Abstract
Objective: Increased expression of tumor necrosis factor a (TNF-α) has emerged as an important inflammatory factor in osteoarthritis (OA) and other joint diseases. The study was performed to investigate whether the expression of TNF-α in human chondrocytes was regulated by miRNAs. Methods: MiRNA-130a and TNF-α expression in cartilage specimens was examined in patients with knee osteoarthritis, chondrocytes and osteoarthritis rat model. Chondrocytes were transfected with siRNAs as a gene silencing methods. Expression of genes and proteins were analyzed by real-time PCR and western blotting respectively. Results: Increased TNF-α and decreased miRNA-130a were observed in tissues from osteoarthritis patients. Moreover, we found a highly negitive correlation between miRNA-130a and TNF-α. Next, miRNA-130a loss-of-function increased the expression of TNF-α and promoted inflammation in chondrocytes. It was reasonable that miRNA-130a regulated a distinct underlying molecular and pathogenic mechanism of OA by forming a negative feedback loop with TNF-α. Furthermore, there were the abnormalities of bone metabolism in OA rat, which showed the miRNA-130a and TNF-α dysfunction that was one of important factors for the occurrence and development of OA. Conclusions: Our results indicated that miR-130a played an important role in regulating the expression of TNF-α in human chondrocytes and identified miR-130a as a novel therapeutic target in OA.
Keywords: Osteoarthritis, chondrocytes, miRNA-130a, TNF-α, bone
Introduction
Osteoarthritis (OA) is a widely prevalent disease characterized by the progressive destruction of articular cartilage and is responsible for a majority of the health and economic burden in the elderly population worldwide [1,2]. Although multiple factors are involved in triggering OA, the cartilage destruction appears to be a result of imbalance between extracellular matrix (ECM) synthesis and degradation [3]. During the development of OA, chondrocytes become metabolically active and disrupt the equilibrium between anabolic and catabolic effects [4]. On the other hand, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are involved in articular chondrocytes functional or molecular dysfunction [5]. A meta-analysis shows that TNF-α gene polymorphisms is associated with an increased risk of OA patients, which have a higher frequency of the AA genotype and GA genotype compared with the control population [6]. In vitro research shows that IL-1β and/or TNF-α up-regulate matrix metalloproteases-1 (MMP-1) and -3 (MMP-3) mRNA in chondrocyte potentially pathogenic in OA [7]. However, the underlying molecular mechanisms regulating chondrogenesis during osteoarthritis development are still poorly understood.
MicroRNAs (miRNAs) are small nonencoded RNAs serving as important post-transcriptional gene regulators. The key feature of miRNAs control cell proliferation and differentiation of various cell types [8,9]. A growing number of studies have demonstrated that the pathogenic change in tissues has been linked to miRNAs [10,11]. Altered miRNAs expression has been implicated in the pathogenesis of different forms of arthritis, including rheumatoid arthritis (RA) and OA [12,13]. Many studies have demonstrated that altered expression of miRNAs in synovia, peripheral blood mononuclear cells (PBMCs) or T cells from patients with RA or OA is associated with innate immunity and inflammation [14-16]. A recent study shows that miRNA-145 [2], miRNA-21 [1] and miRNA-25 [17] are over-expressed in the articular chondrocytes of osteoarthritis, and miRNA-146a [12] and miRNA-149 [18] are suppressed. The above research results show that miRNAs post-transcriptional regulatory is critical in the activity of chondrocytes and its pathophysiological significance in the development of osteoarthritis. Carrying out a functional study by luciferase assays shows that miR-149 is suppressed in OA chondrocytes, and this phenomenon seems to be correlated to increased expression of pro-inflammatory cytokines such as TNFα, IL1β and IL6 [18]. On the contrary, inhibition of miR-155 reduces TNF-α production. Further study indicates that miRNA-155-deficient mice are resistant to collagen-induced arthritis, with profound suppression of antigen-specific Th17 cell and autoantibody responses and markedly reduced articular inflammation [19].
MiRNA-130a has recently been found to be implicated in many critical processes in various types of human diseases, such as hepatocellular carcinoma [20], ventricular arrhythmias [21], hepatic insulin sensitivity and liver steatosis [22], endothelial progenitor cell dysfunction [23] and human colorectal cancer [24]. However, the underlying molecular mechanisms of miRNA-130a regulating chondrogenesis during the development of osteoarthritis are still poorly understood. In this study, we found that the increasing of TNF-α and decreasing of miRNA-130a was highly correlated in chondrocytes from OA patients. The study further suggested that decreased expression of miRNA-130a, both in vitro and in vivo, correlated with TNF-α in the development of osteoarthritis.
