Abstract
Arginase is upregulated in some tissues under diabetes states. Arginase can compete with nitroxide synthase (NOS) for the common substrate L-arginine and thus increases oxidative stress by NOS uncoupling. We want to analyze whether arginase is upregulated and contribute to oxidative stress in H9c2 cells during high glucose treatment. H9c2 cells were cultured in normal or high glucose DMEM. Arginase activity increased in parallel with increased cell death and oxidative stress. Arginase inhibitor N ω-hydroxy-nor-l-arginine (nor-NOHA) and NOS inhibitor N ω-nitro-l-arginine methyl ester (L-NAME) could reverse these effects. Despite of upregulated NOS activity, NO production was impaired which could be preserved by nor-NOHA, suggesting a decreased substrate availability of NOS due to increased arginase activity. L-arginine supplementation decreased superoxide production while it could not protect cells from death. Upregulated arginase activity in H9c2 treated with high glucose can cause NOS uncoupling and subsequently reactive oxygen species augmentation and cell death. These findings suggest that arginase will be a novel therapeutic target for treatment of diabetic cardiomyopathy.
Keywords: Arginase, cardiomyocyte, diabetes, oxidative stress, NOS uncoupling
Introduction
Diabetic cardiomyopathy is a distinct primary disease process, which is independent of coronary artery disease and hypertension [1,2]. Although the underlying mechanisms are still incompletely understood, the increased reactive oxygen species (ROS) and cell death in cardiomyocyte induced by hyperglycemia definitely contribute to the pathological process [3,4]. However, a number of clinical trials could not confirm a benefit of antioxidants administration in diabetic cardiomyopathy [5-7]. Hence, strategies associating new targets of reactive oxygen species in diabetic cardiomyopathy are of great importance.
Recent reports issued that upregulation of arginase activity contributed to oxidative stress in endothelial cells in a variety of pathophysiological conditions, such as atherosclerosis, hypertension, diabetes, and so on [8-13]. As arginase shares the common substrate L-arginine with nitric oxide syntheses (NOS), it can compete L-arginine with NOS, leading to NOS uncoupling, a state which characterized by decreased NO production and increased reactive oxygen species (ROS). Arginase was also found being expressed in cardiomyocyte, and was involved in the cardiac pathological process in heart failure [14], chagas disease [15], myocardial infarction/reperfusion injury [16], hypertension [9-12], left ventricular hypertrophy [17], and so on. Jochen et al. found Arginase II alone was ex-pressed in rat cardiomyocyte mitochondria and modulated myocardial contractility by a nitric oxide synthase 1-dependent mechanism [18]. These studies suggest that arginase may play an important role in cardiac function and cardiomyocyte fate in cardiovascular disease.
In streptozotocin induced diabetic rats, increased arginase activity involves in vascular endothelial dysfunction by decreasing L-arginine availability to NO synthase [13]. A recent study showed that plasma arginase activity was increased in type II diabetic subjects with impaired NOS activity, correlating with the degree of hyperglycemia, and was reduced by physiologic hyperinsulinemia [19]. More recently, several clinical studies found that arginase activity was upregulated in diabetic state [20-22]. These suggest that arginase activity may be changed by glucose concentration. However, it remains unknown whether arginase is related to cardiomyocyte injury by oxidative stress under hyperglycemia. Therefore, we hypothesized that arginase activity may contribute, at least partly, to increased oxidative stress in cardiomyocyte induced by high glucose.
Materials and methods
Experimental protocol for cells
Neonatal rat heart-derived H9c2 cells were gifts presented by Professor Christopher HK Cheng. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum(Hyclone Labs., Logan, UT), penicillin (100 U/ml) and streptomycin (100 lg/ml), at 37°C in an atmosphere of 5% CO2 and 95% air. Cells were passaged when they grew to 80% confluence. When cells reached 40-50% confluence, the cultures were switched to DMEM supplemented with 1% FBS containing normal glucose (5.6 mM) or high glucose (35 mM) [23] or high glucose with L-arginine (Sigma) 2 mM [24] in varying time. N ω-hydroxy-nor-l-arginine (nor-NOHA, Enzo) 100 μM [25,26], N ω-nitro-l-arginine methyl ester (L-NAME, Sigma) 100 μM [27] were administered 30 min prior to high glucose exposure.
