Abstract
Purpose: Chemical burn in cornea may cause permanent visual problem or complete blindness. In the present study, we investigated the role of microRNA 206 (miR-206) in relieving chemical burn in mouse cornea. Method: An alkali burn model was established in C57BL/6 mice to induce chemical corneal injury. Within 72 hours, the transient inflammatory responses in alkali-treated corneas were measured by opacity and corneal neovascularization (CNV) levels, and the gene expression profile of miR-206 was measured by quantitative real-time PCR (qPCR). Inhibitory oligonucleotides of miR-206, miR-206-I, were intrastromally injected into alkali-burned corneas. The possible protective effects of down-regulating miR-206 were assessed by both in vivo measurements of inflammatory responses and in vitro histochemical examinations of corneal epithelium sections. The possible binding of miR-206 on its molecular target, connexin43 (Cx43), was assessed by luciferase reporter (LR) and western blot (WB) assays. Cx43 was silenced by siRNA to examine its effect on regulating miR-206 modulation in alkali-burned cornea. Results: Opacity and CNV levels, along with gene expression of miR-206, were all transiently elevated within 72 hours of alkali-burned mouse cornea. Intrastromal injection of miR-206-I into alkali-burned cornea down-regulated miR-206 and ameliorated inflammatory responses both in vivo and in vitro. LR and WB assays confirmed that Cx43 was directly targeted by miR-206 in mouse cornea. Genetic silencing of Cx43 reversed the protective effect of miR-206 down-regulation in alkali-burned cornea. Conclusion: miR-206, associated with Cx43, is a novel molecular modulator in alkali burn in mouse cornea.
Keywords: Cornea, alkali, miR-206, Cx43
Introduction
The transparent cornea has a highly ordered structure that transmits light and serves as a barrier between external and intraocular environments. Once the cornea is injured, visual deterioration is inevitable and irreversible. The common immediate or short-term causes resulting in corneal injuries include infectious diseases, Stevens-Johnson Syndrome (SJS) or chemical burn [1-4]. Other long-term causes, such as severe dry eye, often lead to eyelid deformities and may account for as many 46% of the ocular complications in all patients with corneal diseases [4]. However, despite the advanced understanding on the pathology of corneal diseases in human, little is known about the molecular mechanisms underlying those short-term or long-term abnormalities, especially irreversible injuries induced chemical burn, of cornea.
MicroRNAs (miRNAs) are families of noncoding and small size, around 18 to 22 nucleotides long, RNAs, that exert transcriptional regulations in many types of human and animal tissues [5,6]. Studies have demonstrated that a large number of miRNAs are expressed in mammalian eyes with distinctly different expression patterns and molecular functions [7,8]. Among them, microRNA 206 (miR-206), was shown to be expressed in both vertebrate and non-vertebrate eyes [8,9], and play important role in the development of photoreceptor in drosophila eyes [10]. However, little is known whether miR-206 is involved in any molecular pathways underling the pathology of chemical burn in cornea.
Connexins (Cxs) are a family of structurally related trans-membrane proteins that assemble to form gap junctions to participate various biological processes cross the cell membrane in many of the sensory organs, such as inner ears and eyes [11-15]. Of Cx family, connexin 43 (Cx43) is known to be expressed in mammalian cornea and its expression pattern is highly associated with the inflammatory responses in injured cornea [16,17]. Moreover, study had demonstrated that upregulating Cx43 in cornea facilitated the wound healing in damaged cornea [17], suggesting that targeting Cx43 could be a future therapeutic option to treat corneal injury in patients.
In our study, we investigated whether transiently modulating mR-206 and its possible down streaming target Cx43, within 72 hours of alkali-induced burn, would have any effects on ameliorating the inflammatory responses or facilitating the healing process in mouse cornea. The results of our study would further our understanding on the underlying molecular mechanisms of chemical burn in cornea, as well as help developing feasible clinical treatment to help patients with corneal injury.
Materials and methods
Animals
In the present study, six- to eight-week-old male C57BL/6 mice were obtained from Shanghai Experimental Animal Center, Chinese Academy of Sciences in Shanghai, China. All experimental protocols or procedures were reviewed and approved by the Animals Research and Ethics Committee at the First Hospital Affiliated to PLA General Hospital in Beijing, China.
