Abstract
The aim of this study was to determine whether long non-coding RNA PVT1 can participate in the regulation of cardiac hypertrophy. A C57BL/6 mouse cardiac hypertrophic model was established using transverse aortic constriction (TAC). The animals subjected to sham operation were used as controls. Transcripts of PVT1 were analyzed in hearts of model and sham control groups after TAC for 4 weeks using quantitative real-time PCR (qRT-PCR). Additionally, to investigate whether PVT1 was involved in cardiac hypertrophy, 1 μM angiotensin II (Ang II) was used to induce hypertrophy and PVT1 siRNA was performed in the cultured neonatal mouse cardiac cardiomyocytes. Cell size was measured by cell surface area and total protein content analyses in response to Ang II treatment. Moreover, some hypertrophic markers including atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and beta-myosin heavy chain (β-MHC) were also quantified using qRT-PCR. As a result, PVT1 was up-regulated by 2.5-fold (P < 0.05) in hypertrophic hearts after TAC for 4 weeks as compared to sham group. In addition, siRNA of endogenous PVT1 in cardiomyocytes significantly reduced (P < 0.05) Ang II-induced increase of cell size in terms of cell surface area (by 5.6-fold) and total protein content (by 23.0%). PVT1 siRNA also obviously attenuated Ang II-induced ANP and β-MHC expression by 40.9% and 41.5%, respectively (P < 0.05), but had no effect on BNP mRNA expression. Our results demonstrated that PVT1 was essential for the maintenance of cell size of cardiomyocytes and might play a role in the regulation of cardiac hypertrophy.
Keywords: Cardiac hypertrophy, PVT1, long non-coding RNA, cardiomyocytes, transverse aortic constriction
Introduction
Heart failure is still one of the leading causes of mortality and morbidity in the world, even if there has been great improvement in heart disease treatment [1]. Cardiac hypertrophy, a remodeling of myocardium marked by the enlargement of cardiomyocytes, often leads to progression to heart failure and is an independent and major risk factor for cardiovascular disease [2,3]. Although great advances in the identification of genes and signaling pathways involved in cardiac hypertrophy have been shown [4,5], additional regulatory mechanisms remain to be identified.
Previous studies have demonstrated that microRNAs (miRNAs), which are some ~ 22 nucleotide long, evolutionary conserved and noncoding RNA molecules, are implicated in various cardiovascular diseases, including cardiac hypertrophy and heart failure [6-8]. Furthermore, miRNA expression profiling studies have shown that many miRNAs are aberrantly expressed and play either pro- or anti-hypertrophic activity roles in the process of cardiac hypertrophy [3,9-11]. Therefore, miRNAs have the potential to become one of useful clinically diagnostic and therapeutic targets for heart disease.
Long non-coding RNAs (lncRNAs), a group of non-coding RNAs longer than 200 nucleotides in length, constitute another novel class of non-coding RNAs and regulate gene expression, thus having the possibility to modulate disease progression [12]. Recently, some studies are just beginning to understand the biology of lncRNA in the cardiovascular system and the important role of lncRNA in heart development and heart failure [13-16]. Han et al. have demonstrated that Myheart, a novel cluster of long noncoding RNAs, protects the heart from pathological hypertrophy [17]. In addition, some other lncRNAs, such as 2900055J20Rik [18] and CHRF [19], have also been identified to be involved in regulation of cardiac hypertrophy. These observations motivate further investigation of the value of lncRNAs in the cardiac hypertrophy.
The plasmacytoma variant translocation 1 (PVT1) gene, originally identified as a transcriptional unit from a human homologous sequence to Pvt1 [20], is a lncRNA (1.9 kb) that encodes a number of alternative transcripts and a host gene for several miRNAs [21]. When amplified and overexpressed, PVT1 can increase cell proliferation and inhibit apoptosis, indicating that it is an anti-apoptotic molecule [22]. Therefore, PVT1 is frequently a target of genetic gains and amplifications in various cancers [22-25]. Although several previous reports have identified the functional roles of PVT1 implicated in the pathogenesis of the human diseases mentioned above, the role that this gene may play in the development of cardiac hypertrophy is not yet known.
