Abstract
Denervated skin could result in impaired healing of wounds, such as decubitus ulcers and diabetic foot ulcers. Other studies indicated that cutaneous fiber density is reduced after inner nerve transection and that neuropeptide level depletes after denervation, leading to reduced cell proliferation around the wound and thus wound healing problems. Recent studies have revealed that skin-derived precursors (SKPs), which form a neural crest-related stem cell population in the dermis of skin, participate in cutaneous nerve regeneration. We hypothesized that injecting SKPs into denervated wound promotes healing. A bilateral denervation wound model was established followed by SKP transplantation. The wound healing rate was determined at 7, 14, and 21 d after injury. Cell proliferation activity during wound healing was analyzed by proliferating cell nuclear antigen immunohistochemistry (IHC). Nerve fiber density was measured by S-100 IHC. The contents of nerve growth factor, substance P, and calcitonin gene-related peptide were examined by enzyme-linked immunosorbent assay. The rate of epithelization in the SKP-treated group was faster than that in the control group. Wound cell proliferation and nerve fiber density were obviously higher in the SKP-treated group than in the control group. In addition, the content of neuropeptides was higher in the SKP-treated group than in the control group during wound healing. In conclusion, SKPs can promote denervated wound healing through cell proliferation and nerve fiber regeneration, and can facilitate the release of neuropeptides.
Keywords: SKP, denervation, wound healing, cell transplantation, neuropeptides
Introduction
In recent years, the incidence of chronic wounds with injury to peripheral nerves has increased. Wound healing is delayed in denervated wounds. Skin biopsies revealed that the density of axons in the epidermis and dermis decreases after axotomy [1]. Refractory wound healing in diabetic patients is associated with diabetes-induced impairment in peripheral nerves. The mechanism behind this phenomenon is unknown but may be related to the lack of innervation in these wounds. Wound healing is a complicated process consisting of three main phases, namely, inflammation, connective tissue deposition, and remodeling, each of which is impaired by nerve injury. Some studies reported that sensory innervation is crucial in normal wound healing because partial denervation results in enlarged wound and delayed re-epithelialization [2-4]. Moreover, denervated wounds have significantly lower inflammatory cells, particularly macrophages [5]. Some studies suggested that neuropeptides released from sensory nerves participate in wound healing. Denervation alone decreases the level of nerve growth factor (NGF), a neurotrophic factor required for nociceptor nerve ingrowth into wound tissue [6]. Moreover, denervated tissue demonstrates reduced microvascular responses because of the denervation-evoked desensitization of vascular smooth muscle. Reduction in these responses may delay wound healing [7].
The principal treatment strategies for denervated wounds include early debridement, pressure therapy, and hyperbaric oxygen treatment. However, some studies have revealed that stem cells can be used for the repair of wounds, even denervated wounds. On the one hand, epithelial homeostasis depends on the presence of stem cells that participate in wound repair [8-11]. On the other hand, capsaicin denervation impairs stem cell progeny egress from hair follicles, a circumstance associated with epidermal activation [12]. These findings imply that sensory innervation is crucial in the modulation of stem cell physiology during wound healing.
Skin-derived precursors (SKPs) form a novel population of neural crest-derived precursors originated from cells residing within the mesenchymal compartments (the dermal sheath and the dermal papilla) of hair follicles [13]. SKPs have neural and mesodermal lineage potencies and may differentiate into lipocytes, smooth muscle cells, glial cells, osteocytes, chondrocytes, and neurons in vitro [14-16]. Basing on the capability of SKPs to differentiate into neurons and glial cells, researchers have applied SKPs in the repair of injured nerves. Results showed that SKPs transplanted into the demyelinated brain of neonatal shiverer mice can generate Schwann cells that myelinate axons in the central nervous system (CNS). SKPs are a highly accessible source of myelinating cells for the treatment of nervous system injury, congenital leukodystrophies, and demyelinating disorders [17,18]. Moreover, transplanted SKPs survive in the brain and work as dopaminergic neuronal cells that effectively produce dopamine to improve Parkinson disease-like symptoms [19]. Given their ability to generate potentially autologous Schwann-like cells that could survive within the environment of injured nerve, SKPs show promise in improving the regeneration of chronically injured nerves [20]. Recently, Chen et al. [21] have demonstrated that SKPs are neurotropic to injured nerves and that they can differentiate into Schwann cells to myelinate regenerating axons during cutaneous nerve regeneration. Our previous study revealed that SKPs and hyaluronic acid complexes can accelerate and promote wound healing in diabetic rats [22]. In addition, SKP may participate in wound healing when SKPs are still detectable in the hair papillae and whisker follicles until 4 weeks after cell transplantation [23]. These observations are consistent with the idea that SKPs promote cutaneous wound healing and produce progenitors for cutaneous nerve regeneration.