Materials and methods
Serum samples and cell culture
Cartilage tissues were obtained at the time of total knee replacement from OA patients diagnosed according to the American College of Rheumatology criteria for this disease, and patients were announced with written informed consent from The East Hospital of Tongji University on Animal Care. The study was approved by the Ethics Committee of The East Hospital of Tongji University on Animal Care. 25 cartilage tissues of OA patients and 25 cases of normal control were collected between 02/2012 and 8/2014.
Human chondrocytes were maintained in RPMI-1640 (Invitrogen, USA) supplemented with 10% FBS (Invitrogen, USA) at 37°C in a humidified incubator (Thermo, USA), 5% CO2, 95% air atmosphere. The medium was replenished every day. Confluent cells were treated with various concentrations of IL-1β (PeproTech, Rocky Hill, NJ, USA).
Animal treatment
Twelve-month-old male Sprague-Dawley rats (Slac Laboratory Animal, Shanghai, China) were allowed to acclimate to the environment for 1 week. All experimental procedures were carried out in accordance with the guidelines of The Sixth Affiliated Hospital of Shanghai Jiaotong University on Animal Care. The right knee joint was exposed with a medial par patellar approach. The patella was dislocated laterally and the knee placed in full flexion, followed by anterior cruciate ligament transection and medial meniscus resection with micro-scissors. Sham-arthrotomized animals were negative controls. The rats were randomly divided into three groups: (1) Vehicle group (n = 6); (2) OA group (n = 6); (3) OA with etanercept-treated group received etanercept orally at a dose of 20 mg/kg per day (ETA, n = 6). All rats were sacrificed 8 weeks after ETA treatment.
Bone histomorphology
The tibias were decalcified in 0.5 M EDTA (pH = 8.0) and then embedded in paraffin by standard histological procedures. Section of 5 μm were cut and stained with toluidine blue staining and visualized under a microscope (Leica DM 2500).
The trabecular bone microarchitecture of the proximal metaphysis of the tibia was measured using a microtomography scanner (SkyScan 1076, Kontizh, Belgium) with a slice thickness of 22 μm. Bone morphometric parameters, including bone volume over total volume (BV/TV), trabecula number (Tb.N), trabecula thickness (Tb. Th) and bone mineral density over total volume (BMD/TV) were obtained by analyzing the VOI.
Quantitative real-time PCR
Chondrocytes (1.0 × 105/well) were plated and treated in 6-well plates (three wells per group) with various concentrations of IL-1β for 24 h or the combination IL-1β with etanercept (ETA). The Chondrocytes RNA extraction was performed according to the TRIzol manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Synthesis of cDNAs was performed by reverse transcription reactions with 4 μg of total RNA using moloney murine leukemia virus reverse transcriptase (Promega, Switzerland) with oligo dT (15) primers (Fermentas) as described by the manufacturer. The first strand cDNAs served as the template for the regular polymerase chain reaction (PCR) performed using a DNA Engine (ABI 9700). The cycling conditions were 2-min polymerase activation at 95°C followed by 40 cycles at 95°C for 15 s and 55°C for 60 s. PCR with the following primers: miRNA-130a, Forward 5’-TTGCGATTCTGTTTTGTGCT-3’ and Reverse 5’-GTGGGGTCCTCAGTGGG-3’; TNF-α, Forward 5’-TCAGCCGAGGTGATACGTAT -3’ and Reverse 5’-CACGATTAGCCTACATACAACGC-3’; GAPDH, Forward 5’-ACAGGGGAGGTGATAGCATT-3’ and Reverse 5’-GACCAAAAGCCTTCATACATCTC-3’. Glyceraldehyde-phosphate dehydrogenase (GAPDH) as an internal control was used to normalize the data to determine the relative expression of the target genes. The reaction conditions were set according to the kit instructions. After completion of the reaction, the amplification curve and melting curve were analyzed. Gene expression values are represented using the 2-ΔΔCt method.
Western blotting
The chondrocytes were homogenized and extracted in NP-40 buffer, followed by 5-10 min boiling and centrifugation to obtain the supernatant. Samples containing 60 μg of protein were separated on 10% SDS-PAGE gel, transferred to PVDF Transfer Membrane (Millipore). After saturation with 5% (w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with the following antibodies, TNF-α, TGF-β, MMP-9 and CAII (Santa Cruz, USA), at dilutions ranging from 1:500 to 1:2,000 at 4°C over-night. After three washes with TBST, membranes were incubated with secondary immunoglobulins (Igs) conjugated to IRDye 800CW Infrared Dye (LI-COR), including donkey anti-goat IgG and donkey anti-mouse IgG at a dilution of 1:10,000-1:20,000. After 1 hour incubation at 37°C, membranes were washed three times with TBST. Blots were visualized by the Odyssey Infrared Imaging System (LI-COR Biotechnology). Signals were densitometrically assessed (Odyssey Application Software version 3.0) and normalized to the β-actin signals to correct for unequal loading using the monoclonal anti-β-actin antibody (Bioworld Technology, USA).
