Abstract
Notoginsenoside R1 (NR1) is the main bioactive component in panaxnotoginseng, an old herb medicine widely used in Asian countries in the treatment of microcirculatory diseases. However, little is known about the effect of NR1 on antihypertension and the underlying mechanisms are still not clear. This study is aim to investigate the effect and elicit the mechanism of NR1 in antihypertension. Firstly, to assess the ability of NR1 in antihypertension, NR1 was injected in spontaneously hypertensive rats (SHR) via the vena caudalis. Then we examined the rats systolic blood pressure and inducible nitric oxide synthase (iNOS) activation in rats thoracoabdominal aortic. To further investigate the molecular mechanism of NR1 reduce blood pressure, primary SHR and WYK rat vascular endothelial cells (RVECs) were used for next study. LncRNAs related to hypertension were gained from bioinformatics analysis. The role of LncRNAs was finally characterized in RVECs by siRNA. Our results showed that NR1 significantly reduce blood pressure in SHR and induce nitric oxide (NO) generation through increasing the phosphorylation of iNOS. Through bioinformatics analysis and knockdown LncRNA AK094457 in RVECs, we also found LncRNA AK094457 promoted iNOS expression and NO concentration. Thus, we conclude that NR1 reduces the caudal blood pressure of SHR through induction of iNOS regulated by long non-coding RNA AK094457. These findings may have important implications for understanding the mechanisms of NR1 regulation blood pressure.
Keywords: Spontaneously hypertensive rats, notoginsenoside R1 (NR1), bioinformatics analysis, long non-coding RNA AK094457, nitric oxide synthase (iNOS)
Introduction
Hypertension is a major public health challenge due to its high prevalence and concomitant increase in the risk for cardiovascular disease (CVD) and all-cause mortality. As a complex trait, hypertension is influenced by multiple environmental and genetic determinants, such as nitric oxide (NO) [1] and is characterized by impaired vascular endothelial function. Disruption of endothelial cell function is characterized by impaired bioavailability of NO [2] and induces vascular disease. At present, despite the considerable advances in diagnosis and adjuvant therapy, the clinical outcome of hypertension patients has not been improved markedly. Thus, pathogenesis, diagnosis and medical treatments of hypertension still are substantial clinical challenges. Accordingly, there is an urgent necessity to improve the molecular characterization of hypertension, in order to facilitate understanding of hypertension pathogenesis and improvement on diagnostic and therapeutic efficiency.
Panax notoginseng, one of the most frequently used traditional Chinese medicine, is well known for its efficacy in promoting blood circulation, ameliorating pathological hemostasis, alleviating pain [3-6]. It is one of the famous traditional medicinal herbs that have been used for hundreds of years in many East Asian countries [7]. P.N consists of two major ingredients: The first is the panaxadiol group, which includes R1, Rb2, Rb3, Rc, Rd, Rg3, Rh2, and Rs1. The second is the panaxatriol group, which includes Re, Rf, Rg1, Rg2, and Rh1. Individual ginsenosides exert different effects via different mechanisms in various tissues. The combination of ginsenosides in ginseng extracts may be important for providing more powerful therapeutic and pharmacological effects [8-10]. Clinical reports suggest that P.N plays an important role in the treatment of inflammation, coronary heart disease, stroke and immunological disease [11-13].
Abnormalities in nitric oxide (NO) play an important role in the pathophysiology of hypertension [14-16] and are related to resistant hypertension [17]. Therefore its relationship to essential hypertension has been the subject of intense investigation. As with coronary artery disease and diabetes initial evidence suggesting a NO-dependent component of the disease came from studies assessing endothelium-dependent vasodilatation. A number of studies have demonstrated the impairment of NO-mediated vasodilatation in brachial [18], coronary [19] and renal arteries [20] in patients with essential hypertension compared to controls. In addition to the involvement of NO during the process of inflammation, the vascular hemodynamics is greatly affected by NO production. However, many aspects of the development of hypertension at the molecular level are still unknown.
Long non-coding RNA (LncRNA) is a class of new found non-coding RNAs that longer than 200 nucleotides in length. LncRNAs remain nearly completely unexplored in hypertension research. However, emerging evidence indicates lncRNAs may have functional significance in the development, physiology or diseases of the heart and the vasculature [21,22]. The significance of non-protein-coding RNA in general is being increasingly recognized in hypertension research [23-25]. Therefore, identifying the relationship between LncRNAs regulated by NR1 may help to understand the function of NR1 in pathogenesis of Hypertension.