Alkali-induced chemical burn to mouse cornea
An experimental paradigm of using alkali to induce chemical burn in mouse cornea was slightly modified according to methods described in previous studies [18,19]. Briefly, mice were anesthetized with intraperitoneal injection of 40 mg/kg ketamine and 10 mg/kg xylazine. A piece of filter paper (Sigma-Aldrich, USA) was cut into round shapes with diameter of 1.5 mm, soaked in 1 N NaOH for 10 min, then applied to the center cornea of the right eyes for 2 minutes under a microscope. The eyes were then immediately irrigated with 50 ml of sterile pyrogen-free saline.
Opacity score and corneal neovascularization score
Corneal opacity score was measured at 0, 6, 12, 24, 36 and 72 hours after alkali injury, according the method described in a previous study [20]. Briefly, the degree of opacification was defined between scale 0 and 5 +, with scale 0 equals completely transparent cornea and scale 5 + equals complete corneal opacity with total obscuration of the anterior chamber. Corneal neovascularization (CNV) score was measured according the method in a previous study [21]. Briefly, the score was scaled between 0 and 12, averaged between 0 and 3 per corneal quadrant with an increment of 0.5 per measurement of centripetal neovascular branch outgrowth from the corneoscleral limbus. All measurement was implemented by a skilled technician in a double-blinded manner. And the measured differences in opacity or CNV scores were assessed by a non-parametric Wilcoxon signed-rank test.
Quantitative real-time PCR (qPCR)
Total RNA of corneal tissues was extracted using the miRNeasy kit (Qiagen, USA) per manufacturer’s instructions. RNA was then quantified using the NanoDrop 1000 (Thermo Fisher, USA). Complementary DNA (cDNA) was then generated with a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, CBA) from 1 µg total RNA and Taqman miRNA-specific RT primer (Invitrogen, USA) per manufacturer’s instructions. Quantitative real-time PCR was performed using Taqman Universal PCR Master Mix on PRISM 7900HT Sequence Detection System (Applied Biosystems, USA), with U6 as control to normalize the PCR product of miR-206.
Synthesis of miR-206 inhibitor and intrastromal injection
The mouse anti-miR-206 oligonucleotides inhibitor, miR-206-I, as well as its non-specific microRNA control, miR-C, were commercially purchased from Guangzhou RiboBio (RiboBio, Guangzhou, China). To apply miRNA oligonucleotides into cornea, the method of intrastromal injection was slightly modified from previous study [22]. Briefly, one hour after alkali burn, the corneal surface was desensitized by topical application of 2% lidocaine for 5 minutes. A total volume of 40 μL of miR-206-I (0.1 mM) or miR-C (0.1 mM) was subconjunctivally injected near the limbus adjacent to the pathologic blood vessels extending into the cornea. After 30 minutes, a second injection of same amount was given intrastromally directed toward the distal end of cornea.
Immunohistochemistry
Mice eyeballs were fixed with 10% buffered formalin in optimal cutting temperature (OCT) compound (Sigma Aldrich, USA). The paraffin-embedded serial sections (4 μm) were then deparaffinized by xylene and stained for hematoxylin and eosin (H&E). The sections were prepared on an inverted microscope (Zeiss, Germany) at 10X magnification for imaging, and the inflammatory infiltration on corneal epithelium was evaluated.
Luciferase reporter assay
Total cDNA of mouse cornea was generated by reverse PCR as described above. The DNA sequence of 3’UTR of Cx43 was digested by restriction enzymes Hie RI and Rsa I, and then cloned into a pMir-Report plasmid (Ambion, USA) to create luciferase vector containing wild type Cx43 3’UTR, Luc-Cx43-WT. The mutated 3’TUR of Cx43, containing modified sequence at suspected binding site to miR-206, was constructed by a Site-Directed Mutagenesis Kit (SBS Genetech, China), and then cloned into pMir-Report plasmid to create mutant luciferase vector Luc-Cx43-MU. Both vectors were verified by DNA sequencing. Correspondingly, a non-specific control luciferase vector, Luc-C was also constructed. All three vectors were than co-transfected with β-galactosidase and mouse miR-206 mimics (RiboBio, Guangzhou, China) into HEK293T cells. Twenty-four hours after transfection, the relative luciferase activities were measured by a dual luciferase reporter assay system (Promega, USA), and normalized to the values of Luc-C, per manufacturer’s instructions.