To shed light on a potential effect of PVT1 on hypertrophy, we analyzed its expression level in hearts of hypertrophy model mice subjected to transverse aortic constriction (TAC). With small interference RNA (siRNA) approach, we further demonstrated an essential role of PVT1 in the regulation of angiotensin II (Ang II)-induced increase of cardiomyocytes size.
Materials and methods
Cardiac hypertrophy animal model
To determine the expression changes of PVT1 in hypertrophic hearts we applied a well-established mouse cardiac hypertrophy model by TAC as described [3,26]. Briefly, C57BL/6 mice of 8-10 weeks old (22-24 g) were anesthetized by intraperitoneal injection of 3.6% chloral hydrate. Under sterile conditions, a longitudinal incision of 2-3 mm was made in the proximal sternum to allow visualization of the aortic arch and then transverse aorta binding constriction was performed with an overlaying blunted 27-gauge needle and a 6-0 silk suture. After that, the needle was quickly removed to create a defined constriction. Moreover, a sham surgical operation was performed in which the transverse aorta was exposed but not banded. All animal experiments in this study were performed with the approval of the Animal Care Committee of Laboratory Animal Centre & Nanjing Hospital Affiliated to Nanjing Medical University (SYXK2009-0015). Cardiac hypertrophy was evaluated by echocardiography analysis of heart size, including left ventricular posterior wall thickness at end-systole (LVPWs), left ventricular posterior wall thickness at end-diastole (LVPWd), interventricular septum thickness at end-diastole (IVSd) and interventricular septum thickness at end-systole (IVSs) as outcome indicators. The body mass (BM), heart mass (HM), left ventricular mass were also measured to calculate the ratios of HM/BM and LVM/BM.
Neonatal mouse cardiomyocyte culture
Neonatal mouse cardiomyocytes were prepared as described previously [1,27]. In brief, hearts were obtained from 1 to 3 days old C57BL/6 mice and placed in ice-cold phosphate-buffered saline (PBS) solution. After repeated rinsing, the ventricular tissues were minced with scissors and enzymatically dissociated with 0.25% trypsin (Beyotime, China) at 37°C under 100 rpm rotation. After dissociation, cardiomyocytes were enriched using Percoll (Amersham) step gradients, and then cultured in DMEM (Hyclone) with 10% fetal bovine serum (FBS, Hyclone) and seeded into six-well plates.
Hypertrophic stimulation and small interference RNA (siRNA)
To induce hypertrophy, cardiomyocytes were stimulated with 1 μM Ang II as previously described [28]. The Stealth™ siRNAs targeting PVT1 (CCUGCAUAACUAUCUGCUUTT) and a scrambled control siRNA were synthesized and cells were transfected with the siRNAs using lipofectamine 2000, according to the manufacturer’s instructions (Invitrogen). The obtained cardiomyocytes were plated at a density of 5 × 105 cells and cultured for 24 h in serum-containing media, and then divided into 4 groups: control group, sequentially cultured in complete medium; starvation group, subjected to 6 h serum starvation; (starvation + Ang II) group, starved for 6 h following 1 μM Ang II stimulation; (starvation + Ang II + siRNA) group, cultured for 6 h in serum-free media followed by PVT1 siRNA transfection for 24 h prior to stimulation of 1 μM Ang II. The serum-free medium was replaced 6 hours after starvation by the regular culture medium.