In the present study, we sought to examine the functional role of NR1 in antihypertension. We also found that iNOS protein is highly expressed in rats thoracoabdominal aortic and in rats vascular endothelial cells after treated with notoginsenosideNR1 than non-treatment group. In order to understand the mechanism of NR1 regulates hypertension, we further compare the LncRNA expression profile difference between hypertension patients and control groups. Finally, we also investigated the impact of altered LncRNA AK094457 levels, the most obvious change LncRNA, on the phenotypes of rat vascular endothelial cells (RVECs) in vitro. To our knowledge, our findings for the first time revealed the mechanism of NR1 in antihypertension through regulation of LncRNAs.
Material and methods
Materials
P. notoginseng (P.N., Sanchi in Chinese, Yunnan, China) was collected and subjected to ethanol extraction. The yield rate of notoginsenosides R1 from P. notoginseng (P.N.) was around 10%. Antibodies against iNOS and GAPDH were purchased from Cell Signaling technologies (Danvers, MA). Rabbit antibodies conjugated with horseradish peroxidase (HRP) and sheep anti-mouse-HRP were purchased from Zhong San Jin Qiao (Bei Jing, China). All others chemical reagents were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Cell culture
Primary SHR and WYK rat vascular endothelial cells (RVECs) were purchased from Pricells Co. Ltd. (Wuhan, China). Cells were cultured in M199 medium with 2 mM L-glutamine, 100 U/mL penicillin-streptomycin, 100 μg/mL heparin, 30 μg/mL endothelial cell growth supplement and 10% FBS in 75 cm2 tissue culture flasks at 37°C in a humidified atmosphere of 5% CO2.
Animals and measurement of blood pressure
Male 11-week-old SHR and Wistar Kyoto (spontaneously hypertensive rats, WKY) rats weighing 250 to 320 g were purchased from Beijing Vital Laboratory Cell culture and drug treatment. Technology (Beijing, China) and housed under standard conditions at 25°C with a 12/12-hour light-dark cycle. SHR and WKY rats were randomly divided into 4 groups, weighed, and the conscious animals’ blood pressures were measured on a rat tail-cuff (Model RBP-1) for 30 min, followed by administration of P.N. extracts (100 mg/kg) through the caudal vein compared with the clinical dose of the frozen dried powder of NR1 is 400 mg/day. Final BP measurements were the mean of 5-6 determinations for each individual animal. All animal care and experimental protocols conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, and approval was granted by the IACUC of PKU (approval reference number IMM-LiJ-1).
NO production assay
Various concentrations of NR1 were prepared in phenol red-free DMEM medium to reduce assay interference by phenol red. Cell culture supernatants were collected after treatment with different compounds (or PBS in the controls) for 3 h. NO production was detected spectrophotometrically by measuring its final stable equimolar degradation products, nitrite and nitrate, by using nitrate reductase (Assay Design Inc.) and the acid-catalyzed diazotation reaction by using sulfanylamide and naphtylethylenediamine (Griess reaction). Total nitrite was quantified after the reduction of all nitrates with nitrate reductase. Nitrite levels in culture supernatants were within the linearity range of calibration curves that were generated from a solution of sodium nitrite. Total nitrite concentration was calculated from a standard curve constructed over the linear range of the assay and expressed as 100 pmol per 105 cells.
Choice of differentially expressed LncRNAs list using heat map analysis
We obtained the microarray date from Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), and the GEO accession number is GSE38783. The date was generated using the genechipAffymetrix Human Genome U133 Plus 2.0 Array GPL570 (HG-U133_Plus_2), which completely coverage Human Genome U133 Set plus 6500 additional genes for analysis of over 47,000 transcripts. Observations with adjusted p-values ≥0.05 were removed, and thus excluded from further analysis. The heat map of the 50 LncRNAs most obvious differences was created using a method of hierarchical clustering by GeneSpring GX, version 7.3 (Agilent Technologies, California, United States).
Chosen LncRNAs were finally confirmed for altered transcription level using qRT-PCR between WKY and SHR rat thoracoabdominal aortic tissues. Primers used in qRT-PCR were as follows: AK094457: 5’-tggctttagggaaacat-3’ (forward probe); 5’-aagatccaagaaccgatg-3’ (reverse probe). GAPDH: 5’-ggaccaatacgaccaaatccg-3’ (forward probe), 5’-agccacatcgctcagacac-3’ (reverse probe). Other LncRNAs primer sequences are available upon request.