RNA interference
1.0 × 105 cells per well were seeded in 6-well plates the day before transfection. The next day, Lenti-anti-miR130a (Shanghai Genepharma, China) was added into chondrocytes with 1 ml fresh DMEM/F12 containing 10% FBS, 5 μg/ml Polybrene (Sigma, USA). Twelve hours later, the medium was removed and replaced with fresh culture medium. Three days later, the cells were collected for subsequent culture. The experiment was repeated three times.
Statistical analysis
The data from these experiments were reported as mean ± standard errors of mean (SEM) for each group. All statistical analyses were performed by using PRISM version 4.0 (GraphPad). Inter-group differences were analyzed by one-way ANOVA, and followed by Tukey’s multiple comparison test as a post test to compare the group means if overall P < 0.05. Differences with P value of < 0.05 were considered statistically significant.
Results
The expression of miRNA-130a and TNF-α in chondrocytes from OA patients
We initially assessed miRNA-130a and TNF-α expression levels in osteoarthritis patients. As shown by real-time PCR analysis, the expression level of miRNA-130a was significantly decreased, and the expression of TNF-α was markedly increased (superior to 3 folds) in tissues from osteoarthritis patients compared to tissues from traumatic amputees (Figure 1A and 1B). Interestingly, when the correlation between miRNA-130a and TNF-α was analyzed as a continuous variable, we found a highly negitive correlation between miRNA-130a and TNF-α in tissues from osteoarthritis patients (Figure 1C). As shown in Figure 1D, the western blotting results showed that the down-regulation of TNF-α (P < 0.05) in tissues from osteoarthritis patients compared to that of the control group.
Figure 1.
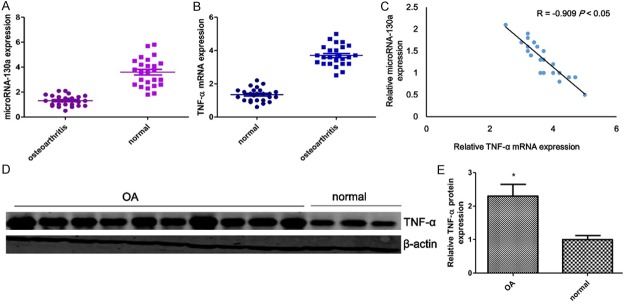
The expression of miRNA-130a and TNF-α in chondrocytes from OA patients. MiRNA-130a expression is measured by Quantitative real-time PCR (A). The mRNA (B) and protein (D) expression of TNF-α are respectively measured by Quantitative real-time PCR and western blotting in chondrocytes from OA patients. Correlation between miRNA-130a and TNF-α (C). Densitometric quantification for western blotting (E). Values are expressed as mean ± SEM, n = 25 in each group. *P < 0.05, versus normal control group.
IL-1β induces TNF-α expression and suppresses miRNA-130a expression
To explore the effect of IL-1β on chondrocytes at the molecular level, the expression of TNF-α and miR-130a induced by IL-1β were evaluated, after exposure to IL-1β for 24 h with with various concentrations. In this experiment, we shown that the expression of miRNA-130a was suppressed when the concentration of IL-1β more than 0.5 ng/ml (Figure 2A). Of note, the mRNA and protein expression of TNF-α increased in a concentration dependent manner when the chondrocytes exposured to IL-1β (Figure 2B-D). Etanercept, TNF-α inhibitor, treatment significantly reversed the expression of TNF-α and miRNA-130a in response to IL-1β.
Figure 2.