In the present study, we made denervated skin areas on nude mouse by dissection of the spinal hemicord [3] and then delivered SKPs into the cutaneous wound. During wound healing, the SKP-treated wounds demonstrated superior histological recovery and nerve fiber regeneration than the control-treated wounds. These results suggest that SKPs have promising functions for the tissue regeneration of denervated cutaneous wounds.
Materials and methods
Kunming mice aged 1 d to 3 d (specific pathogen-free) and 60 BALB/C-nu (nude mice) aged 6 weeks to 8 weeks were purchased from the Experimental Animal Center of Sun Yat-Sen University. All animal procedures were approved under the guidelines of the ethics committee of the first affiliated hospital of Sun Yat-Sen University.
Cultivation of SKPs
SKPs were isolated as previously reported [16,24]. The Kunming mice were immersed in 75% ethanol for 5 min before sacrificing. The skins on the abdomen and dorsal torso were obtained, and the subcutaneous tissues were removed. The skins were cut into 2 mm2 to 3 mm2 pieces, washed thrice in Hank’s balanced buffered saline (HBSS; GIBCO BRL, Rockville, MD), and then digested with 0.1% trypsin at 37°C for 40 min and with 0.1% DNase at room temperature for 1 min. Digestion was terminated by Dulbecco’s modified Eagle’s medium (DMEM)/F12 (GIBCO BRL). The skins were washed once with trypsin neutralizing solution (Cell Applications, San Diego, CA), and then centrifuged at 1,200 rpm/min for 10 min. The supernatant was removed, and the precipitants were washed twice with DMEM/F12. The skin pieces were mechanically dissociated and passed through a 200 mesh cell strainer. The filtrate was centrifuged at 1,200 rpm/min for 10 min, and the pellets were resuspended in 10 ml of DMEM/F12 supplemented with 2% B27 (GIBCO, BRL), 20 ng/ml epidermal growth factor (Peprotech EC LTD, London, UK), and 40 ng/ml basic fibroblast growth factor (Peprotech EC LTD, London, UK). The cells were cultured in 25 cm2 tissue culture flasks (Corning) in a 37°C, 5% CO2 tissue-culture incubator. To facilitate the passage of floating clusters of cells, medium containing spheres was centrifuged, the pellet was mechanically dissociated with a fire-polished Pasteur pipette, and the cells were reseeded in fresh medium containing B-27 and the above-mentioned growth factors. The cells were passaged every 6 d to 7 d. For transplantation, the cultured SKPs were centrifuged, the growth factor-containing supernatant was removed, and the spheres were resuspended to 2 × 106 cells/ml in HBSS.
Surgical methods and experimental groups
The nude mice were fasted for 24 h and anesthetized by intraperitoneal injection of 50 mg/kg sodium pentobarbital. Then, denervated cutaneous defects were made as previously described [3]. Briefly, a midline incision was made at the 13th costovertebral angle (2 cm in length). The skins and subcutaneous tissues were separated, and the T9 to L1 vertebrae were exposed (Figure 1A). The nerve roots were exposed bilaterally, the nerve approximately 0.5 cm in length was transected distal to the point of trifurcation, and the wound was closed. After recovery from anesthesia, the mice were stimulated with a needle at the wound site, and no response indicated successful nerve resection. After 2 d, these mice were re-anesthetized with sodium pentobarbital. In the denervated skins, two 5 mm full-thickness excisional skin wounds were created on each side of the midline (Figure 1B). The nude mice with denervated wounds were randomly divided into two groups (n = 30 per group). In the SKP-treated group, 1 × 105 SKPs in 50 μl HBSS were injected intradermally around the wound at four injection sites. The mice in the control group were injected with 50 μl of HBSS. The wounds were covered with semi-occlusive dressings (Tegaderm; 3 M Health Care, St. Paul, Minnesota). The animals were housed individually. We tested the adhesive on the mouse skins prior to this experiment and did not observe any skin irritation or allergic reaction.
Figure 1.