Immunostaining and measurement of cell surface area
After experimental treatment for 72 h, cardiomyocytes were subsequently washed three times with cold PBS and then fixed with 4% paraformaldehyde for 10 min. After fixation, cells were washed three times with cold PBS and permeabilized with 0.5% Triton X-100 in PBS for 10 min. Non-specific binding of the fixed cells was blocked by incubation in 5% BSA solution for 1 h at room temperature, followed by incubation with anti-α-actinin antibody (1:200; Beyotime, China) at 4°C overnight. After washing, the cells were incubated with Alexa Fluor™ 568-conjugated goat anti-mouse antibody (1:1000; Molecular Probes, Eugene, OR) at 37°C for 1 h. Hoechst 33342 (Beyotime, China) staining was used to visualize the nucleus. The cells were examined and photographed using a fluorescence microscope (Olympus, Japan) and cell surface areas were measured using NIH Image J software. Approximately 100 cells were counted from randomized captured images and regarded as an independent experiment, and three independent experiments were performed.
Total protein concentration measurement
To assess cardiomyocytes hypertrophy in all groups, total protein content was also measured [29]. After 72 h of experimental treatment, cardiomyocytes were lysed in RIPA sample buffer (Boster, China), scraped, and collected in cold lysis buffer, and protein concentration was measured by Bradford assay (Bio-Rad Laboratories, Hercules, CA).
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from both hearts of cardiac hypertrophy mouse model and cultured neonatal mouse cardiac cardiomyocytes of all groups using RNeasy mini kit (Qiagen, Germany). First-strand cDNA was synthesized from 1 μg of total RNA using SuperScript II RNase H-Reverse Transcriptase (Invitrogen) according to the manufacturer’s protocol. The sequences of primers (Shenggong Bioengineering Co., Shanghai, China) used for quantification measurement of hypertrophic markers atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), beta-myosin heavy chain (β-MHC) and PVT1 mRNA were shown in Table 1, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as reference gene. The specificity of the PCR amplification was confirmed by agarose gel electrophoresis. Real-time reactions were run and analyzed by using a Real-Time PCR system (Applied Biosystems ABI Prism 7500). The amplification was used to calculate the CT value (ΔCT) of the target genes and the difference between the ΔCT of those genes and GAPDH gene. In addition, the equation 2-ΔΔCT was used to determine the relative amount of mRNA in specific target genes.
Table 1.
The sequences of the primers used for qRT-PCR
| Primer | Sequences (5’-3’) | |
|---|---|---|
| PVT1 | Forward | CCTCTTGGTCCCTGATGCA |
| Reverse | GATTCCCATGCCTCTCATCCT | |
| ANP | Forward | TGAGTGAGCAGACTGAGGAA |
| Reverse | TGGATCTTCGTAGGCTCCGA | |
| BNP | Forward | ACAGAAGCTGCTGGAGCTGA |
| Reverse | CCGATCCGGTCTATCTTGTG | |
| β-MHC | Forward | TATCGATGACCTGGAGCTGA |
| Reverse | AGTATTGACCTTGTCTTCCTC | |
| GAPDH | Forward | ACAGCAACAGGGTGGTGGAC |
| Reverse | TTTGAGGGTGCAGCGAACTT |
ANP: atrial natriuretic peptide; BNP: B-type natriuretic peptide; β-MHC: beta-myosin heavy chain; GAPDH: glyceraldehydes-3-phosphate dehydrogenase.
Statistical analysis
The results were expressed as mean ± SD of at least three independent experiments. Comparisons among multiple groups were made by analysis of variance (ANOVA) followed by Tukey post hoc test using SPSS 16.0. Two groups were analyzed by Student’s t test. P < 0.05 was considered statistically significant.
Results
PVT1 was up-regulatede in hypertrophic hearts
The TAC was applied to induce cardiac hypertrophy, and development of cardiac hypertrophy and regression of the established cardiac hypertrophy were confirmed by echocardiography analysis. After 4 weeks’ constriction, the TAC hearts showed significantly higher (P < 0.05) HM/BM, LVM/BM, LVPWs, LVPWd, IVSd and IVSs values than the sham group (Table 2), implying the hypertrophic growth. qRT-PCR analysis demonstrated that the relative expression level of PVT1 was significantly higher by 2.5-fold (P < 0.05) in TAC than in sham (Figure 1).