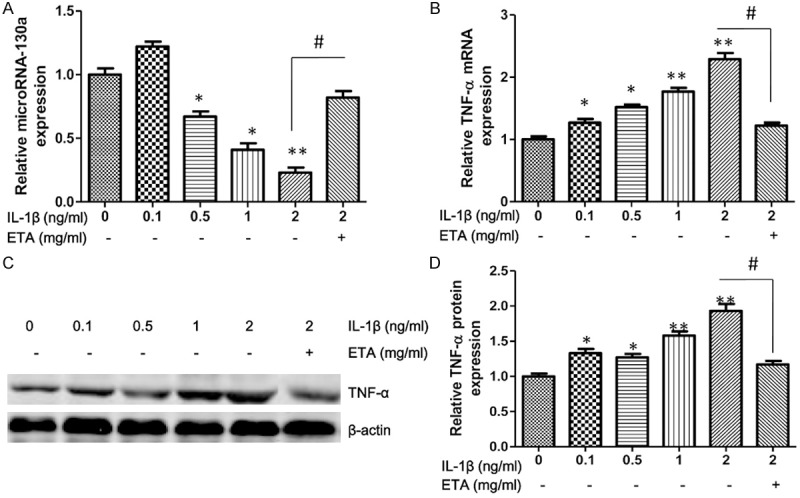
IL-1β induces TNF-α expression and suppresses miRNA-130a expression. Chondrocytes are treated with IL-1β (ng/ml) for various concentrations or combined with etanercept (ETA, 2 mg/ml) for 48 h, RNA expression of miRNA-130a is measured by Quantitative real-time PCR in chondrocytes (A). The mRNA (B) and protein (C) expression of TNF-α are respectively measured by Quantitative real-time PCR and western blotting in chondrocytes. Densitometric quantification for western blotting (D). Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05, versus normal control group; #P < 0.05, versus IL-1β (2 ng/ml) treatment group.
MiRNA-130a loss-of-function in chondrocytes
We investigated the genes expression of catabolic and anabolic factors and inflammatory cytokines in chondrocytes because matrix-degrading enzymes and proinflammatory cytokines have a key role in the pathogenesis of osteoarthritis [25]. MiRNA loss-of-function caused a decrease in the gene expression pattern of type-II-collagen, type-X-collagen and Runx2, and the expression of MMP9 was up-regulated (Figure 3A). Moreover, the miRNA-130a inhibitor could promote inflammation in chondrocytes and caused significantly increased expression of IL-6, IL-8 and MCP-1 (Figure 3B). Because miRNA-130a was markedly decreased, and TNF-α was significantly increased in osteoarthritis, it may function as a promoter of osteoarthritis. To clarify the correlation between miRNA-130a and TNF-α, we tested whether downregulation of miRNA-130a in chondrocytes would affect TNF-α expression. In response to anti-miRNA-130a, the mRNA and protein expression of TNF-α were increased by approximately 3 times compared to chondrocytes transfected with control miRNA (Figure 3C-E).
Figure 3.
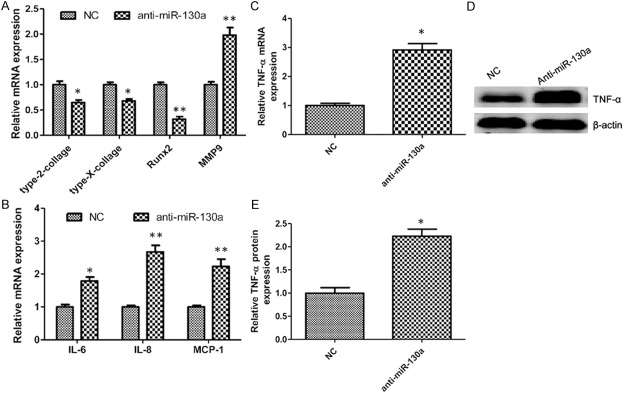
MiRNA-130a loss-of-function in chondrocytes. Chondrocytes transfected with an anti-miR-130a precursor showed decreased expression of chondrogenesis markers compared with control group (A). Levels of inflammatory cytokines were measured in chondrocytes transfected with an anti-miR-130a precursor (B). The mRNA (C) and protein (D) expression of TNF-α are respectively measured by Quantitative real-time PCR and western blotting in chondrocytes transfected with an anti-miR-130a precursor. Densitometric quantification for western blotting (E). Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05, **P < 0.01 versus control group.