Quantitative real-time PCR
The regulation of iNOS mRNA expression by NR1 was determined by qRT-PCR analysis. Total RNA was isolated using an RNA miniprep kit according to the manufacturer’s instructions (Stratagene, CA, USA). qRT-PCR was carried out using the selective primers for the LncRNA AK094457 and iNOS genes. Primers specific for iNOS: 5’-caacctgcacgccaagaac-3’ (forward probe); 5’-tccacaactcgctccaagatg-3’ (reverse probe) was used, PCR were performed for 30 cycles using the following conditions: denaturation at 95°C for 0.5 min, annealing at 49°C for 0.5 min and elongation at 68°C for 1.5 min.
Western blot
The mouse thoracoabdominal aortic and RVECs were lysed in RIPA buffer (Boston Bioproducts, MA) with protease inhibitors, and proteins were resolved by SDS/PAGE gel and transferred to a PVDF membrane (Millipore, MA). After being blocked for 1 h in TBS-T with 5% nonfat milk, the PVDF membrane was then probed with primary antibodies polyclonal rabbit anti-iNOS (1:2000) and anti-GAPDH (1:2000, Cell Signaling), at 4°C overnight and then incubated (1 hour) in TBST/2.0% BSA containing horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies (1:5000). The proteins were visualized with an ECL detection system (Amersham). Semi-quantifications were performed with densitometric analysis by Metamorph software.
Transfections of small interfering RNA (siRNA)
Non-targeting siRNA (siNT) and siLncRNA-AK094457 (Invitrogen), at a concentration of 10 nM, were transfected into RVECs using RNAiMAX reagent (Invitrogen) following the manufacturer’s instructions. After 24 h, cells were seeded onto chambered slides or 24-well plates, and allowed to grow for another 24-48 h prior to RNA isolation or the start of experiments. Knockdown of gene expression was confirmed by qRT-PCR. The sequences of siRNAs were as follows: si-AK094457-1: ccgggccgtcatatattggtcttga; si-AK094457-2: cgggccgtcatatattggtctttgat; si-AK094457-3: ggccgtcatatattggtcttgatgt; si-Scramble: ccgccgtcatatattggtctggtga.
Statistic analysis
All values are expressed as mean ± SD. Relaxation in response to herbs is presented as percent change in tension from pre-constriction levels. When multiple vessel rings were studied from one rat, responses were averaged, and n represents the number of rats per group. Comparisons were made by using a one-way ANOVA with repeated measures, followed by the Student-Newman-Keuls test to detect individual differences. P<0.05 was considered as statistically significant.
Results
NR1 reduces blood pressure of spontaneously hypertensive rats
To determine the activity of NR1 in hypertension suppression, P.N. extract was injected into the tail of SHRs (n=9) and WKY (n=9) rats at the dosage of 100 mg/kg and a fall in the tail blood pressure in SHRs was noticed at6 weeks post-injection. There was a significant change in the NR1-treated SHRs (201±8.3 vs. 169±7.8 mmHg, P<0.05) after treatment of 6 weeks (Figure 1A). However, the same dosage of NR1 did not significantly alter blood pressure in WKY rats (125±9.1 vs. 123±9.2 mmHg, P>0.05). These data indicated that NR1 can significantly reduce blood pressure of Spontaneously Hypertensive Rats.
Figure 1.

The effect of notoginsenoside R1 on rats systolic blood pressure (SBP). WYK, Wistar-Kyoto rats; SHR, spontaneously hypertensive rats; NR1, notoginsenoside R1. A. Blood pressures were measured on a rat tail-cuff (Model RBP-1). Data are expressed as means ± SD from five rats in each group and analyzed by one-way ANOVA. B. Western blotting analysis for iNOS expression in four groups, GAPDH was used as an internal control. C. Quantification of iNOS was normalized to GAPDH and the results are expressed as means ± SD, n=3.
To evaluate the molecular mechanism of NR1 in hypertension suppression, the expression levels of iNOS in NR1-treated groups were determined by qRT-PCR and Western Blot. As shown in Figure 1B, after NR1 treatment, the expression of iNOS was significantly increased in SHR rats thoracoabdominal aortic tissues compared to non-treated group. As shown in Figure 1C, the level of iNOS mRNA was also increased approximately 4.0-fold in rats thoracoabdominal aortic compare to non-treated group.