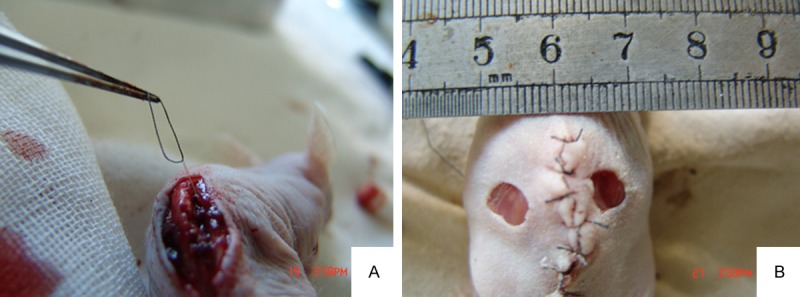
(A, B) exposed posterior spinal cord. The right posterior spinal cord at T9 was placed under traction using string after which the nerve was dissected to create a denervated skin (A). Two 5 mm full-thickness excisional skin wounds were created on denervated regions of the mouse dorsum (B).
Wound analysis
Wound analysis was made as described previously [25]. Digital photographs of wounds were taken at days 7, 14, and 21. The wound area was measured by tracing the wound margin and calculated using an image analysis program (NIH Image). Immediately after the wound was made, the wound area was recorded as the initial area, and the remaining area of unhealed wound was determined at different time points. Area of healed wound = initial area - area of unhealed wound. Wound healing rate = [area of healed wound/initial area] × 100. Time to wound closure was defined as the time at which the wound bed was completely re-epithelialized. Analysis was performed with Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA). The investigators who measured the samples were blinded to the groupings and treatments. The mice were sacrificed at days 7, 14, and 21, at which times skin samples including the wound and 4 mm of the surrounding skin were harvested using a 10 mm biopsy punch for enzyme-linked immunosorbent assay (ELISA), histological, and immunohistochemical (IHC) analyses. Briefly, the samples were bisected into two halves. One half was immediately frozen for ELISA, and the other was fixed in 10% paraformaldehyde, embedded in paraffin, and cut into 7 μm sections for IHC analysis.
ELISA measurements
ELISA was performed as previously described [26]. Briefly, the cutaneous wound tissue of the mice was dissected, weighed, frozen immediately in liquid nitrogen, and then stored at -80°C until further processing. Liquid nitrogen-frozen wound tissue was pulverized in a dismembrator (B. Braun Biotech International, Allentown, PA, USA) to determine the contents of substance P (SP) and calcitonin gene-related peptide (CGRP). SP and CGRP were sequentially extracted from the pulverized tissue samples and then subjected to liquid/liquid extraction with petroleum ether (Aldrich Chemical CO., Inc., Milwaukee, WI, USA) to remove lipids. The homogenates were centrifuged at 2000× g for 10 min, and the supernatants were lyophilized. The contents of SP and CGRP were determined using SP and CGRP ELISA kits (Peninsula, St. Helens, Merseyside, UK), respectively, according to the manufacturer’s protocol. NGF was also extracted from the pulverized tissue samples using a phosphate buffer (100 nM, pH 7.4) containing 0.4 M NaCl, 0.5% bovine serum albumin, 0.1 mM benzethonium chloride, 0.1 mM phenylmethylsulphonyl fluoride, 10 mM ethylenediaminetetraacetic acid, and 10 U/ml aprotinin. The content of NGF was measured in an NGF Emax TM ImmunoAssay System according to the manufacturer’s protocol (Promega, Mannheim, Germany). The resulting tissue pellets were vacuum-dried, and their dry weight was determined to quantitate protein levels.
IHC analysis
The slides were treated with xylene for deparaffinization and with gradient alcohol for debenzolization and then rehydrated. Endogenous peroxidase activity was inactivated with 3% hydrogen peroxide for 10 min. The sections were incubated with antibodies against PCNA (proliferating cell nuclear antigen, β-subunit, mouse 1:500; Sigma Aldrich, St. Louis, MO) or S-100 (mouse 1:500; Sigma Aldrich, St. Louis, MO) at 4°C overnight and then washed with phosphate-buffered saline (PBS). Subsequently, the sections were sequentially treated with biotin-conjugated secondary antibodies and SP complex for 20 min. The substrates were added, and color development was performed with diaminobenzidine for 5 min. After the slides were washed with water, the sections were counterstained with hematoxylin, dehydrated in gradient alcohol, and then cleared in xylene. The slides were mounted with Permount and then observed under a light microscope. In the negative controls, the primary antibody was replaced with PBS, and all other procedures were the same as those aforementioned.