Bone tissue morphology parameters
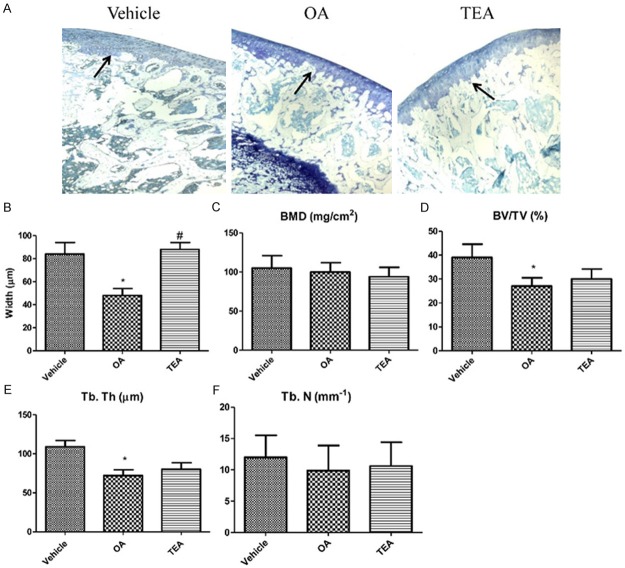
Toluidine blue staining was performed to observe the articular cartilage at the proximal metaphysis of the tibia. The thickness of articular cartilage was reduced in the proximal tibia of OA group (Figure 4A and 4B). The black arrow indicated that articular cartilage suffered seriously injured, so not in its integrity. TEA reversed OA-induced articular cartilage damage and maintained integrity of articular cartilage in the proximal epiphysis of tibia (Figure 4A and 4B). Moreover, toluidine blue staining showed the increased disconnections and separation of trabecular bone network in the proximal epiphysis of tibia as well as the reduction of trabecular bone mass of primary and secondary spongiosa throughout the proximal epiphysis of tibia in OA group. Importantly, TEA reversed OA-induced trabecular deleterious effects and stimulated bone remodeling (Figure 4A and 4B). The trabecular bone mass at the proximal epiphysis of the tibia was quantified using micro-CT scanning. Analyses of the data from the proximal epiphysis of the tibia revealed that OA rat exhibited significantly lower trabecular BV/TV and Tb. Th compared to that of the control group. Notably, treatment with TEA for OA rat resulted in increasing the BV/TV ratio and Tb. Th (Figure 4D and 4E). However, the results obtained in the OA group compared to the control group showed that there were no differences in BMD and Tb. N (Figure 4C and 4F).
Figure 4.
Bone tissue morphology parameters in OA rat. The thickness of articular cartilage is shown by black arrows (A, magnification, × 50) and the width of the articular cartilage is quantified (B). BMD/TV bone mineral density over total volume (C), BV/TV bone volume over total volume (D), Tb. N trabecula number (E) and Tb. Th trabecula thickness (F) are measured by micro-CT in the proximal tibia of rat. Values are expressed as mean ± SEM, n = 6 in each group. *P < 0.05, versus vehicle group; #P < 0.05, versus OA group.
OA regulates bone metabolism
We assessed miRNA-130a and TNF-α expression levels in OA animal model. Consistent with the clinical specimen results, the expression level of miRNA-130a was significantly decreased, and the expression of TNF-α was markedly increased in OA group compared to those of the normal controls. Interestingly, treatment with TEA resulted in further up-regulation of miRNA-130a and down-regulation of TNF-α (Figure 5A, 5C and 5D). To determine the level of the key regulators for bone metabolism, TNF-α (pro-inflammatory cytokine), TGF-β (pro-osteogenesis cytokine), CAII (for resorption on bone inorganic substance) and MMP-9 (for resorption on bone organic component) were measured. The results showed that the mRNA and protein expression of CAII, TNF-α and MMP-9 were significantly increased, and TGF-β was suppressed in the OA group compared to those of the normal controls (Figure 5B-D). Interestingly, treatment with TEA could reverse bone metabolism dysfunction in OA group.
Figure 5.
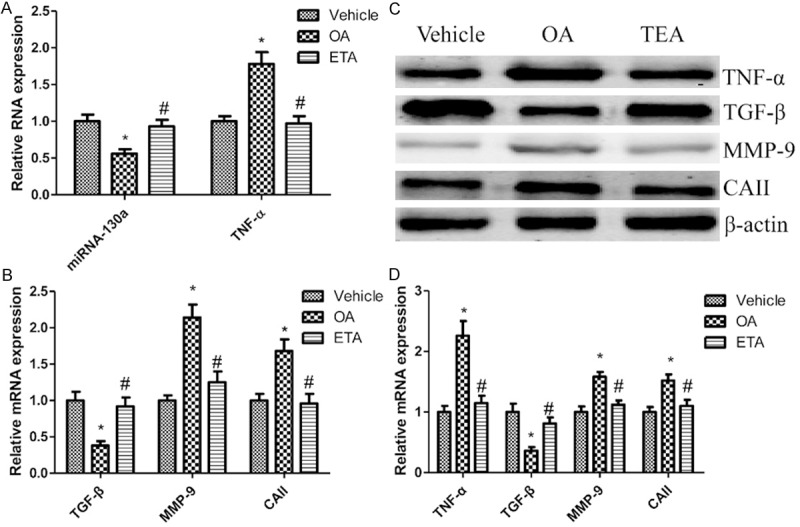
OA regulates bone metabolism. The expression of miRNA-130a and TNF-α in the proximal tibia of rat (A). mRNA (B) and protein (C) expression of bone metabolism markers are respectively measured by Quantitative real-time PCR and western blotting in the proximal tibia of rat. Densitometric quantification for western blotting (D). Values are expressed as mean ± SEM, n = 6 in each group. *P < 0.05, versus vehicle group; #P < 0.05, versus OA group.