Effect ofNR1 on the expression of iNOS and NO production in RVECs
To further investigate the molecular mechanism of NR1 reduce blood pressure, primary SHR and WYK rat vascular endothelial cells (RVECs) were used for next study. After NR1 treatment, the expression of iNOS in RVECs of SHR was significantly increased, but also lower than in WYK control group (Figure 2A). We also examined NO levels in the culture medium using the NO production assay kit. We found that the NO production was increased after treatment of NR1 in SHR RVECs (Figure 2B). These results show that NR1 stimulates the expression of iNOS and NO production in rat endothelial cells.
Figure 2.
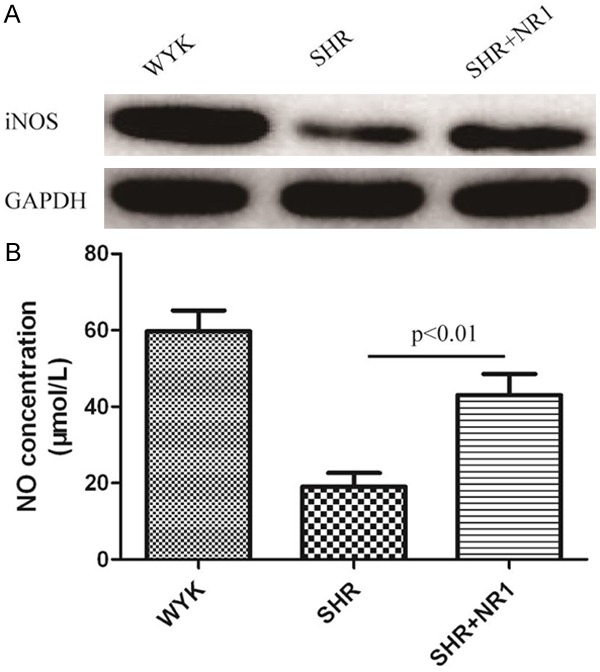
The effect of notoginsenoside R1 on iNOS protein expression and NO concentration in rats vascular endothelial cells. WYK, Wistar-Kyoto rats cells; SHR, spontaneously hypertensive rats cells; NR1, notoginsenoside R1. A. Western blotting analysis for iNOS expression in three group cells, GAPDH was used as an internal control. B. NO concentration was measured by NO assay kit from Beyotime (China).
Choice of differentially expressed LncRNAs list using heat map analysis between hypertension patients and control groups
We used the Arraystar Human LncRNA Array v2.0, submitted to the Gene Expression Omnibus (GEO) by Varambally at Sep 17, 2005 and last update at Nov 26, 2014, to analyze differential LncRNAs expression between hypertension patients and control groups. Firstly, hierarchical clustering analysis was used to compare differential LncRNA expression of the top 50 LncRNAs. The clustered heat map of 50 LncRNAs for v2.0 is shown in Figure 3A. Then, we further confirmed the selected 10 up-regulation of LncRNAs in rat vascular endothelial cells through qRT-PCR. As shown in Figure 3B, comparing to WYK, the expression of LncRNA AK094457 was significantly increased about 5.4-fold in SHR rats. However, all other 9 LncRNAs have a weak up-regulation. Our data indicated that LncRNA AK094457 was aberrant high expression in SHR vascular endothelial cells.
Figure 3.

Heatmap analysis of the LncRNA expression of hypertension patients and control groups. A. The heat map analysis of known hypertension related marker genes was created using a method of hierarchical clustering by GeneSpring GX, version 7.3. Rows: samples; Columns: LncRNAs; Color key indicates LncRNA expression value, red: highest, blue lowest. B. Chosen LncRNAs was finally confirmed for altered transcription level using qRT-PCR. C. The expression of LncRNAAK094457 was concentration-dependently regulated by NR1.
It is interesting that the expression of LncRNA AK094457 was down-regulated by NR1 (Figure 3C). And the effect of regulation is a dose-dependent manner. This data suggest that long non-coding RNA AK094457 may play an important role in the effect of NR1 anti-hypertension.
Knockdown of LncRNA AK094457 in RVECs cells results in enhancement of iNOS expression and NO concentration
In order to investigate the functional role of LncRNA AK094457 in anti-hypertension, siRNA experiment was used to silence LncRNA AK094457 in RVECs. As shown in Figure 4A, the mRNA level of LncRNA AK094457 in siRNAs transfected cells down to 0.8-fold, 0.2-fold and 0.4-flod respectively, comparing with scramble group. These results indicated that LncRNA AK094457 was efficiently silenced in RVECs by si-AK094457-2. Therefore si-AK094457-2 was used for all subsequent LncRNA AK094457 silencing experiments.