Cell apoptosis and death assessment
Cell apoptosis was measured by Annexin V-PI Apoptosis Detection Kit (BD Biosciences, CA, USA) according to the manufacturer’s protocol. The cells were analyzed by FACScanTM flow cytometer (BD Biosciences, CA, USA). The percentages of total apoptotic cells were calculated by summing the percentages of cells in early apoptosis (Annexin V-positive but PI-negative) and late apoptosis (Annexin V-positive and PI-positive).
For cell death determination, cells were suspended in 0.4% trypan blue in PBS (pH 7.4), and counted using Hemacytometer. The percentages of stained cells over total cells were used as an index of death.
Arginase activity assay
Arginase activity was determined as previously described with slight modification [13]. Briefly, cells were collected, washed twice with PBS, and then sonicated at 20 kHz for 30 s in 50 μl of 0.1% Triton X-100 containing protease inhibitors cocktails. Then the cell lysate was added into 50 μl of 10 mM MnCl2, 50 mM Tris-HCl (pH 7.5) and arginase was activated by heating the mixture at 55°C for 10 minutes. Arginine hydrolysis was performed by incubating the mixture with 25 μl of 0.5 M arginine (pH 9.7) at 37°C for 60 minutes and stopped by adding 400 μl of an acid mixture containing H2SO4, H3PO4 and H2O (1:3:7). For colorimetric determination of urea, 25 μl of 9% ISPF (dissolved in 100% ethanol) was added and the mixture was heated at 100°C for 45 min. Keep the samples in dark for 10 minutes and the OD was read at 540 nm in a microplate reader (BioTek Instruments, Inc., Burlington, VT).
Measurement of NOS activity
NOS activity was determined by NOS activity assay kit (Beyotime, Shanghai, China) according to the manufacturer’s protocol. Briefly, the medium was removed from the 96-well plate and cells were washed with PBS twice. The cells were loaded with DAF-FM DA (5 μM) and incubated with L-arginine and 0.1 mM NADPH at 37°C for 60 minutes. Fluorescence readings were made in triplicate in a 96-well plate at Ex/Em = 495/515 nm.
Intracellular ROS and NO detection
For ROS detection, H9c2 cells were loaded with 4 μM H2DCF-DA in PBS at 37°C for 15 minutes. For NO production assay, the cells were incubated with DAF-FM DA (5 μM) at 37°C for 60 minutes. Images were acquired by a fluorescence microscope (Axio Vert.A1, Zeiss) at Ex/Em = 488/525 nm (H2DCF-DA) or Ex/Em = 495/515 nm (DAF-FM DA) wavelengths at room temperature, and fluorescence intensity was measured using Image-Pro® Plus software.
RT-PCR
Total RNA was extracted from cells using Trizol (Invitrogen Life Technologies, California, USA) according to the manufacturer’s instructions. 4 μg of isolated RNA from each sample was used as a template for reverse transcription with reverse transcriptase (Promega, WI, USA) according to the standard protocols. The cDNA product was used for subsequent PCR amplification with specific primers, Arginase II: sense 5’-GTGTATCCTCGTTCAGTGGGC-3’, antisense 5’-CTATTGCCAGGCTGTGGTCTC-3’, 115 bp. β-actin: sense 5’-CCTCTATGCCAACACAGTGC-3’, antisense 5’-GTACTCCTGCTTGCTGATCC-3’, 210 bp. The cDNA was amplified with an initial incubation at 94°C for 4 min followed by 34 cycles (1 min at 94°C, 45 s at 61°C, and 45 s at 72°C) and an additional extension step for 10 min at 72°C at the end of the last cycle. The PCR amplified products were analyzed on 2.0% agarose gels visualized by staining with ethidium bromide and photographed under UV light. The ratios of band density of specific product to β-actin were evaluated.