Western blot analysis
Lysates of mouse cornea were prepared with a lysis buffer containing 50 mM Tris (pH 7.6), 150 mM NaCl, 1 mM EDTA, 10% glycerol, and 0.5% NP-40 and protease inhibitor cocktail (Invitrogen, USA). The total protein were then dissolved on 10% SDS-PAGE gel and transferred to the nitrocellulose membranes. The primary antibody was against Cx43 (1:3000, Sigma Aldrich, USA), and the secondary antibody was horseradish peroxidase-conjugated one (Bio-Rad, USA). GAPDH was the internal control. The visualization of western blot analysis was conducted using an enhanced chemiluminesence system (Amersham Biosciences, USA) per manufacturer’s instructions.
SiRNA synthesis and corneal application
Genetic knocking down Cx43 in mouse cornea was achieved by the application of siRNA. Cx43 specific mouse siRNA, siRNA-Cx43, and a non-specific scrambled mouse siRNA, siRNA-C, were synthesized by Guangzhoiu RiboBio (RiboBio, Guangzhou, China). One hour after intrastromal injection of miR-206-I, a total volume of 50 μL siRNA-Cx43 (1 uM) or siRNA-C (1 uM) was delivered to cornea by another intrastromal injection. To examine the efficiency of knocking down corneal Cx43 by siRNA, normal corneas (without alkali burn) were injected with siRNAs and assessed with Western blotting in 24 hours.
Statistical analysis
The data in the present study were presented as the mean values ± standard errors. Comparison between data was made by student’s t-test with windows-based SPSS software (version 12.0). Significant difference was determined if P < 0.05. All experiments were repeated at least three times.
Results
MicroRNA-206 was transiently upregulated in alkali-burned cornea
In general study of corneal wounds, the total time duration for wound closure may last up to 2 or 3 weeks after initial injury. However, the molecular events occurred during the first couple days are critical for the healing of corneal wounds or regeneration of corneal epithelial tissues. Thus, in the present study, we focused on the first 72 hours after corneal burn.
The corneas of six- to eight-week-old male C57BL/6 mice were treated with 1N NaOH for 10 minutes. The control corneas were treated with normal saline only. Corneal opacity (Figure 1A) and CNV development (Figure 1B) were examined at 6, 12, 24, 36 and 72 hours after alkali injury. The results showed that, both opacity and CNV levels were significantly increased in alkali-burned corneas, as compared to the saline-treated ones, in time-dependent manners (Figure 1A, 1B, *: P < 0.05). Interestingly, our miRNA-array screening demonstrated miR-206, a micro-RNA that was lowly expressed in normal mouse cornea, was significantly upregulated during alkali treatment. Our subsequent qPCR examination confirmed this result, showing that the expression levels of miR-206 were markedly upregulated from 6 hours to 72 hours after alkali burn, also in a time-dependent manner (Figure 1C, *: P < 0.05).
Figure 1.