Figure 4.
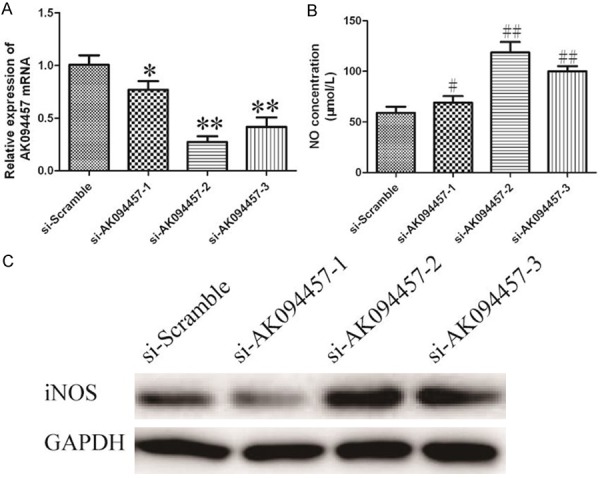
The effect of LncRNA AK094457 on iNOS expression and NO concentration in rats vascular endothelial cells was detected using siRNA assay. A. Silencing efficiency of AK094457 was verified by qRT-PCR. *P<0.05, **P<0.01 vssi-Scramble group. B. iNOS expression was detected by Western blotting analysis, GAPDH was used as an internal control. C. NO concentration was measured by NO assay kit from Beyotime (China). Values represent mean ± SD of three independent experiments. #P<0.05, ##P<0.01 vssi-Scramble group.
To explore the effect of LncRNA AK094457 regualted NO concentration, si-AK094457-2 was transfected into RVECs and NO concentration was measured by NO assay kit from Beyotime (China). As shown in Figure 4B, data shows a notable increase of NO concentration in SHRRVECs after si-AK094457-2 transfection (P<0.01).
We also investigated the influence of iNOS expression caused by silencing of LncRNA AK094457. The iNOS expression was measured by Western blot. As shown in Figure 4C, a marked increase of iNOS in SHRRVECs also observed. These results showed that silencing LncRNA AK094457 up-regulated the expression of iNOS and increase NO generation in RVECs.
Discussion
In the present study, we report a novel finding: Notoginsenoside R1 (NR1) reduces the caudal blood pressure of SHR through induction of iNOS regulated by long non-coding RNA AK094457.
Previous studies suggest that Ginsenosides from P. ginseng decrease the blood pressure in both experimental animals and hypertensive patients [26-29]. The antihypertensive effect of ginsenosides may result from their ability to inhibit vascular tone. In addition P.N. extract, specifically the major ingredients, NR1 and Rg1, were used to elucidate their role in vasorelaxation in small animal models, demonstrated that these compounds relaxed murine coronary arteries primarily by increasing NO production through the PI3K/Akt/eNOS and Larginine/eNOS/NO pathways, as well as increased cationic amino acid transporter expression. These observations provided additional evidences to previous reports that suggest an Rg1-induced increase of vascular endothelial growth factor in endothelial cells through theactivation of a PI3K/Akt pathway [30,31].
Although clinical trials are lacking, in vitro studies using P. Notoginseng do suggest possible cardiovascular effects. One study that used purified notoginsenoside R1, extracted from P. notoginseng, on human umbilical vein endothelial cells showed a dose- and time-dependent synthesis of tissue-type plasminogen activator without affecting the synthesis of plasminogen activating inhibitor, thus enhancing fibrinolytic parameters [32]. Another study suggests that P. notoginsengsaponins may inhibit atherogenesis by interfering with the proliferation of smooth muscle cells [33]. According to a recent study, it appears that P. notoginseng exerts its therapeutic effects on atherosclerosis through an anti-inflammatory action and regulation of the blood lipid profile [12]. Although many studies suggest that the protective effects of ginsenosides have been widely studied and shown to have new beneficial effects on hypertension [28] and various diseases, such as atherosclerosis, cancer, and thrombosis [12,34-36], the role of P. notoginseng in the treatment of hypertension is less certain, since it causes vasodilation or vasoconstriction depending on concentration and the target vessel.