Western blot
Cells were collected and washed twice with PBS. Then cell lysis buffer (Jiancheng, Nanjing, China) supplemented with 1 mM PMSF, 10 mg/ml aprotinin, 10 mg/ml leupeptin, and 10 mg/ml pepstatin A) was added and cells were lysised for 30 min. After centrifugation at 12,000 g for 15 min, the supernatant was collected. Protein concentration was determined using the modified Bradford method. Equal amounts of protein were mixed with SDS sample buffer (0.125 M Tris-HCl, pH 6.8, 10% glycerol, 2% β-mercaptoethanol, 2% SDS and 0.1% bromophenol blue) and boiled for 8 min. Then samples were electrophoresed on SDS-PAGE and electrotransferred to PVDF membrane. Nonspecific protein binding was blocked by incubating the membranes with 5% non-fat dry milk for 2 h at room temperature. Then membranes were incubated with primary antibodies for nNOS (1:1000, Abcam), eNOS (1:1000, Chemicon), nitrotyrosine (1:1000, Cayman) and GAPDH (1:1000, CWbiotech) at 4°C overnight. HRP-conjugated secondary antibodies were incubated for 2 h at room temperature. The protein bands were visualized with enhanced chemiluminescence reagents (Pierce) according to the instructions of the vendor.
Immunocytology
H9c2 cells were grown in cover slips and treated as described above. Cells were rinsed twice with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature. Then after washed with PBS for three times, cells were permeabilized with 0.2% triton-X100 for 30 min at 4°C. Wash cells three times before blocking them with 10% goat serum for 60 min at room temperature. Then incubate the cells with nitrotyrosine antibody at 4°C overnight. Rinse the cells with 0.05% Triton X-100 three times, and then incubate the cells with FITC-labelled secondary antibody for 60 min at room temperature. Rinse the cells with PBS three times and stain the nucleus with DAPI for 1 min. After three times washing with PBS, the cells were photographed under fluorescence microscope.
Statistics
Data are expressed as mean ± SEM, unless otherwise indicated. Statistical significance was assessed by Student’s t test or one-way ANOVA with subsequent post hoc Turkey test where appropriate. All statistics was calculated by GraphPad 5.0. An error probability of P<0.05 was regarded as significant.
Results
Inhibition of arginase and NOS but not L-arginine supplementation protected cells from high glucose- induced apoptosis
High glucose has been demonstrated to induce apoptosis in cardiomycytes [3]. To examine the contribution of arginase and NOS to high glucose-induced death and apoptosis, we incubated H9c2 cells in normal glucose, high glucose or high glucose with L-arginine, arginase inhibitor nor-NOHA, or NOS inhibitor L-NAME, respectively. H9c2 viability was determined by typan blue staining as well as flow cytometry. High glucose significantly increased cell death and apoptosis as compared with normal glucose (Figure 1A and 1B). Incubation with nor-NOHA or L-NAME significantly decreased cell death and apoptosis, while L-arginine leaded to increased cell injury (Figure 1A and 1B). These results demonstrated that arginase activation induced cell death in H9c2 cells.
Figure 1.