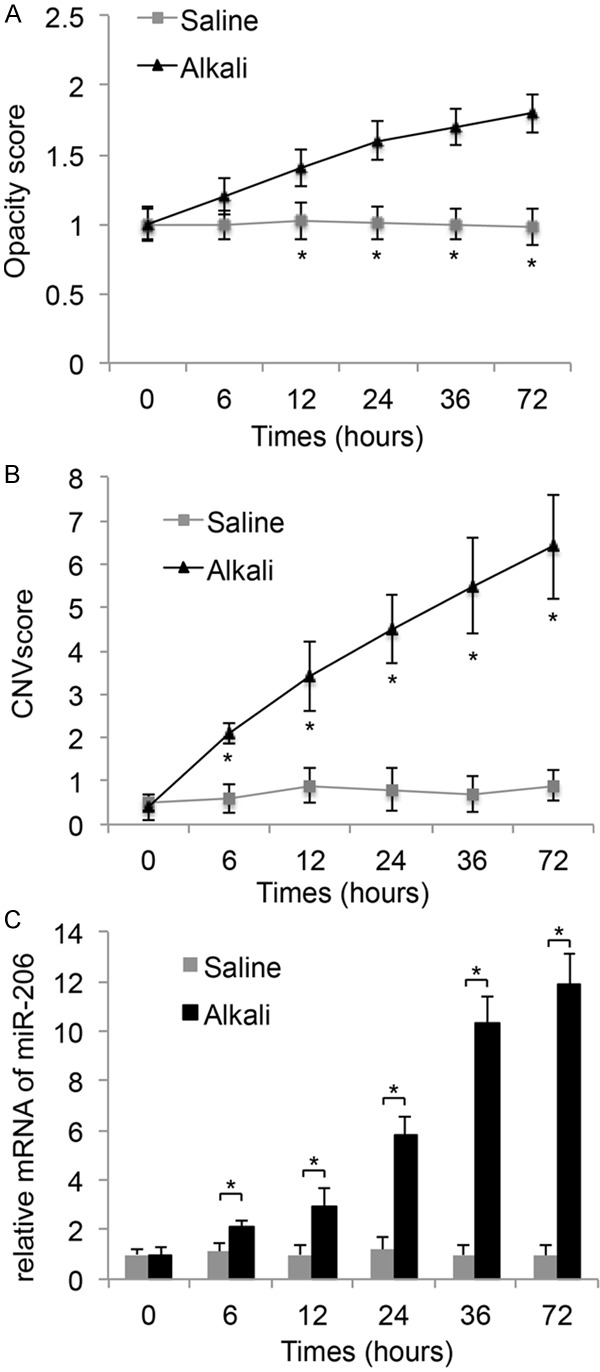
Transient responses and miRNA regulation to Alkali-burn in mouse cornea. 1 N NaOH was applied to corneas of 6 to 8 weeks old male C57BL/6 mice for 10 min. The control corneas were treated with normal saline. Transient responses, including relative levels of opacity scores (A) and corneal neovascularization (VNV) scores (B) were measured and compared between the corneas treated with alkali and saline, at 0, 6, 12, 24, 36 and 72 hours. (C) QPCR was also applied to compare the relative expression levels of miR-206 between corneas treated with alkali and saline. (*: P < 0.05).
Downregulation of miR-206 ameliorated alkali-induced corneal burn
As we discovered that miR-206 was actively upregulated by alkali burn, we wondered whether it would play any function role during the chemical injury in mouse cornea. To address this hypothesis, we synthesized mouse miR-206 inhibitor, miR-206-I, to exogenously suppress miR-206 in cornea. It was then delivered into cornea through the method of intrastromal injection. A non-specific miRNA, miR-C, was also injected into corneal as parallel control experiment.
First, the inhibitory efficiency by synthesized oligonucleotide inhibitor was examined by qPCR in normal mouse cornea. The result showed that miR-206-I was able to significantly reduce the endogenous production of corneal miR-206 by about 40% (Figure 2A, *: P < 0.05).
Figure 2.

Transient downregulation of miR-206 reduced alkali burn in mouse cornea. (A) Normal mouse corneas were intrastromally injected with miR-206-I (0.1 mM) or miR-C (0.1 mM). The expression levels of miR-206 were examined in 24 hours by qPCR. (B) In vivo microscopic examination (upper panel) and in vitro H&E staining of alkali-injured corneas treated with miR-206-I or miR-C were conducted 3 days after injury. Continuous assessments of corneal opacity (C) and corneal neovascularization (CNV) (D) on alkali-injured corneas treated with miR-206-I or miR-C were conducted 0, 6, 12, 24, 36 and 72 hours after injury, respectively. (*: P < 0.05).
Then, miR-206-I or miR-C was injected into alkali-burned cornea, 1 hour after initial injury. Three days later, in vivo examination of the corneas under the microscope showed that inflammatory responses were significantly reduced in miR-206-I treated corneas (arrows, Figure 2B, upper panel). The examination of in vitro H&E staining confirmed the in vivo observation, showing that inflammatory epithelium was seen in miR-C treated corneas, whereas much healed and organized epithelium was seen in miR-206-I injected corneas (Figure 2B, lower panel).