Table 2.
Outcome indicators for cardiac hypertrophy
| Parameters | Control | TAC |
|---|---|---|
| HM/BM (%) | 0.39 ± 0.01a | 0.67 ± 0.12b |
| LVM/BM (%) | 0.29 ± 0.06a | 0.53 ± 0.13b |
| LVPWs (mm) | 1.20 ± 0.08a | 1.38 ± 0.12b |
| LVPWd (mm) | 0.74 ± 0.03a | 1.07 ± 0.18b |
| IVSd (mm) | 0.71 ± 0.03a | 1.04 ± 0.10b |
| IVSs (mm) | 1.16 ± 0.07a | 1.47 ± 0.09b |
Note: Means with different letters (a and b) were significantly different (p < 0.05). BW, body mass; HW, heart mass; LVM, left ventricularma mass; LVPWs, left ventricular posterior wall thickness at end-systole; LVPWd, left ventricular posterior wall thickness at end-diastole; IVSd, interventricular septum thickness at end-diastole; IVSs, interventricular septum thickness at end-systole.
Figure 1.

The expression level of PVT1 in hearts of transverse aortic constriction (TAC) mouse model and sham control after 4 weeks (n = 4 for each group). The different letters (a and b) indicated significant differences (P < 0.05).
Down-regulation of PVT1 reduced the cell surface area of cardiomyocytes induced by Ang II
To investigate whether endogenous PVT1 played a significant role in cardiomyocytes hypertrophy, RNA interference was performed to silence PVT1 gene expression through siRNA. Compared with the starvation and Ang II treatment groups, PVT1 siRNA was sufficient to down-regulate endogenous PVT1 expression in cultured cardiomyocytes by 33.0% and 55.5%, respectively, as analyzed with qRT-PCR (Figure 2A, P < 0.05). Moreover, Ang II stimulation induced significantly increased (P < 0.05) expression of PVT1 compared to starvation group (Figure 2A). The size of cells was measured by relative cell surface areas and showed that the size of cardiomyocytes transfected with PVT1 siRNA was obviously reduced by 5.6-fold to baseline level as compared with that of Ang II stimulation (Figure 2B and 2C, P < 0.05), suggesting that the endogenous PVT1 might be required to maintain cell size of cardiomyocytes and might be involved in the Ang II-induced cell size enlargement of cardiomyocytes.
Figure 2.

PVT1 siRNA rescued hypertrophic responses in cardiomyocytes. A. After 72 h of culture, relative fold change of PVT1 expression in the cultured neonatal mouse cardiac cardiomyocyte of starvation group, (starvation + Ang II) group and (starvation + Ang II + siRNA) group vs. control group, respectively. B. Representative photograph of cardiomyocytes transfected with PVT1 siRNA after Ang II treatment (Hoechst 33342 staining). C. Cell surface areas of the cardiomyocytes were measured using Image J software (n = 100). Scale bars, 20 μm. The different letters (a, b and c) indicated significant differences (P < 0.05).
Down-regulation of PVT1 diminished Ang II-induced protein content
To assess cardiomyocyte hypertrophy in vitro, protein synthesis was measured by total protein content by Bradford Assay. Ang II stimulation significantly increased the protein quantity by 2.0- and 3.0-fold vs. control and starvation groups, respectively (P < 0.05). This was reduced by 23.0% with PVT1 siRNA pretreatment (P < 0.05) vs. non-siRNA preteated Ang II-stimulated cells (Figure 3).
Figure 3.