The percentages of PCNA-positive nuclei were determined by counting the numbers of PCNA-positive nuclei and total nuclei in five random fields per section using an image analysis program (NIH Image). Four successive sections per wound were analyzed. The number of intraepidermal nerve fibers was counted based on S-100-positive cells. For each section, areas with high levels of PCNA expression were found at low magnification (40×), and five fields in each section were randomly selected at high magnification (400×). A total of 100 epithelial cells and the number of PCNA-positive cells were counted followed by averaging. The percentage of PCNA-positive cells was calculated. For each section, areas with high levels of S-100 expression were found at low magnification (40×), and five fields in each section were randomly selected at high magnification (200×). The number of S-100-positive cells was counted, and then the percentage of S-100-positive cells was calculated.
Statistical analysis
Data were presented as x̅ ± s, and SPSS13.0 statistical software was used for analysis. One-way ANOVA was used for comparisons between groups. A value of P < 0.05 was considered to indicate statistical significance. Graphics were performed with the Photoshop CS3.
Results
Skin and wound morphology
At 7 d after injury, granulation tissue was observed in both groups accompanied by newly generated epithelia. The wound area was decreased. There is no significant difference between two groups in epithelialization, with no apparent wound swelling and only a mild inflammatory response. At 14 d after SKP treatment, Wound healing rate was 87.42 ± 6.63 in SKP-treated group, with mild wound contraction. Granulation tissue in the control group was fresh. The wound base was lightly red, and the wound margin was clear and accompanied by newly generated epithelia and wound contraction. At 21 d after SKP treatment, complete wound healing was noted in SKP-treated mice, and wound contraction was more profound in the control group than in the SKP-treated group.
SKPs significantly promote wound healing
The wound healing rates at 7 d after SKPs treatment were 18.87% ± 3.22% and 15.32% ± 1.74% in the SKP-treated and control mice, respectively (P < 0.05). The wound healing rates at 14 d after treatment in the SKP-treated and control groups were 100.00% ± 0.00% and 72.35% ± 3.20%, respectively, suggesting that the wound healing rate significantly increased in the SKP-treated group (P < 0.05). Complete wound healing was observed at 21 d in the control group (Table 1).
Table 1.
The percentage of wound healing after treatment (%)
| group | wounds | days post-wound | ||
|---|---|---|---|---|
|
| ||||
| 7 | 14 | 21 | ||
| SKPs | 20 | 15.65 ± 2.34 | 87.42 ± 6.63# | 100.00 ± 0.00# |
| control | 20 | 15.32 ± 2.65 | 69.36 ± 3.72 | 80.52 ± 3.21 |
Each values is the mean ± SE of 20 wounds.
P < 0.05.
Changes in PCNA expression during wounding healing
The amount of PCNA in the wound was measured using IHC to test the difference in PCNA expression during wound healing with or without SKPs. As shown in Figure 2, PCNA-positive cells were yellow-brown. At 7 d after SKP treatment, positive cells were mainly found in the basal cell layer, spine cell layer, and granule cell layer of the epidermis and in the fibroblasts of the dermis. At 14 d, positive cells mainly lie in the stratum germinativum. Moreover, the PCNA expression level of the SKP-treated group was obviously higher than that of the control group at days 14 (P < 0.01) and 21 (P < 0.05). The PCNA expression level peaked in the control group at 21 d. However, the levels of PCNA were markedly greater in the SKP-treated group than in the control group and then peaked at day 14, indicating that the proliferation rate decreased during wound healing.
Figure 2.
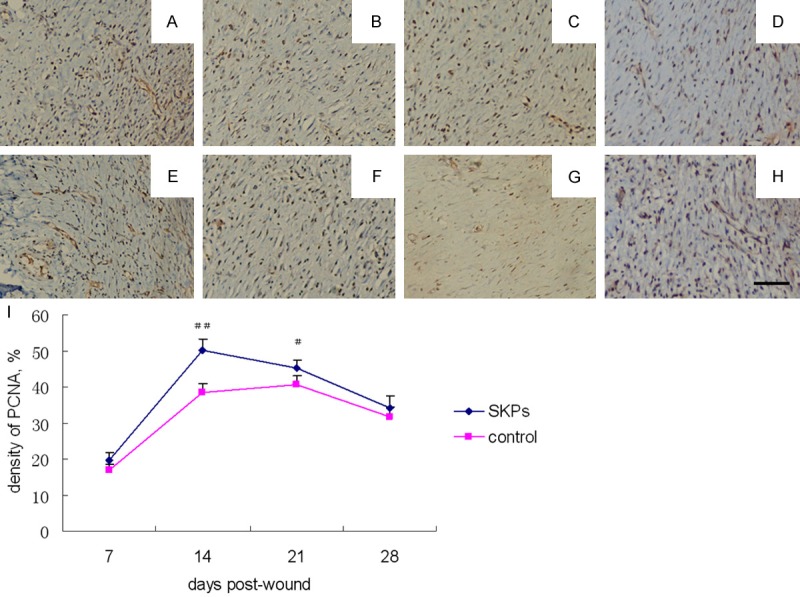
PCNA expression of IHC analysis in wound of SKP-treated (A-D) and control (E-H) nude mice. Photomicrographs were obtained on post-wound day 7 (A, E), 14 (B, F), 21 (C, G) and 28 (D, H). Scale bar in h 100 μm for all panels. (I) Comparison of the percentage of PCNA expression between SKP-treated and control groups at different post-wound days. ##P < 0.01, comparison between SKP-treated and control groups; #P < 0.05, comparison between SKP-treated and control groups.