Furthermore, continuous in vivo biomicroscopic examinations revealed that, about 12 hours after alkali burn injury, opacity levels (Figure 2C, *: P < 0.05) and CNV levels (Figure 2D, *: P < 0.05) were significantly decreased in miR-206-I treated alkali-burned corneas.
Taken together, the results of our experiments support the hypothesis that transient downregulation of miR-206 reduces the chemical burn injury in cornea.
Connexin43 was directly regulated by miR-206 in mouse cornea
As we discovered that miR-206 had functional role in regulating the biological responses of cornea to alkali burn, we wondered what the molecular targets would be during the process of miR-206 modulation in mouse cornea. For that purpose, we conducted a bioinformatic search at the website of TargetScan (http://www.targetscan.org) to identify the possible targets of miR-206 in mouse corneal tissues. As the search result, we identified that Cx43 was very likely to be the direct down streaming target of miR-206 in mouse (Figure 3A).
Figure 3.
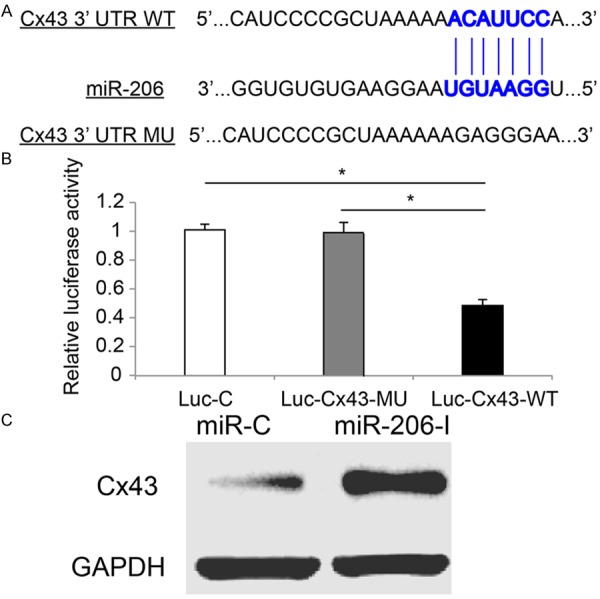
Cx43 was regulated by miR-206 in alkali-burned mouse cornea. A. The schematic diagram of miR-206 binding the 3’UTR of wild type (WT) Cx43 was drawn (source: TargetScan). A mutated (MU) sequence corresponding to the binding site of 3’UTR of Cx43 was also drawn. B. In a dual-luciferase reporter assay, HEK 293T cells were transfected with mouse miR-206 mimics, β-galactosidase, and one of the three luciferase vectors, Luc-C, Luc-Cx43-MU and Luc-Cx43-WT. One day later, relative luciferase activities were measured (*: P < 0.05). C. In alkali-burned corneas, intrastromal injection of either miR-206-I or miR-C was conducted one hour after initial injury. Two days later, western blotting analysis was performed on those corneas to examine the protein expressions of Cx43.
Then, we tested this possibility with a dual-luciferase reporter assay. We constructed three luciferase vectors. One was to contain wild type corneal Cx43 3’UTR at suspected miR-206 binding site (Luc-Cx43-WT). One was to contain mutated corneal Cx43 3’TUR of Cx43 (Luc-Cx43-MU). The third one was to contain a non-specific control vector (Luc-C). These vectors were then co-transfected with β-galactosidase and mouse miR-206 mimics into HEK293T cells. Twenty-four hours later, the dual-luciferase reporter assay demonstrated that the relative luciferase activity of Luc-Cx43-WT was dramatically decreased as compared to the luciferase activities of either Luc-C or Luc-Cx43-MU (Figure 3B, *: P < 0.05). Thus, our results confirmed that, in mouse cornea, Cx43 was the direct target of miR-206.
Moreover, using a western blot assay, we examined the protein expression levels of Cx43 in alkali-burned corneas treated with miR-206-I or miR-C. The result showed that the protein of Cx43 was dramatically up-regulated while miR-206 was suppressed by miR-206-I (Figure 3C); further confirming that Cx43 was directly regulated by miR-206 in mouse cornea.