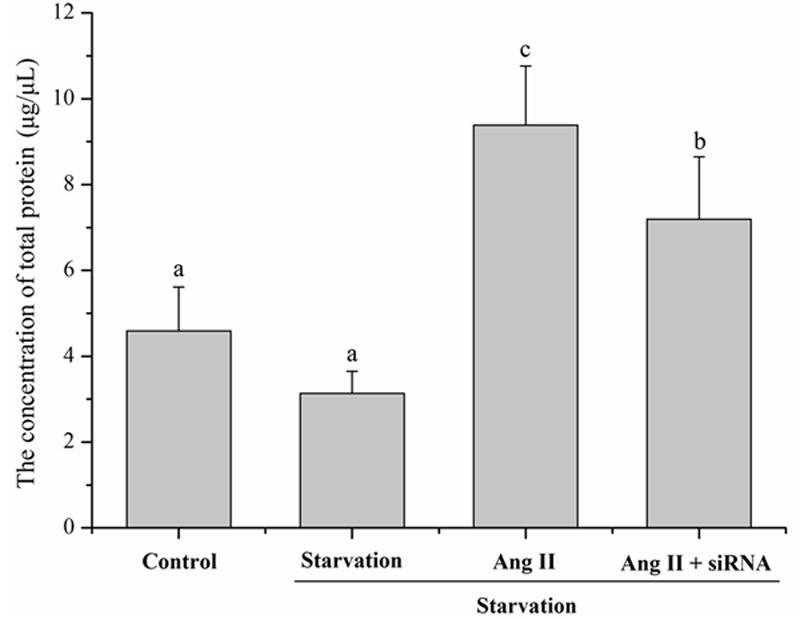
After 72 h of culture, cardiomyocytes hypertrophy was assessed by total protein content measurement in all groups by Bradford Assay. The different letters (a, b and c) indicated significant differences (P < 0.05).
PVT1 siRNA attenuated Ang II-induced ANP and β-MHC expression but had no effect on BNP
Ang II treatment dramatically increased expression levels of ANP and β-MHC, molecular markers of cardiomyocyte hypertrophy, by 4.8- and 3.0-fold (P < 0.05 vs. respective controls for both). PVT1 siRNA transfection markedly diminished this ANP and β-MHC expression by 40.9% and 41.5%, respectively, vs. Ang II alone (Figure 4A and 4C). However, Ang II stimulation and PVT1 siRNA pretreatment did not affect BNP expression (P > 0.05, Figure 4B).
Figure 4.
After 72 h of culture, relative fold change of (A) ANP, (B) BNP and (C) β-MHC expression in the cultured neonatal mouse cardiac cardiomyocyte of treatment groups vs. control group, respectively. The different letters (a, b and c) indicated significant differences (P < 0.05). ANP: atrial natriuretic peptide; BNP: B-type natriuretic peptide; β-MHC: beta-myosin heavy chain.
Discussion
Previous reports have shown that lncRNAs are involved in several aspects cardiac development and pathophysiology, including cardiac hypertrophy and heart failure [13,16,18,19]. In present study, we revealed that lncRNA PVT1 had an essential role in cell size maintenance of cardiomyocytes during hypertrophy. Initially, we showed that PVT1 was up-regulated about 2.5-fold after TAC for 4 weeks. Then using siRNA approaches in cultured cardiomyocytes, we further revealed that down-regulation of PVT1 in cardiomyocytes could attenuate the Ang II-induced increase of cell size (by cell surface area measurement), total protein contents as well as expression levels of ANP and β-MHC (molecular markers of cardiomyocyte hypertrophy).
Mammalian genomes encode numerous lncRNAs which have been defined to have important functions in RNA processing, chromatin modification, structural scaffolds, and modulation of apoptosis and invasion [30,31]. Therefore, unsurprisingly, these molecules are emerging as important players in several human pathologies, including cardiovascular diseases [13]. A recent report has demonstrated that lncRNA CHRF performs as endogenous sponge RNA to regulate cardiac hypertrophy by inhibiting miR-489 expression and activity [19]. A cardioprotective lncRNA Myheart has also been identified to protect the heart from pathological hypertrophy [17]. In addition, it has been shown that the lncRNA 2900055J20Rik may be involved in regulation of cardiac hypertrophy [18]. In our present study, lncRNA PVT1 was also found to be implicated in hypertrophied heart and hypertrophy of cardiomyocytes. The discovery of the novel lncRNA (PVT1) in cardiac hypertrophy further reveals the function of lncRNAs participating in regulating hypertrophy and may shed new lights on understanding the complex molecular mechanism of cardiac hypertrophy.