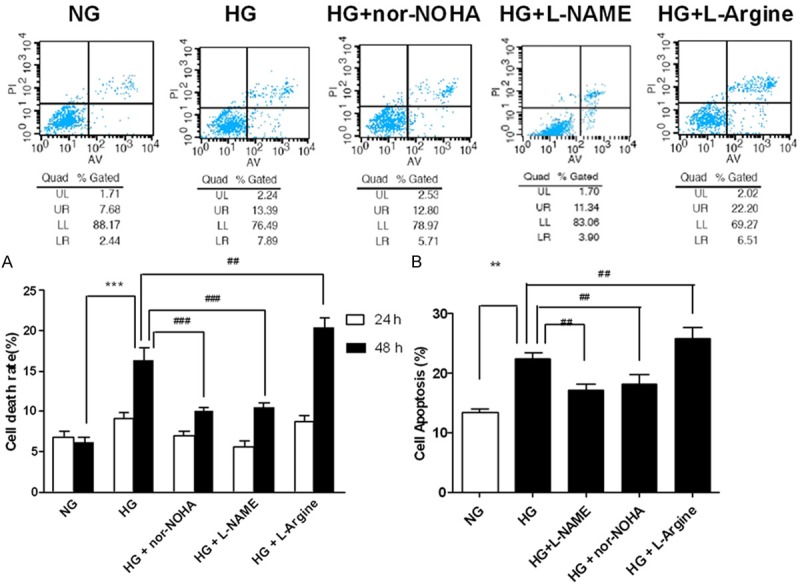
Effects of arginase on death and apoptosis of H9c2 cells exposed to high glucose. A. Trypan blue staining of H9c2 cells exposed to high glucose for 24 h and 48 h. B. Flow cytometry of H9c2 cells exposed to high glucose for 48 h. NG = normal glucose (5.56 mM), HG = high glucose (35 mM); nor-NOHA, the arginase inhibitor; L-NAME, the NOS inhibitor. ***P<0.001 vs. NG; **P<0.01 vs. NG; ###P<0.001 vs. HG; ##P<0.01 vs. HG.
High glucose increased arginase activity in H9c2 cells
To investigate the role of arginase in high glucose-induced death of H9c2 cells, we determined the effect of high glucose on the arginase activity in H9c2 cells. In comparison with normal glucose treated cells, arginase activity was increased following treatment with high glucose for 48 h and 72 h (Figure 2A).
Figure 2.
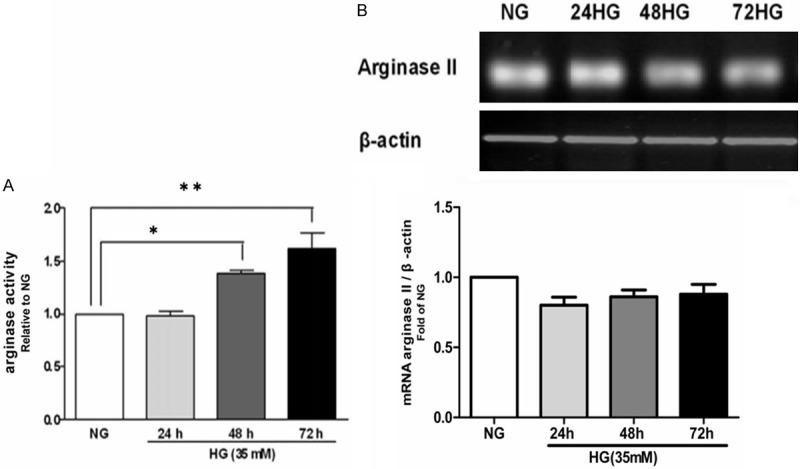
Expression and activity of arginase in H9c2 cells exposed to high glucose. A. Expression of arginase mRNA in H9c2 cells exposed to high glucose at different time points. B. Activity of arginase in H9c2 cells exposed to high glucose for 24 h, 48 h, and 72 h. NG = normal glucose (5.56 mM), HG = high glucose (35 mM). *P<0.05 vs. NG; **P<0.01 vs. NG.
To clarify the isoform-specific activation of arginase, we examined the mRNA expression of both arginase I and arginase II isoforms in H9c2 cells. Only arginase II was detected (Figure 2B). Thus arginase II was the predominant isoform contributed to high glucose-induced upregulation of the arginase activity in H9c2 cells. Up-regulation of the arginase activity may be not due to alteration of arginase II expression since the mRNA levels was similar between normal and high glucose-incubated cells (Figure 2B).
NOS activity was enhanced by high glucose state while NO was reduced
NOS activity was increased by high glucose stimulation (Figure 3A) while NO production was decreased (Figure 3B). Interestingly, nor-NOHA increased both NOS activity and NO production (Figure 3A and 3B).