NO is crucial to the maintenance of normal blood pressure [37] and therefore its relationship to essential hypertension has been the subject of intense investigation. Several studies have demonstrated impaired endothelial dysfunction in essential hypertension, which is associated with a blunted response to NO-mediated effects. Although it is unclear whether this represents reduced synthesis or increased consumption of NO. The vasodilator effects of the NO-donor sodium nitroprusside, was identical in both hypertensive and normotensives [38], suggesting that the cGMP-signalling pathway in smooth muscle cells is normal. Interestingly, hypertensive subjects have defective vascular responses to acetylcholine, but normal responses to β-adrenergic stimulation by bradykinin. These findings suggested that endothelial dysfunction associated with essential hypertension is due to a selective abnormality of the signalling pathways leading to NOS activation. Indeed, the hypotensive effects of angiotensin-converting enzyme (ACE) inhibitors in hypertensive subjects, has been shown to occur through increased formation of bradykinin and leading to enhanced NO formation. Another study has suggested that the depressed levels of basal NO in hypertensive subjects could be due to increased circulating levels of ADMA [39], although this has not been confirmed. Thus the reduced effect of NO is probably due to a variety of contributing factors.
The nitric oxide pathway is thought to be involved in the regulation of vascular tone in hypertension and stoke [40]. NO is produced from the guanidino group of L-arginine in an NADPH-dependent reaction catalyzed by a family of NOS enzymes [41]. Recent reports indicate that NO production is dependent on the availability and delivery of extracellular L-arginine that can be mediated by several different classes of cationic amino acid transporters, which have been characterized in terms of their ion dependency, substrate specificity, and relative affinity [42]. In endothelial cells, the delivery of extracellular L-arginine is mediated by the Na+-independent system y+ carrier [43].
Long noncoding RNAs have been found play widespread roles in many tumor cellular processes, including regulation cell proliferation, apoptosis and cell cycle arrest in recent years. LncRNAs remain nearly completely unexplored in hypertension research. However, emerging evidence indicates lncRNAs may have functional significance in the development, physiology or diseases of the heart and the vasculature. LncRNA-ANRIL plays a pivotal role in regulation of cardiac Cdkn2a/b expression and suggests that this region affects CAD progression by altering the dynamics of vascular cell proliferation [44]. ANRIL expression levels were directly correlated with the severity of atherosclerosis and Chr9p21 genotype in a large cohort [45] LncRNA NPPA codes for a precursor of atrial natriuretic peptide (ANP) that protects the cardiovascular system from the volume and pressure overload by decreasing vascular smooth muscle tone, which suggested that it may participate the occurrence and development of hypertension [46]. The significance of non-protein-coding RNA in general is being increasingly recognized in hypertension research.
In this study, we found that NR1 is apparent, as evidenced by significant lowering of the caudal blood pressure (Figure 1A), this implies that NR1 may also play a role in treatment of hypertension. Hence, in the present study, using spontaneously hypertensive rats (SHR), after NR1 treatment, we found NO production and induce iNOS expression increases in rats thoracoabdominal aortic and in rats vascular endothelial cells. Thus, we ensure NR1 is critical in maintaining blood pressure. Furthermore, we demonstrated that the expression level of LncRNAAK094457is upregulated in hypertension rat tissue (Figure 3A). Moreover, silencing AK094457 by siRNA could induce higher expression of iNOS and concentration of NO compared with the control group in RVECs (Figure 4B, 4C). Our data identified an important role for AK094457 in antihypertension.
However, there are several limitations in our study that should be mentioned. For example, in this study we do not use clinical samples, we use animal models. Although we found LncRNA AK094457 expression is related to expression of iNOS and concentration of NO, the detailed regulation mechanisms are not understood. The mechanism how NR1 regulates LncRNA AK094457 is still unknown.
In conclusion, we have described a new role for NR1 in antihypertension, stimulating NO release in rat vein endothelial cells. We also provided a systematic characterization of lncRNAs and identified several lncRNAs that might be involved in hypertension. Our data indicated that NR1 reduces blood pressure in spontaneously hypertensive rats through the long non-coding RNA AK094457. These findings provide a new foundation for lncRNA research in hypertension and expand current understanding of the effect of NR1 antihypertension, which could improve the therapeutic approaches for hypertension.
Acknowledgements
This study was sponsored by the Shanghai Science and Technology commission to guide project (10411968200).
Disclosure of conflict of interest
None.