PVT1 is a lncRNA (1.9 kb) that encodes a number of alternative transcripts [32]. Although there are a few reports showing that PVT1 plays an important role in the pathogenesis of many human diseases [21,22,25], it is not yet clear whether PVT1 is involved in the regulation of cardiac hypertrophy. In our study, PVT1 was found to be substantially up-regulated in TAC model and in response to hypertrophic stimulation (Ang II), suggesting that the endogenous PVT1 might be required to maintain cell size of cardiomyocytes. Inhibition of PVT1 could attenuate enlargement of cardiomyocytes size, which indicating that PVT1 was essential for regulation of cardiac hypertrophy. However, some research limitations still existed in the present work. The downstream targets of PVT1 and exact molecular mechanism are still unclear. It is an interesting scientific topic and we will focus on that in our future research.
Our present study identified for the first time that PVT1 was essential for cell size maintenance of cardiomyocytes and for regulation of cardiac hypertrophy, suggesting that PVT1 might be a potential therapeutic target for cardiac hypertrophy.
Disclosure of conflict of interest
None.
References
- 1.Song XW, Li Q, Lin L, Wang XC, Li DF, Wang GK, Ren AJ, Wang YR, Qin YW, Yuan WJ. MicroRNAs are dynamically regulated in hypertrophic hearts, and miR-199a is essential for the maintenance of cell size in cardiomyocytes. J Cell Physiol. 2010;225:437–443. doi: 10.1002/jcp.22217. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong MH, Lee JS, Kim DH, Park WJ, Yang DK. Identification of novel microRNAs negatively regulating cardiac hypertrophy. Biochem Biophys Res Commun. 2012;428:191–196. doi: 10.1016/j.bbrc.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 4.Feng H, Ouyang W, Liu J, Sun Y, Hu R, Huang L, Xian J, Jing C, Zhou M. Global microRNA profiles and signaling pathways in the development of cardiac hypertrophy. Braz J Med Biol Res. 2014;47:361–8. doi: 10.1590/1414-431X20142937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Wang N, Liu W, Zhi X, Zhang TC. Signaling Pathways in Cardiac Hypertrophy; Proceedings of the 2012 international conference on applied biotechnology (ICAB 2012); Springer; 2014. [Google Scholar]
- 6.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 7.Gladka MM, Da Costa Martins PA, De Windt LJ. Small changes can make a big difference-microRNA regulation of cardiac hypertrophy. J Mol Cell Cardiol. 2012;52:74–82. doi: 10.1016/j.yjmcc.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42:1137–1141. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Rooij E, Sutherland LB, Liu N, Williams AH, Mcanally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jentzsch C, Leierseder S, Loyer X, Flohrschütz I, Sassi Y, Hartmann D, Thum T, Laggerbauer B, Engelhardt S. A phenotypic screen to identify hypertrophy-modulating microRNAs in primary cardiomyocytes. J Mol Cell Cardiol. 2012;52:13–20. doi: 10.1016/j.yjmcc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Martins PaDC, De Windt LJ. MicroRNAs in control of cardiac hypertrophy. Cardiovasc Res. 2012;93:563–572. doi: 10.1093/cvr/cvs013. [DOI] [PubMed] [Google Scholar]
- 12.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Papait R, Kunderfranco P, Stirparo GG, Latronico MV, Condorelli G. Long noncoding RNA: a new player of heart failure? J Cardiovasc Transl Res. 2013;6:876–883. doi: 10.1007/s12265-013-9488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonrock N, Harvey RP, Mattick JS. Long noncoding RNAs in cardiac development and pathophysiology. Circ Res. 2012;111:1349–1362. doi: 10.1161/CIRCRESAHA.112.268953. [DOI] [PubMed] [Google Scholar]