Figure 3.
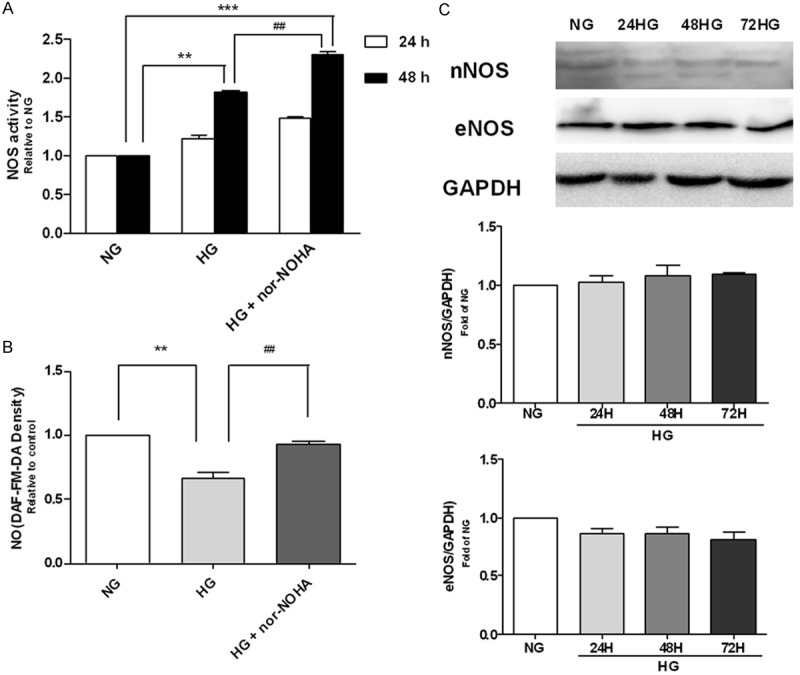
NO production and NOS activity in H9c2 cells exposed to high glucose. A. NO amounts in H9c2 cells exposed to normal glucose (NG, 5.56 mM) and high glucose (HG, 35 mM) for 48 h. B. Activity of NOS in H9c2 cells exposed to normal glucose (NG, 5.56 mM) and high glucose (HG, 35 mM) for 24 h and 48 h. nor-NOHA, the arginase inhibitor. C. Western blot with anti-nNOS and anti-eNOS antibody of cellular proteins from H9c2 cells exposed to high glucose for 24 h, 48 h, and 72 h. **P<0.01 vs. NG; ***P<0.001 vs. NG; ##P<0.01 vs. HG.
Treatment of H9c2 cells with high glucose did not affect eNOS and nNOS expression (Figure 3C). iNOS expression was not detected.
Upregulated arginase activity increased oxidative stress in cells exposed to high glucose
Previous study have demonstrated that levels of oxidative stress were increased in diabetic hearts and high glucose-treated cardiomyocytes as shown by elevated levels of ROS and nitrotyrosine formation, a marker for ONOO-production [3]. Similar to these results, we observed that treatment of H9c2 cells with high glucose significantly increased superoxide generation compared with normal glucose (Figure 4A), as well as nitrotyrosine staining (Figure 4B). However, the effects of high glucose was completely blocked by the NOS inhibitor L-NAME, confirming that NOS should be a prominent source of oxidative stress in high glucose-treated H9c2 cells. Furthermore, treatment with the arginase inhibitor nor-NOHA also significantly reduced ROS generation, suggesting that oxidative stress in high glucose-treated H9c2 cells was closely associated with increased arginase activity and following NOS uncoupling. We also examined L-arginine supplementation on superoxide levels and as expected L-arginine significantly reduced superoxide levels. These data indicate that arginase and therefore NOS uncoupling are sources of superoxide in H9c2 cells in response to high glucose stimulation.
Figure 4.