References
- 1.Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 2.Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich) 2006;8:17–29. doi: 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 4.Sun HX, Pan HJ, Pan YJ. Haemolytic activities and immunologic adjuvant effect of Panax notoginseng saponins. Acta Pharmacol Sin. 2003;24:1150–1154. [PubMed] [Google Scholar]
- 5.Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 6.Dong TT, Cui XM, Song ZH, Zhao KJ, Ji ZN, Lo CK, Tsim KW. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J Agric Food Chem. 2003;51:4617–4623. doi: 10.1021/jf034229k. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa M, Murakami T, Ueno T, Yashiro K, Hirokawa N, Murakami N, Yamahara J, Matsuda H, Saijoh R, Tanaka O. Bioactive saponins and glycosides. VIII. Notoginseng (1): new dammarane-type triterpene oligoglycosides, notoginsenosides-A, -B, -C, and -D, from the dried root of Panax notoginseng (Burk.) F.H. Chen. Chem Pharm Bull (Tokyo) 1997;45:1039–1045. doi: 10.1248/cpb.45.1039. [DOI] [PubMed] [Google Scholar]
- 8.Qi LW, Wang CZ, Yuan CS. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 10.Huu Tung N, Uto T, Morinaga O, Kim YH, Shoyama Y. Pharmacological effects of ginseng on liver functions and diseases: a minireview. Evid Based Complement Alternat Med. 2012;2012:173297. doi: 10.1155/2012/173297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia ZY, Liu XY, Zhan LY, He YH, Luo T, Xia Z. Ginsenosides compound (shen-fu) attenuates gastrointestinal injury and inhibits inflammatory response after cardiopulmonary bypass in patients with congenital heart disease. J Thorac Cardiovasc Surg. 2005;130:258–264. doi: 10.1016/j.jtcvs.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YG, Zhang HG, Zhang GY, Fan JS, Li XH, Liu YH, Li SH, Lian XM, Tang Z. Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin Exp Pharmacol Physiol. 2008;35:1238–1244. doi: 10.1111/j.1440-1681.2008.04997.x. [DOI] [PubMed] [Google Scholar]
- 13.Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 14.Watson T, Goon PK, Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 15.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 16.Sandrim VC, Yugar-Toledo JC, Desta Z, Flockhart DA, Moreno H Jr, Tanus-Santos JE. Endothelial nitric oxide synthase haplotypes are related to blood pressure elevation, but not to resistance to antihypertensive drug therapy. J Hypertens. 2006;24:2393–2397. doi: 10.1097/01.hjh.0000251899.47626.4f. [DOI] [PubMed] [Google Scholar]
- 17.Yugar-Toledo JC, Ferreira-Melo SE, Consolim-Colombo FM, Irigoyen MC, Coelho OR, Moreno H Jr. Cyclic guanosine monophosphate phosphodiesterase-5 inhibitor promotes an endothelium NO-dependent-like vasodilation in patients with refractory hypertension. Nitric Oxide. 2007;16:315–321. doi: 10.1016/j.niox.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Panza JA, Garcia CE, Kilcoyne CM, Quyyumi AA, Cannon RO 3rd. Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–1738. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 19.Treasure CB, Klein JL, Vita JA, Manoukian SV, Renwick GH, Selwyn AP, Ganz P, Alexander RW. Hypertension and left ventricular hypertrophy are associated with impaired endothelium-mediated relaxation in human coronary resistance vessels. Circulation. 1993;87:86–93. doi: 10.1161/01.cir.87.1.86. [DOI] [PubMed] [Google Scholar]
- 20.Higashi Y, Oshima T, Ozono R, Watanabe M, Matsuura H, Kajiyama G. Effects of L-arginine infusion on renal hemodynamics in patients with mild essential hypertension. Hypertension. 1995;25:898–902. doi: 10.1161/01.hyp.25.4.898. [DOI] [PubMed] [Google Scholar]
- 21.Schonrock N, Harvey RP, Mattick JS. Long noncoding RNAs in cardiac development and pathophysiology. Circ Res. 2012;111:1349–1362. doi: 10.1161/CIRCRESAHA.112.268953. [DOI] [PubMed] [Google Scholar]
- 22.Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Boon RA, Dimmeler S. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114:1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 23.Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, Nelson-Williams C, Raja KM, Kashgarian M, Shulman GI, Scheinman SJ, Lifton RP. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306:1190–1194. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heggermont WA, Heymans S. MicroRNAs are involved in end-organ damage during hypertension. Hypertension. 2012;60:1088–1093. doi: 10.1161/HYPERTENSIONAHA.111.187104. [DOI] [PubMed] [Google Scholar]