Discussion
Research into the pathogenesis of OA is difficult, because this disease is caused by multiple factors such as age, metabolism, inflammation and genetic influences [26]. In the present study we made several important observations. First, increased TNF-α and decreased miRNA-130a were observed in tissues from osteoarthritis patients. Moreover, we found a highly negitive correlation between miRNA-130a and TNF-α. Next, miRNA-130a loss-of-function increased the expression of TNF-α and promoted inflammation in chondrocytes. It was reasonable that miRNA-130a regulated a distinct underlying molecular and pathogenic mechanism of OA by forming a negative feedback loop with TNF-α. Furthermore, there were the abnormalities of bone metabolism in OA rat, which showed the miRNA-130a and TNF-α dysfunction that was one of important factors for the occurrence and development of OA. Together, these observations suggested that miRNA-130a and TNF-α played an important role in the pathological courses of OA.
In this study, we identified that miRNA-130a expression was significantly up-regulated in chondrocytes in response to IL-1β stimulation. IL-1β plays a vital role in cartilage degradation during OA development [2]. Dozens of studies have been identified miRNAs participating in the processes of disrupted cartilage homeostasis in response to IL-1β [2,11,27-29]. The expression of miRNA-140 is suppressed in human chondrocytes through exposure to IL-1β [28]. In contrast to that the expression of miR-145 increased in OA chondrocytes in response to IL-1β stimulation [2]. Moreover, over-expression of miRNA-9, miRNA-98 and miRNA-146 reduces IL-1β-mediated production of TNF-α, and miRNA-9 gain-of-function reduces IL-1β induced MMP13 protein release suggests that these miRNAs may play a protective role in OA disease [30]. Consistent with previous studies results, we revealed that the expression of miRNA-130a was suppressed when chondrocytes expose to IL-1β. Functional studies revealed that activation of the inflammatory was observed in miRNA-130a loss-of-function chondrocytes, and it led to the high activity of bone absorption. Thus, the abnormal expression pattern of miRNA-130a correlated with the imbalance of anabolic-catabolic responses in OA. Recent studies have suggested that miRNA-130a inhibits HCV replication by restoring the innate immune response [31], regulates autophagy of endothelial progenitor cells through Runx3 [32] and contributes to cisplatin resistance in ovarian cancer cells [33], while there is no reported on osteoarthritis and related conditions.
Of note, the mRNA and protein expression of TNF-α in chondrocytes increased in the presence of IL-1β. Rho-associated protein kinase inhibitor Y27632 can block TNF-α-induced F-actin overexpression and cellular stiffness, and impaired cell stiffening in response to the contractile agonist histamine in chondrocytes [5]. Glycation end product induces TNF-α and MMP-13 expression through inhibition NF-κB activation in Chondrocytes. Pioglitazone dose-dependently inhibits the expression of TNF-α and MMP-13 induced by AGEs [34]. Moreover, TNF-α can induce cartilage matrix destruction, which reverses by donepezil [35]. Above research result indicates that TNF-α over-expression negatively regulates chondrocytes function. Our findings showed that miRNA-130a was down-regulated in OA cartilage, while over-expression negatively affects TNF-α level suggests that reduced miRNA-130a expression in OA cartilage could be a factor in the promotion of an inflammatory in the development of osteoarthritis. MiRNA-130a was not predicted to directly target to TNF-α. However, the fact that IL-1β-induced production of TNF-α was significantly reduced by TNF-α inhibitor.
In conclusion, we could not predict that TNF-α was a directly regulating target of miRNA-130a. But there was a high correlation between TNF-α and miRNA-130a in chondrocytes. The underlying mechanism of OA was mediated, at least partially, through down-regulation of miRNA-130a and up-regulation of TNF-α. Moreover, these results suggested that miRNA-130a might be a potential target for the treatment of OA.
Acknowledgements
This study was supported by Shanghai medical key subject construction project (No. ZK2012A28), Key National Clinical Discipline construction project (2013).
Disclosure of conflict of interest
None.