Changes in S-100 expression during wound healing
Cells with S-100 expression were yellow-brown and mainly located around the vessels in the dermis. Beaded or wave-like small nerve branches were noted. The number of S-100 positive nerves significantly increased in SKP-treated group; the nerves in this group were straight and large. The expression of S-100 at days 14, 21, and 28 was markedly higher in SKP-treated group than in the control group. The density of S-100-positive nerves peaked in both groups (Figure 3).
Figure 3.
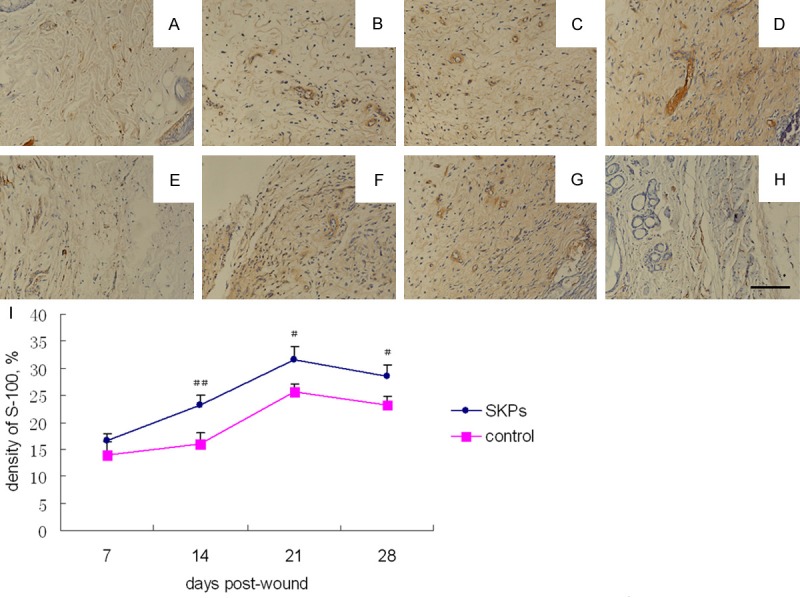
S-100 expression of IHC analysis in wound of SKP-treated (A-D) and control (E-H) nude mice. Photomicrographs were obtained on post-wound day 7 (A, E), 14 (B, F), 21 (C, G) and 28 (D, H). Scale bar in h 100 μm for all panels. (I) Comparison of the percentage of S-100 expression between SKP-treated and control groups at different post-wound days. ##P < 0.01, comparison between SKP-treated and control groups; #P < 0.05, comparison between SKP-treated and control groups.
NGF, SP, and CGRP levels are elevated in the SKP engraftment site
NGF, SP, and CRGP were quantified by ELISA to evaluate the level of neuropeptides in wounds. The content of NGF increased at day 7 and then declined at day 21. The contents of SP and CGRP showed similar increasing and decreasing trends during wound healing. The contents of NGF, SP, and CRGP in the SKP-treated group were significantly higher than those in the control group at days 14, 21, and 28. No significant differences in the contents of the three neuropeptides were observed between the SKP-treated and control groups (Figure 4).
Figure 4.