- 25.Liang M, Liu Y, Mladinov D, Cowley AW Jr, Trivedi H, Fang Y, Xu X, Ding X, Tian Z. MicroRNA: a new frontier in kidney and blood pressure research. Am J Physiol Renal Physiol. 2009;297:F553–558. doi: 10.1152/ajprenal.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung J, Han KH, Zo JH, Park HJ, Kim CH, Oh BH. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med. 2000;28:205–216. doi: 10.1142/S0192415X00000258. [DOI] [PubMed] [Google Scholar]
- 27.Kim CS, Park JB, Kim KJ, Chang SJ, Ryoo SW, Jeon BH. Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacol Sin. 2002;23:1152–1156. [PubMed] [Google Scholar]
- 28.Jeon BH, Kim CS, Park KS, Lee JW, Park JB, Kim KJ, Kim SH, Chang SJ, Nam KY. Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen Pharmacol. 2000;35:135–141. doi: 10.1016/s0306-3623(01)00096-9. [DOI] [PubMed] [Google Scholar]
- 29.Han KH, Choe SC, Kim HS, Sohn DW, Nam KY, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Effect of red ginseng on blood pressure in patients with essential hypertension and white coat hypertension. Am J Chin Med. 1998;26:199–209. doi: 10.1142/S0192415X98000257. [DOI] [PubMed] [Google Scholar]
- 30.Leung KW, Pon YL, Wong RN, Wong AS. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–36288. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- 31.Leung KW, Cheng YK, Mak NK, Chan KK, Fan TP, Wong RN. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006;580:3211–3216. doi: 10.1016/j.febslet.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Wojta J, Binder BR. Effect of notoginsenoside R1 on the synthesis of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in cultured human umbilical vein endothelial cells. Arterioscler Thromb. 1994;14:1040–1046. doi: 10.1161/01.atv.14.7.1040. [DOI] [PubMed] [Google Scholar]
- 33.Lin SG, Zheng XL, Chen QY, Sun JJ. Effect of Panax notoginseng saponins on increased proliferation of cultured aortic smooth muscle cells stimulated by hypercholesterolemic serum. Zhongguo Yao Li Xue Bao. 1993;14:314–316. [PubMed] [Google Scholar]
- 34.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 35.Jin YR, Yu JY, Lee JJ, You SH, Chung JH, Noh JY, Im JH, Han XH, Kim TJ, Shin KS, Wee JJ, Yun YP. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Liu L, Yu Y, Chen B, Tang C, Li X. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Mol Med Rep. 2012;5:1295–1298. doi: 10.3892/mmr.2012.808. [DOI] [PubMed] [Google Scholar]
- 37.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 38.Calver A, Collier J, Moncada S, Vallance P. Effect of local intra-arterial NG-monomethyl-L-arginine in patients with hypertension: the nitric oxide dilator mechanism appears abnormal. J Hypertens. 1992;10:1025–1031. [PubMed] [Google Scholar]
- 39.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 40.Kublickiene KR, Cockell AP, Nisell H, Poston L. Role of nitric oxide in the regulation of vascular tone in pressurized and perfused resistance myometrial arteries from term pregnant women. Am J Obstet Gynecol. 1997;177:1263–1269. doi: 10.1016/s0002-9378(97)70048-6. [DOI] [PubMed] [Google Scholar]
- 41.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 42.Closs EI, Simon A, Vekony N, Rotmann A. Plasma membrane transporters for arginine. J Nutr. 2004;134:2752S–2759S. doi: 10.1093/jn/134.10.2752S. discussion 2765S-2767S. [DOI] [PubMed] [Google Scholar]
- 43.Zharikov SI, Block ER. Characterization of L-arginine uptake by plasma membrane vesicles isolated from cultured pulmonary artery endothelial cells. Biochim Biophys Acta. 1998;1369:173–183. doi: 10.1016/s0005-2736(97)00191-0. [DOI] [PubMed] [Google Scholar]
- 44.Visel A, Zhu Y, May D, Afzal V, Gong E, Attanasio C, Blow MJ, Cohen JC, Rubin EM, Pennacchio LA. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–412. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holdt LM, Beutner F, Scholz M, Gielen S, Gabel G, Bergert H, Schuler G, Thiery J, Teupser D. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol. 2010;30:620–627. doi: 10.1161/ATVBAHA.109.196832. [DOI] [PubMed] [Google Scholar]
- 46.Annilo T, Kepp K, Laan M. Natural antisense transcript of natriuretic peptide precursor A (NPPA): structural organization and modulation of NPPA expression. BMC Mol Biol. 2009;10:81. doi: 10.1186/1471-2199-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]