- 15.Ounzain S, Crippa S, Pedrazzini T. Small and long non-coding RNAs in cardiac homeostasis and regeneration. Biochim Biophys Acta. 2013;1833:923–933. doi: 10.1016/j.bbamcr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Scheuermann JC, Boyer LA. Getting to the heart of the matter: long non-coding RNAs in cardiac development and disease. EMBO J. 2013;32:1805–1816. doi: 10.1038/emboj.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han P, Li W, Lin CH, Yang J, Shang C, Nuernberg ST, Jin KK, Xu W, Lin CY, Lin CJ. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514:102–6. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Hamad E, Vausort M, Funakoshi H, Feldman A, Wagner D, Devaux Y. Long non-coding RNAs and cardiac hypertrophy. Cardiovasc Res. 2014;103:S10–S10. [Google Scholar]
- 19.Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Cardiovasc Res. 2014;114:1377–1388. doi: 10.1161/CIRCRESAHA.114.302476. [DOI] [PubMed] [Google Scholar]
- 20.Graham M, Adams JM. Chromosome 8 breakpoint far 3’of the c-myc oncogene in a Burkitt’s lymphoma 2; 8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J. 1986;5:2845. doi: 10.1002/j.1460-2075.1986.tb04578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck-Engeser GB, Lum AM, Huppi K, Caplen NJ, Wang BB, Wabl M. Pvt1-encoded microRNAs in oncogenesis. Retrovirology. 2008;5:4. doi: 10.1186/1742-4690-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan Y, Kuo WL, Stilwell JL, Takano H, Lapuk AV, Fridlyand J, Mao JH, Yu M, Miller MA, Santos JL. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13:5745–5755. doi: 10.1158/1078-0432.CCR-06-2882. [DOI] [PubMed] [Google Scholar]
- 23.Shtivelman E, Bishop JM. The PVT gene frequently amplifies with MYC in tumor cells. Mol Cell Biol. 1989;9:1148–1154. doi: 10.1128/mcb.9.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huppi K, Pitt JJ, Wahlberg BM, Caplen NJ. The 8q24 gene desert: an oasis of non-coding transcriptional activity. Frontiers in genetics. 2012;3:69. doi: 10.3389/fgene.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonoki T. PVT1: A Cancer-associated Non-coding Gene Revisited. Cloning & Transgenesis. 2014 [Epub ahead of print] [Google Scholar]
- 26.Chen X, Wang SC, Cao LH, Yang GQ, Li M, Su JC. Comparison between radial head replacement and open reduction and internal fixation in clinical treatment of unstable, multi-fragmented radial head fractures. Int Orthop. 2011;35:1071–1076. doi: 10.1007/s00264-010-1107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadoshima JI, Jahn L, Takahashi T, Kulik T, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992;267:10551–10560. [PubMed] [Google Scholar]
- 28.Hu CM, Chen YH, Chiang MT, Chau LY. Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo. Circulation. 2004;110:309–316. doi: 10.1161/01.CIR.0000135475.35758.23. [DOI] [PubMed] [Google Scholar]
- 29.Essick EE, Ouchi N, Wilson RM, Ohashi K, Ghobrial J, Shibata R, Pimentel DR, Sam F. Adiponectin mediates cardioprotection in oxidative stress-induced cardiac myocyte remodeling. Am J Physiol Heart Circ Physiol. 2011;301:H984–H993. doi: 10.1152/ajpheart.00428.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 31.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez ML, Distefano JK. The role of non-coding RNAs in diabetic nephropathy: potential applications as biomarkers for disease development and progression. Diabetes Res Clin Pract. 2013;99:1–11. doi: 10.1016/j.diabres.2012.10.010. [DOI] [PubMed] [Google Scholar]