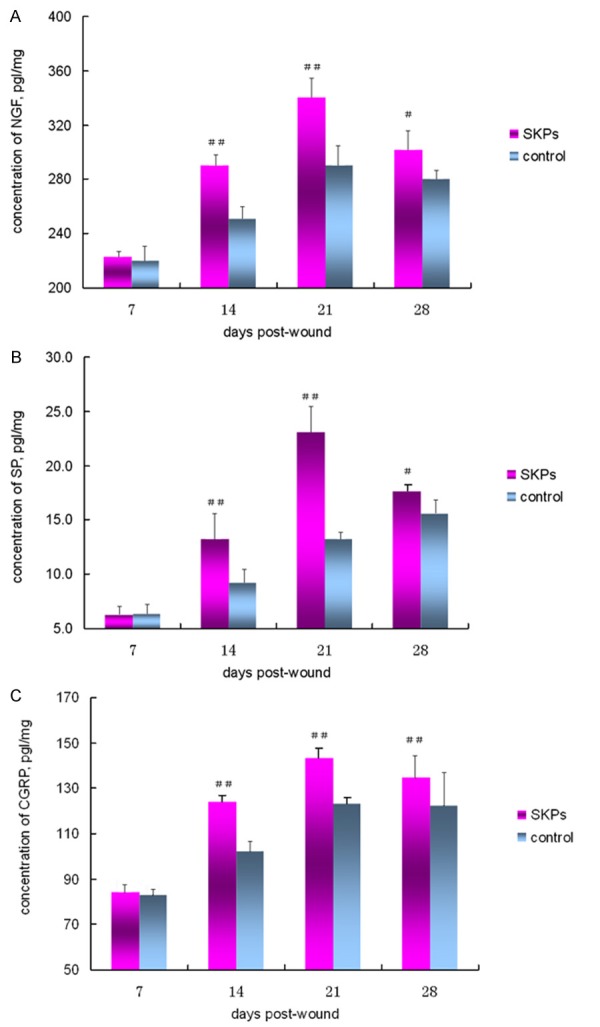
ELISA analysis of wound tissue for concentration of NGF (A), SP (B), and CGRP (C). Comparison of the concentration of NGF (A), SP (B), and CGRP (C) between SKP-treated and control groups at different post-wound days. ##P < 0.01, comparison between SKP-treated and control groups; #P < 0.05, comparison between SKP-treated and control groups.
Discussion
Components of fetal cutaneous regeneration and peripheral nerve regeneration are mutually dependent. On the one hand, fetal cutaneous regeneration may be driven by the peripheral nerve. On the other hand, denervation disrupted both epidermal wrinkle regeneration and dermal regeneration at distinct developmental stages [27]. Denervated wounds, including decubitus ulcers and diabetic foot ulcers, are difficult to heal. In addition, minimal improvement has been obtained in promoting wound repair in the past few decades. The best available treatment for denervated wounds achieves only a 50% healing rate that is often temporary [28]. The development of innovative treatments to enhance wound healing and regeneration is needed. Research has shown that stem cell transplantation can promote the repair of peripheral nerve trunks and partly facilitate the functional recovery of organs innervated by injured nerves. To date, little is known about the biological behavior and activity of stem cells in denervated wounds and about the effects of stem cell therapy on denervated wound healing. In the present study, SKP transplantation enhanced wound healing in denervated cutaneous wounds by promoting re-epithelialization, cell proliferation, neuropeptide secretion, and nerve regeneration.
We used a denervated cutaneous wound model to assess the effect of SKPs in denervated wounds [3,29]. The results of the present study indicate that implantation of SKPs significantly promotes wound healing. In the peripheral nerves, S-100 is mainly located in the perineurium and the cytoplasm and membrane of Schwann cells. The increased expression of S-100 during neurogeneration suggests the importance of Schwann cells in this process. In the present study, the regeneration of axons in the skin was gradually achieved during wound healing, accompanied by a gradual increase in the expression of S-100. The expression of S-100 in the SKP-treated mice was dramatically higher than that in the control mice. We postulated that SKPs are implicated in the repair of injured nerves in the wound area. Consistent with our findings, SKPs have been recently shown as a physiologic source of progenitors for cutaneous nerve regeneration in the skin [21]. With respect to the functions of these regenerated nerves, more studies are needed and an animal model with favorable skin sensitivity and reactivity is required.
Currently, the mechanisms underlying the regeneration of damaged nerves in the skin remain unclear. Repair can be achieved by the extension of residual axons and/or by the proliferation and differentiation of local stem cells. Therefore, wound repair and neuroregeneration can be achieved when the environment is beneficial for the extension of residual axons and/or stem cell differentiation into neurons. Recent studies have suggested that the neuropeptides released from cutaneous nerve fibers, such as NGF, SP, and CGRP, participate in wound healing [5,30-32]. These findings might be associated with the neuropeptides secreted after SKP transplantation in the present study. With the stimulation of these factors, SKPs can differentiate into neurons and/or glial cells, which guide neuroregeneration. Moreover, the results of the PCNA immunohistochemical assay suggest that these neuropeptides may be important for the proliferation of epidermal and dermal cells. In the present study, the PCNA-positive cells were primarily found in the actively proliferating epithelial cells and fibroblasts. The expression of PCNA was significantly higher in the SKP-treated mice than in the control mice. This result can be attributed to neurotrophic factors directly or indirectly secreted by the SKPs, which promote epithelial proliferation and accelerate epithelialization, resulting in wound healing.
