s disease patients and controls. PLoS One. 2014;9:e88604. doi: 10.1371/journal.pone.0088604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W Jr, Rongvaux A, Van Rooijen N, Haberman AM, Iwakura Y, Kuchroo VK, Kolls JK, Bluestone JA, Herold KC, Flavell RA. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dambacher J, Beigel F, Zitzmann K, De Toni EN, Goke B, Diepolder HM, Auernhammer CJ, Brand S. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut. 2009;58:1207–1217. doi: 10.1136/gut.2007.130112. [DOI] [PubMed] [Google Scholar]
- 23.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, Pierce RH, McClanahan T, Kastelein RA. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lackner AA, Mohan M, Veazey RS. The gastrointestinal tract and AIDS pathogenesis. Gastroenterology. 2009;136:1965–1978. doi: 10.1053/j.gastro.2008.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing Y, Deng Z, Yao Z, Tsun A, Li B. FOXP3 and RORgammat: transcriptional regulation of Treg and Th17. Int Immunopharmacol. 2011;11:536–542. doi: 10.1016/j.intimp.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fazekas de St Groth B, Zhu E, Asad S, Lee L. Flow cytometric detection of human regulatory T cells. Methods Mol Biol. 2011;707:263–279. doi: 10.1007/978-1-61737-979-6_17. [DOI] [PubMed] [Google Scholar]