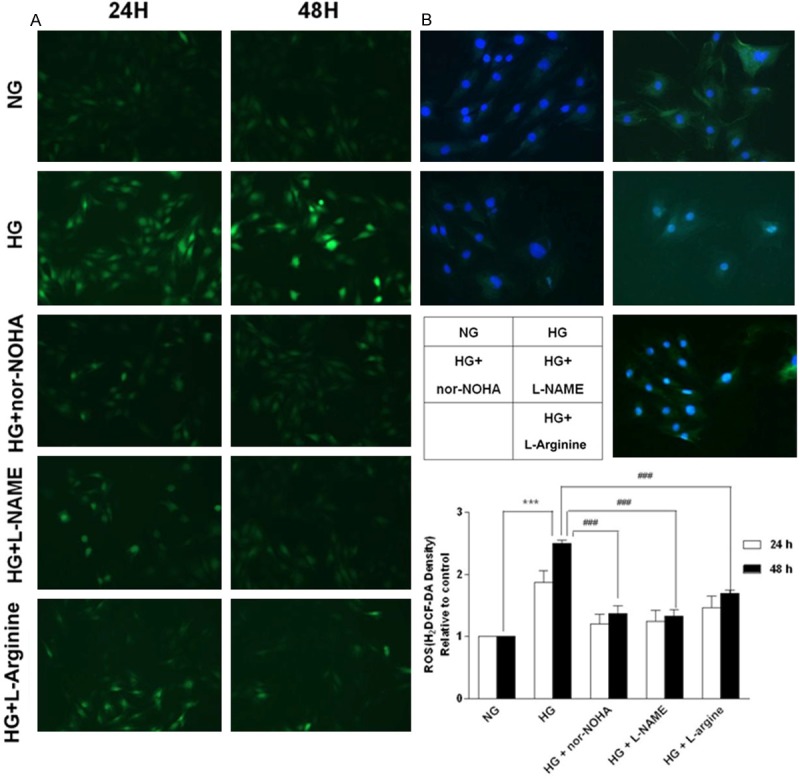
Assessment of oxidative/nitrosative stress of H9c2 cells exposed to high glucose. A. Reactive oxygen species (ROS) generation at different time points. B. Immunocytology with an anti-nitrotyrosine antibody of cellular proteins from H9c2 cells exposed to high glucose for 48 h. NG = normal glucose (5.56 mM), HG = high glucose (35 mM); nor-NOHA, the arginase inhibitor; L-NAME, the NOS inhibitor. Data were obtained from three independent experiments. ***P<0.001 vs. NG; ###P<0.001 vs. HG.
Discussion
In our present study, the results showed that arginase activity and NOS activity were upregulated while NO production was reduced in H9c2 cells exposed to high glucose condition. Arginase inhibitor nor-NOHA, as well as NOS inhibitor L-NAME decreased the superoxide levels and protected cells from death induced by high glucose. L-arginine supplementation could abate ROS production, but increase cell death under high glucose state.
As a major participant in L-arginine catabolism competing with NOS, arginase has been demonstrated to prefer to inflammation and oxidative stress which are main pathophysiological features in diabetes [15,25,27,28]. Actually, arginase activity was increased by diabetes [19-21], resulting in impairment of vascular endothelial function. Furthermore, in STZ induced diabetes rat, impaired vasorelaxation to acetylcholine was correlated with the increase in reactive oxygen species and arginase activity. Increased arginase activity contributed to excessive ROS through NOS uncoupling [12]. In type II diabetic subjects, an increased plasma arginase activity and impaired NOS activity was found to be correlates with the degree of hyperglycemia [19]. In this regard, we demonstrated that increased arginase activity contributed to high glucose-induced cardiomyocyte death and superoxide levels, providing further support for the causal role of increased arginase activity in diabetic cardiomyopathy.