References
- 1.Zhang Y, Jia J, Yang S, Liu X, Ye S, Tian H. MicroRNA-21 controls the development of osteoarthritis by targeting GDF-5 in chondrocytes. Exp Mol Med. 2014;46:e79. doi: 10.1038/emm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang B, Kang X, Xing Y, Dou C, Kang F, Li J, Quan Y, Dong S. Effect of microRNA-145 on IL-1beta-induced cartilage degradation in human chondrocytes. FEBS Lett. 2014;588:2344–2352. doi: 10.1016/j.febslet.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 3.Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001;15:805–829. doi: 10.1053/berh.2001.0195. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Wu L, Li L, Chen S. Monotropein exerts protective effects against IL-1beta-induced apoptosis and catabolic responses on osteoarthritis chondrocytes. Int Immunopharmacol. 2014;23:575–580. doi: 10.1016/j.intimp.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Xie J, Rajappa R, Deng L, Fredberg J, Yang L. Interleukin-1beta and tumor necrosis factor-alpha increase stiffness and impair contractile function of articular chondrocytes. Acta Biochim Biophys Sin (Shanghai) 2014 doi: 10.1093/abbs/gmu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kou S, Wu Y. Meta-analysis of tumor necrosis factor alpha -308 polymorphism and knee osteoarthritis risk. BMC Musculoskelet Disord. 2014;15:373. doi: 10.1186/1471-2474-15-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.unisch E, Kinne RW, Alsalameh RJ, Alsalameh S. Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and -3 mRNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: in situ hybridization studies on a single cell level. Int J Rheum Dis. 2014 doi: 10.1111/1756-185X.12431. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Gioia U, Di Carlo V, Caramanica P, Toselli C, Cinquino A, Marchioni M, Laneve P, Biagioni S, Bozzoni I, Cacci E, Caffarelli E. miR-23a and miR-125b regulate neural stem/progenitor cell proliferation by targeting Musashi1. RNA Biol. 2014;11:1105–12. doi: 10.4161/rna.35508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y, Li D, Liang Q, Liu S, Zuo X, Li L, Sun X, Li W, Guo M, Huang Z. miR-638 regulates differentiation and proliferation in leukemic cells by targeting cyclin-dependent kinase 2. J Biol Chem. 2015;290:1818–1828. doi: 10.1074/jbc.M114.599191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weilner S, Grillari-Voglauer R, Redl H, Grillari J, Nau T. The role of microRNAs in cellular senescence and age-related conditions of cartilage and bone. Acta Orthop. 2015;86:92–9. doi: 10.3109/17453674.2014.957079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirzamohammadi F, Papaioannou G, Kobayashi T. MicroRNAs in cartilage development, homeostasis, and disease. Curr Osteoporos Rep. 2014;12:410–419. doi: 10.1007/s11914-014-0229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CR, Shih KS, Liou JP, Wu YW, Hsieh IN, Lee HY, Lin TC, Wang JH. Denbinobin upregulates miR-146a expression and attenuates IL-1beta-induced upregulation of ICAM-1 and VCAM-1 expressions in osteoarthritis fibroblast-like synoviocytes. J Mol Med (Berl) 2014 doi: 10.1007/s00109-014-1192-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Lu MC, Yu CL, Chen HC, Yu HC, Huang HB, Lai NS. Increased miR-223 expression in T cells from patients with rheumatoid arthritis leads to decreased insulin-like growth factor-1-mediated interleukin-10 production. Clin Exp Immunol. 2014;177:641–651. doi: 10.1111/cei.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulci V, Scappucci G, Sebastiani GD, Giannitti C, Franceschini D, Meloni F, Colombo T, Citarella F, Barnaba V, Minisola G, Galeazzi M, Macino G. miR-223 is overexpressed in T-lymphocytes of patients affected by rheumatoid arthritis. Hum Immunol. 2010;71:206–211. doi: 10.1016/j.humimm.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10:R101. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, Shi Y, Harley JB, Shen N, Qian Y. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Kim SJ. Overexpression of microRNA-25 by withaferin A induces cyclooxygenase-2 expression in rabbit articular chondrocytes. J Pharmacol Sci. 2014;125:83–90. doi: 10.1254/jphs.13232fp. [DOI] [PubMed] [Google Scholar]
- 18.Santini P, Politi L, Vedova PD, Scandurra R, Scotto d’Abusco A. The inflammatory circuitry of miR-149 as a pathological mechanism in osteoarthritis. Rheumatol Int. 2014;34:711–716. doi: 10.1007/s00296-013-2754-8. [DOI] [PubMed] [Google Scholar]