Connexin43 inhibition reversed the protective effect by miR-206 downregualtion in alkali-burned mouse cornea
Finally, we investigated whether Cx43 would functionally modulate the protective effect of miR-206 downregulation in alkali-burned mouse cornea. We constructed siRNAs to specifically knock down endogenous gene expression of Cx43 in mouse cornea. The efficiency of siRNA application was verified by western blotting analysis of normal mouse cornea intrastromally injected with either Cx43 specific siRNA (siRNA-Cx43, 1 uM) or non-specific scrambled siRNA (siRNA-C, 1 uM). The result showed that Cx43 protein level was significantly decreased in corneas injected with siRNA-Cx43, as compared to the protein level of Cx43 in siRNA-C injected corneas, thus confirming the specificity and efficiency of siRNA-43 (Figure 4A).
Figure 4.
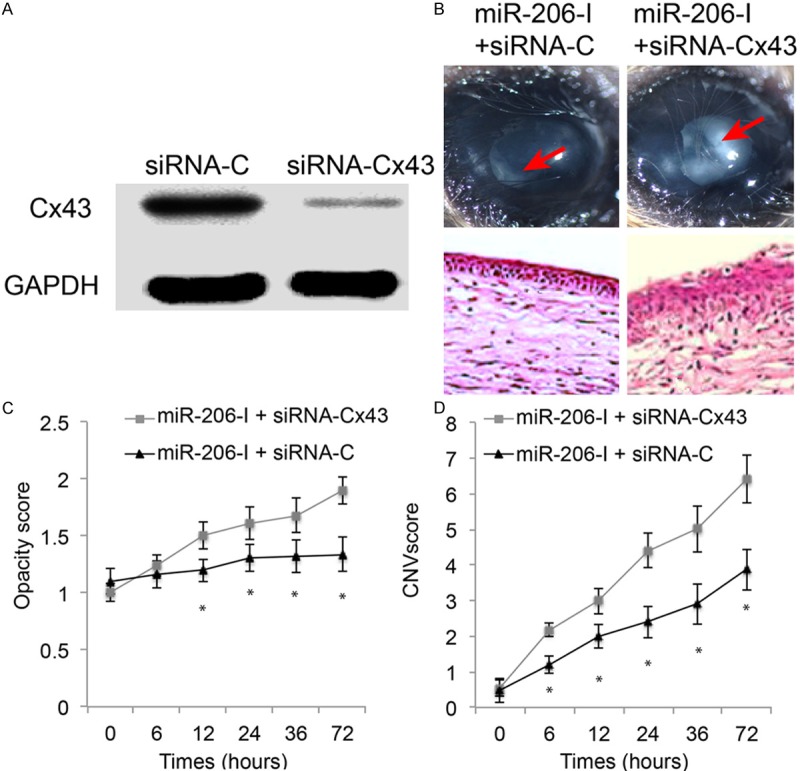
Inhibition of Cx43 reserved the protection of miR-206 down-regulation in alkali-burned mouse cornea. (A) Cx43 specific siRNA (siRNA-43, 1 uM), or non-specific scrambled control siRNA (siRNA-C, 1 uM) was intrastromally injected into normal mouse cornea. One day later, the effect of knocking down corneal Cx43 by siRNA was assessed by western blotting analysis. (B) Alkali-burned mouse cornea was treated with miR-206 downregulation (miR-206-I), followed by intrastromal injection of siRNA-Cx43 or siRNA-C in 1 hour. Both in vivo microscopic examination (upper panel) and in vitro H&E staining (lower panel) were applied to assess the effect of inhibiting Cx43 on inflammatory responses in alkali-burned and miR-206 down-regulated mouse cornea. Also, in alkali-injured and miR-206-I down-regulated mouse corneas, time-dependent assessments on transient responses, including corneal opacity (C) and corneal neovascularization (CNV) (D) were conducted while corneas were treated with either siRNA-Cx43 or siRNA-C at 0, 6, 12, 24, 36 and 72 hours after injury, respectively. (*: P < 0.05).