Compared with other types of adult stem cells, SKPs are widely distributed and easy to harvest with minimally invasive techniques. The present study investigated the process of denervated wound healing. Thus, this study may serve as an experimental basis for the treatment of pressure ulcers in paraplegic patients as well as other chronic wounds. The results of this study suggest that SKPs are an alternative source of stem cells for denervated wound reconstruction. However, the mechanism by which regenerated nerve fibers increase neuropeptides remains unknown. Therefore, the association observed in this study needs to be verified in future studies.
Acknowledgements
This study was supported by Health and Family Planning Commission of Guangdong Province (A2013188), Science and Technology Planning Project of Guangdong Province (2013B022000053), and National Natural Science Foundation of China (81272153 and 81372062). And this paper should be attributed to department of burns in the First Affiliated Hospital of Sun Yat-Sen University.
Disclosure of conflict of interest
None.
References
- 1.Ebenezer GJ, McArthur JC, Thomas D, Murinson B, Hauer P, Polydefkis M, Griffin JW. Denervation of skin in neuropathies: the sequence of axonal and Schwann cell changes in skin biopsies. Brain. 2007;130:2703–2714. doi: 10.1093/brain/awm199. [DOI] [PubMed] [Google Scholar]
- 2.Buckley G, Wong J, Metcalfe AD, Ferguson MW. Denervation affects regenerative responses in MRL/MpJ and repair in C57BL/6 ear wounds. J Anat. 2012;220:3–12. doi: 10.1111/j.1469-7580.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukai T, Takeda A, Uchinuma E. Wound healing in denervated rat skin. Wound Repair Regen. 2005;13:175–180. doi: 10.1111/j.1067-1927.2005.130208.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res. 2002;307:281–291. doi: 10.1007/s00441-001-0477-8. [DOI] [PubMed] [Google Scholar]
- 5.Richards AM, Floyd DC, Terenghi G, McGrouther DA. Cellular changes in denervated tissue during wound healing in a rat model. Br J Dermatol. 1999;140:1093–1099. doi: 10.1046/j.1365-2133.1999.02908.x. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds M, Alvares D, Middleton J, Fitzgerald M. Neonatally wounded skin induces NGF-independent sensory neurite outgrowth in vitro. Brain Res Dev Brain Res. 1997;102:275–283. doi: 10.1016/s0165-3806(97)00105-3. [DOI] [PubMed] [Google Scholar]
- 7.Barker AR, Rosson GD, Dellon AL. Wound healing in denervated tissue. Ann Plast Surg. 2006;57:339–342. doi: 10.1097/01.sap.0000221465.69826.b7. [DOI] [PubMed] [Google Scholar]
- 8.Cerqueira MT, Pirraco RP, Santos TC, Rodrigues DB, Frias AM, Martins AR, Reis RL, Marques AP. Human Adipose Stem Cells Cell Sheet Constructs Impact Epidermal Morphogenesis in Full-Thickness Excisional Wounds. Biomacromolecules. 2013;14:3997–4008. doi: 10.1021/bm4011062. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Gu W, Du J, Reid B, Deng X, Liu Z, Zong Z, Wang H, Yao B, Yang C, Yan J, Zeng L, Chalmers L, Zhao M, Jiang J. Electric fields guide migration of epidermal stem cells and promote skin wound healing. Wound Repair Regen. 2012;20:840–851. doi: 10.1111/j.1524-475X.2012.00829.x. [DOI] [PubMed] [Google Scholar]
- 10.Ojeh NO, Navsaria HA. An in vitro skin model to study the effect of mesenchymal stem cells in wound healing and epidermal regeneration. J Biomed Mater Res A. 2014;102:2785–92. doi: 10.1002/jbm.a.34950. [DOI] [PubMed] [Google Scholar]
- 11.Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol. 2012;23:946–953. doi: 10.1016/j.semcdb.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Martínez E, Galván-Hernández CI, Toscano-Márquez B, Gutiérrez-Ospina G. Modulatory role of sensory innervation on hair follicle stem cell progeny during wound healing of the rat skin. PLoS One. 2012;7:e36421. doi: 10.1371/journal.pone.0036421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, Miller FD. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang HK, Min SK, Jung SY, Jung K, Jang da H, Kim OB, Chun GS, Lee ZH, Min BM. The potential of mouse skin-derived precursors to differentiate into mesenchymal and neural lineages and their application to osteogenic induction in vivo. Int J Mol Med. 2011;28:1001–1011. doi: 10.3892/ijmm.2011.785. [DOI] [PubMed] [Google Scholar]