- 19.Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna M, Fraser AR, Stolarski B, McSharry C, Hueber AJ, Baxter D, Hunter J, Gay S, Liew FY, McInnes IB. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci U S A. 2011;108:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B, Huang P, Qiu J, Liao Y, Hong J, Yuan Y. MicroRNA-130a is down-regulated in hepatocellular carcinoma and associates with poor prognosis. Med Oncol. 2014;31:230. doi: 10.1007/s12032-014-0230-2. [DOI] [PubMed] [Google Scholar]
- 21.Osbourne A, Calway T, Broman M, McSharry S, Earley J, Kim GH. Downregulation of connexin43 by microRNA-130a in cardiomyocytes results in cardiac arrhythmias. J Mol Cell Cardiol. 2014;74:53–63. doi: 10.1016/j.yjmcc.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao F, Yu J, Liu B, Guo Y, Li K, Deng J, Zhang J, Wang C, Chen S, Du Y, Lu Y, Xiao Y, Zhang Z, Guo F. A novel function of microRNA 130a-3p in hepatic insulin sensitivity and liver steatosis. Diabetes. 2014;63:2631–2642. doi: 10.2337/db13-1689. [DOI] [PubMed] [Google Scholar]
- 23.Meng S, Cao J, Zhang X, Fan Y, Fang L, Wang C, Lv Z, Fu D, Li Y. Downregulation of microRNA-130a contributes to endothelial progenitor cell dysfunction in diabetic patients via its target Runx3. PLoS One. 2013;8:e68611. doi: 10.1371/journal.pone.0068611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Nie J, Chen L, Dong G, Du X, Wu X, Tang Y, Han W. The oncogenic role of microRNA-130a/301a/454 in human colorectal cancer via targeting Smad4 expression. PLoS One. 2013;8:e55532. doi: 10.1371/journal.pone.0055532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, Crish JF, Bebek G, Ritter SY, Lindstrom TM, Hwang I, Wong HH, Punzi L, Encarnacion A, Shamloo M, Goodman SB, Wyss-Coray T, Goldring SR, Banda NK, Thurman JM, Gobezie R, Crow MK, Holers VM, Lee DM, Robinson WH. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrandiz ML, Terencio MC, Ruhi R, Verges J, Montell E, Torrent A, Alcaraz MJ. Influence of age on osteoarthritis progression after anterior cruciate ligament transection in rats. Exp Gerontol. 2014;55:44–48. doi: 10.1016/j.exger.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Wang JH, Shih KS, Wu YW, Wang AW, Yang CR. Histone deacetylase inhibitors increase microRNA-146a expression and enhance negative regulation of interleukin-1beta signaling in osteoarthritis fibroblast-like synoviocytes. Osteoarthritis Cartilage. 2013;21:1987–1996. doi: 10.1016/j.joca.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Miyaki S, Nakasa T, Otsuki S, Grogan SP, Higashiyama R, Inoue A, Kato Y, Sato T, Lotz MK, Asahara H. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SJ, Cheon EJ, Lee MH, Kim HA. MicroRNA-127-5p regulates matrix metalloproteinase 13 expression and interleukin-1beta-induced catabolic effects in human chondrocytes. Arthritis Rheum. 2013;65:3141–3152. doi: 10.1002/art.38188. [DOI] [PubMed] [Google Scholar]
- 30.Jones SW, Watkins G, Le Good N, Roberts S, Murphy CL, Brockbank SM, Needham MR, Read SJ, Newham P. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17:464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Duan X, Li Y, Liu B, McGilvray I, Chen L. MicroRNA-130a inhibits HCV replication by restoring the innate immune response. J Viral Hepat. 2014;21:121–128. doi: 10.1111/jvh.12131. [DOI] [PubMed] [Google Scholar]
- 32.Xu Q, Meng S, Liu B, Li MQ, Li Y, Fang L, Li YG. MicroRNA-130a regulates autophagy of endothelial progenitor cells through Runx3. Clin Exp Pharmacol Physiol. 2014;41:351–357. doi: 10.1111/1440-1681.12227. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Huang L, Zhao Y, Tan W. Downregulation of miR-130a contributes to cisplatin resistance in ovarian cancer cells by targeting X-linked inhibitor of apoptosis (XIAP) directly. Acta Biochim Biophys Sin (Shanghai) 2013;45:995–1001. doi: 10.1093/abbs/gmt113. [DOI] [PubMed] [Google Scholar]
- 34.Chen C, Ma C, Zhang Y, Zeng Y, Li Y, Wang W. Pioglitazone Inhibits Advanced Glycation End Product-Induced TNF-alpha and MMP-13 Expression via the Antagonism of NF-kappaB Activation in Chondrocytes. Pharmacology. 2014;94:265–272. doi: 10.1159/000369074. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Zhou Y. The protective effects of Donepezil (DP) against cartilage matrix destruction induced by TNF-alpha. Biochem Biophys Res Commun. 2014;454:115–118. doi: 10.1016/j.bbrc.2014.10.046. [DOI] [PubMed] [Google Scholar]