Then, the alkali-burned mouse corneas were injected with miR-206-I, followed by second intrastromal injection of either siRNA-Cx43 (1 uM) or siRNA-C (1 uM) in an hour. Both in vivo and in vitro corneal evaluations were performed within 3 days after siRNA injection. First, at day 3, corneas were examined under microscope. It showed that reduced inflammatory responses in miR-206-I treated alkali-burned cornea were reversed while Cx43 was genetically silenced by siRNA (arrows, Figure 4B, upper panel). Second, the effect by siRNA-Cx43 was also confirmed by in vitro H&E staining, showing much dis-organized and inflammatory corneal epithelium was spotted in siRNA-Cx43 treated alkali-burned corneas (Figure 4B, lower panel). Third, in vivo biomicroscopic examinations demonstrated that, as compared to the alkali-burned and miR-206 down-regulated corneas treated with siRNA-C, the opacity and CNV levels dramatically increased, around 6 hours and 12 hours after siRNA treatment respectively, in corneas treated with siRNA-Cx43 (Figure 4C, 4D, *: P < 0.05).
Thus, the siRNA experiments strongly suggest that miR-206 modulate inflammatory responses to alkali-burn injury through targeting Cx43 in mouse cornea.
Discussions
In the present study, we investigated the transient inflammatory responses of mouse cornea to alkali burn, and showed that within 72 hours of chemical injury, the corneal opacity and CNV levels were all elevated in time-dependent manners. Also, we discovered, for the first time that, miR-206 was up-regulated during the first 72 hours of alkali burn in the cornea. More importantly, we demonstrated novel mechanisms of miR-206 in modulating cornea pathology, as down-regulating miR-206 transiently (within 72 hours of injury) protected the inflammation and re-organized injured epithelium in alkali-burned cornea. It was previously shown that miR-206 was expressed in mammalian eyes [8]. However, possible functional mechanisms of miR-206 in any of the active processes of eye development or pathology have never been reported. Thus, our finding would certainly help to further the understanding on underlying molecular pathways, especially those associated with miRNA regulations, in regulating/protecting chemical injury in mammalian corneas.
To further identify the modulatory effect of miR-206 in chemically injured cornea, we explored the possible down streaming molecular target of miR-206 in mouse cornea. Through dual-luciferase reporter assay and western blot assay, we demonstrated that Cx43 was directly regulated by miR-206 during the process of alkali-burn in cornea. It was shown in a previous study that miR-206 was upregulated and Cx43 was downregulated accordingly during myoblast differentiation [23]. However, little is known of the association of miR-206 and Cx43 in other mammalian tissues or organs other than skeletal muscle. Thus, our study was the first to report such integrated regulation of miR-206 and Cx43 in mammalian cornea.
Moreover, we used genetic silencing method of siRNA to knock down Cx43 in alkali-burned but miR-206 down-regulated mouse cornea. We then showed that inhibiting Cx43 expression could reverse the protective or healing effect of miR-206 down-regulation in chemically injured cornea. This finding was very important, providing the direct evidence of Cx43 involvement in regulating miRNA- related molecular pathways during the process of chemical burn in cornea. It is worth noting that the expression of Cx43 was dynamic during the process of chemical burn in cornea, as shown in a previous study that gap junction channels including Cx43 were initially down-regulated in 24 hours, but up-regulated in 72 hours, and then down-regulated again in 3-weeks after corneal injury [17]. That study also demonstrated that corneal injection of synthetic Cx43 could accelerate the long-term healing process after corneal wound [17]. In our study, we demonstrated that the protective effect by miR-206 downregulation was reduced by knocking down Cx43 in 72 hours of alkali burn. Along with the results of previous study, it strongly suggests that, despite the differences in short-term or long-term expressing patterns of Cx43, its upregulation would always seem to prevent inflammatory responses or augment wound healing in chemically burned cornea.
Taken together, the results of our study presented novel mechanisms of miR-206, as well as its association with gap junction channel Cx43, in regulating injury responses in chemically burned mammalian cornea. It may benefit the effort in seeking new therapeutic strategies to treat corneal injuries in human patients.
Disclosure of conflict of interest
None.