- 15.Lavoie JF, Biernaskie JA, Chen Y, Bagli D, Alman B, Kaplan DR, Miller FD. Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem Cells Dev. 2009;18:893–906. doi: 10.1089/scd.2008.0260. [DOI] [PubMed] [Google Scholar]
- 16.Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 17.McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006;26:6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tunici P, Bulte JW, Bruzzone MG, Poliani PL, Cajola L, Grisoli M, Douglas T, Finocchiaro G. Brain engraftment and therapeutic potential of stem/progenitor cells derived from mouse skin. J Gene Med. 2006;8:506–513. doi: 10.1002/jgm.866. [DOI] [PubMed] [Google Scholar]
- 19.Higashida T, Jitsuki S, Kubo A, Mitsushima D, Kamiya Y, Kanno H. Skin-derived precursors differentiating into dopaminergic neuronal cells in the brains of Parkinson disease model rats. J Neurosurg. 2010;113:648–655. doi: 10.3171/2010.2.JNS091432. [DOI] [PubMed] [Google Scholar]
- 20.Walsh SK, Gordon T, Addas BM, Kemp SW, Midha R. Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Exp Neurol. 2010;223:221–228. doi: 10.1016/j.expneurol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Pradhan S, Liu C, Le LQ. Skin-derived precursors as a source of progenitors for cutaneous nerve regeneration. Stem Cells. 2012;30:2261–2270. doi: 10.1002/stem.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu B, Qi SH, Liu P, Huang Y, Xie JL, Xu YB, Liu XS, Li YY. Influence of skin-derived progenitor cell combining with hyaluronic acid on the wound healing of diabetic rat. Zhonghua Shao Shang Za Zhi. 2007;23:20–24. [PubMed] [Google Scholar]
- 23.Huang Y, Qi SH, Shu B, Chen L, Xie JL, Xu YB, Liu XS. Fibroblast growth factor-binding protein facilitates the growth and migration of skin-derived precursors. J Cutan Med Surg. 2011;15:201–209. doi: 10.2310/7750.2011.10049. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 26.Legat FJ, Jaiani LT, Wolf P, Wang M, Lang R, Abraham T, Solomon AR, Armstrong CA, Glass JD, Ansel JC. The role of calcitonin gene-related peptide in cutaneous immunosuppression induced by repeated subinflammatory ultraviolet irradiation exposure. Exp Dermatol. 2004;13:242–250. doi: 10.1111/j.0906-6705.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 27.Kishi K, Ohyama K, Satoh H, Kubota Y, Tanaka T, Imanishi N, Nakajima H, Kawamura K, Nakajima T. Mutual dependence of murine fetal cutaneous regeneration and peripheral nerve regeneration. Wound Repair Regen. 2006;14:91–99. doi: 10.1111/j.1743-6109.2005.00093.x. [DOI] [PubMed] [Google Scholar]
- 28.Richards AM, Mitsou J, Floyd DC, Terenghi G, McGrouther DA. Neural innervation and healing. Lancet. 1997;350:339–340. doi: 10.1016/s0140-6736(05)63391-0. [DOI] [PubMed] [Google Scholar]
- 29.Engin C, Demirkan F, Ayhan S, Atabay K, Baran NK. Delayed effect of denervation on wound contraction in rat skin. Plast Reconstr Surg. 1996;98:1063–1067. doi: 10.1097/00006534-199611000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Anand P, Foley P, Navsaria HA, Sinicropi D, Williams-Chestnut RE, Leigh IM. Nerve growth factor levels in cultured human skin cells: effect of gestation and viral transformation. Neurosci Lett. 1995;184:157–160. doi: 10.1016/0304-3940(94)11195-o. [DOI] [PubMed] [Google Scholar]
- 31.Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108:122–128. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- 32.Roggenkamp D, Köpnick S, Stäb F, Wenck H, Schmelz M, Neufang G. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol. 2013;133:1620–1628. doi: 10.1038/jid.2012.464. [DOI] [PubMed] [Google Scholar]