References
- 1.Angra SK, Chawdhary S, Zutshi R, Mohan M. Acid burns of cornea--unusual clinical course. Indian J Ophthalmol. 1984;32:231–233. [PubMed] [Google Scholar]
- 2.Morgan SJ. Chemical burns of the eye: causes and management. Br J Ophthalmol. 1987;71:854–857. doi: 10.1136/bjo.71.11.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Ueta M. Epistatic interactions associated with Stevens-Johnson syndrome. Cornea. 2012;31(Suppl 1):S57–62. doi: 10.1097/ICO.0b013e31826a7f41. [DOI] [PubMed] [Google Scholar]
- 5.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 6.Ranganathan K, Sivasankar V. MicroRNAs - Biology and clinical applications. J Oral Maxillofac Pathol. 2014;18:229–234. doi: 10.4103/0973-029X.140762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan DG, Oliveira-Fernandes M, Lavker RM. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006;12:1175–1184. [PubMed] [Google Scholar]
- 8.Karali M, Peluso I, Gennarino VA, Bilio M, Verde R, Lago G, Dolle P, Banfi S. miRNeye: a microRNA expression atlas of the mouse eye. BMC Genomics. 2010;11:715. doi: 10.1186/1471-2164-11-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora A, McKay GJ, Simpson DA. Prediction and verification of miRNA expression in human and rat retinas. Invest Ophthalmol Vis Sci. 2007;48:3962–3967. doi: 10.1167/iovs.06-1221. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Carthew RW. A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell. 2005;123:1267–1277. doi: 10.1016/j.cell.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 12.Crumling MA, Tong M, Aschenbach KL, Liu LQ, Pipitone CM, Duncan RK. P2X antagonists inhibit styryl dye entry into hair cells. Neuroscience. 2009;161:1144–1153. doi: 10.1016/j.neuroscience.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kar R, Batra N, Riquelme MA, Jiang JX. Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys. 2012;524:2–15. doi: 10.1016/j.abb.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez AD, Acuna R, Figueroa V, Maripillan J, Nicholson B. Gap-junction channels dysfunction in deafness and hearing loss. Antioxid Redox Signal. 2009;11:309–322. doi: 10.1089/ars.2008.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volgyi B, Kovacs-Oller T, Atlasz T, Wilhelm M, Gabriel R. Gap junctional coupling in the vertebrate retina: variations on one theme? Prog Retin Eye Res. 2013;34:1–18. doi: 10.1016/j.preteyeres.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Laux-Fenton WT, Donaldson PJ, Kistler J, Green CR. Connexin expression patterns in the rat cornea: molecular evidence for communication compartments. Cornea. 2003;22:457–464. doi: 10.1097/00003226-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Moore K, Bryant ZJ, Ghatnekar G, Singh UP, Gourdie RG, Potts JD. A synthetic connexin 43 mimetic peptide augments corneal wound healing. Exp Eye Res. 2013;115:178–188. doi: 10.1016/j.exer.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotozono C, He J, Matsumoto Y, Kita M, Imanishi J, Kinoshita S. Cytokine expression in the alkali-burned cornea. Curr Eye Res. 1997;16:670–676. doi: 10.1076/ceyr.16.7.670.5057. [DOI] [PubMed] [Google Scholar]
- 19.Sosne G, Szliter EA, Barrett R, Kernacki KA, Kleinman H, Hazlett LD. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293–299. doi: 10.1006/exer.2001.1125. [DOI] [PubMed] [Google Scholar]
- 20.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice--evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54:694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37:2485–2494. [PubMed] [Google Scholar]
- 22.Lipman RM, Epstein RJ, Hendricks RL. Suppression of corneal neovascularization with cyclosporine. Arch Ophthalmol. 1992;110:405–407. doi: 10.1001/archopht.1992.01080150103037. [DOI] [PubMed] [Google Scholar]
- 23.Anderson C, Catoe H, Werner R. MIR-206 regulates connexin43 expression during skeletal muscle development. Nucleic Acids Res. 2006;34:5863–5871. doi: 10.1093/nar/gkl743. [DOI] [PMC free article] [PubMed] [Google Scholar]