Diabetic cardiomyopathy is characterized by oxidative stress which is predominantly derived from increased activity of NADPH oxidase [29-31]. Based on the results in the present study, we believe that NOS is also one of main source of diabetes/high glucose-induced oxidative stress. As arginase activity in H9c2 cells is increased by exposure to high glucose condition, it competes for more L-arginine which is insufficient to NOS, resulting in NOS uncoupling and thereby increased ROS generation. Since the Km of NOS (1-5 μM) is much lower than that of arginase (1-20 mM) and the endothelial L-arginine levels (near the millimolar range) exceeds the Km of eNOS, it seems unlikely that arginase will compete substrate with NOS and cause NOS uncoupling [32]. However, there are several reasons that may explain this “L-arginine paradox”: (1) the catalysis of arginine by arginase is nearly 200 folds greater than that of NOS [33], (2) at certain conditions; arginase activity can be activated by S-nitrosylation. In rat endothelial cells, S-Nitrosylation of C303 stabilizes the arginase1 trimmer and reduces its K m value to 6-fold [34], and (3) the presence of certain arginase pools, especially the one that is accessible to both arginase and NOS but is not exchangeable with extracellular L-arginine, may partially explain the reciprocal regulation of NOS by arginase through substrate limitation [35].
Similar to previous reports on vascular endothelial cells [25], our study showed that L-arginine supplementation decreased ROS in high glucose-treated H9c2 cells as well as L-NAME, suggesting that increased ROS was in part generated from uncoupling NOS. However, in our study, L-arginine could not protect H9c2 cells from death induced by high glucose, in contract, even aggravated apoptosis of high glucose-treated H9c2 cells. It may be associated with the increase in ONOO following the treatment of high glucose and L-arginine, because we found nitrotyrosine staining was increased in H9c2 cells treated with both high glucose and L-arginine. Indeed, the arginase inhibitor nor-NOHA significantly recovered the NO level in H9c2 cells impaired with high glucose and protected cells from death. The results indicate that endogenous (but not exogenous) L-arginine play an important role against injury of cardiomyocytes induced by high glucose. Actually, some reports have shown that there is a special “L-arginine pool” in endothelial cells, which is accessible to eNOS and arginase and is not exchangeable to extracellular L-arginine [31]. Moreover, Hyun et al. demonstrated that arginase II regulated NO production, vascular endothelial function, and vascular stiffness by modulating eNOS activity [33]. However, whether this “L-arginine pool” exists in cardiomyocyte is not known. As arginase II expressed exclusively on mitochondria [18], which is spatially approach to nNOS, activity-enhanced arginase II may also limits L-arginine availability of nNOS, leading to ROS generation from uncoupled eNOS as well as the extracellular impairment of NO production.
Many studies have shown that the impairment of NO production is closely referred to heart and vascular dysfunction in diabetes [13]. In our present experiments, although the NOS activity in H9c2 cells is increased by high glucose, NO level is declined. The increases in total NOS activity may be attributed to the increased activation of NOS by exceed intracellular Ca2+ [36]. The impaired NO production may be because of NOS uncoupling and excessive ROS [37].
In conclusion, our study provides the role of arginase in cardiomyocyte death induced by high glucose. Increased arginase activity appears to be closely associated with high glucose-induced oxidative stress and impairment of NO production in cardiomyocytes. So, arginase will be a novel therapeutic target for treatment of diabetic cardiomyopathy.
Acknowledgements
This study was supported by the National Science Foundation of China (No. 30973534, 81173052).
Disclosure of conflict of interest
None.
References
- 1.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 2.Letonja M, Petrovič D. Is diabetic cardiomyopathy a specific entity. World J Cardiol. 2014;6:8–13. doi: 10.4330/wjc.v6.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium. Diabetes. 2002;51:1938–48. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- 4.Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: a key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol. 2010;88:233–40. doi: 10.1139/Y10-016. [DOI] [PubMed] [Google Scholar]
- 5.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women. Arch Intern Med. 2007;167:1610–18. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortality A, Iii ERM, Pastor-barriuso R, Dalal D, Riemersma RA. Review meta-analysis: high-dosage vitamin E supplementation may increase. Ann Intern Med. 2005;142:37–47. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 7.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr. 2009;90:429–37. doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–32. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 9.Bagnost T, Ma L, da Silva RF, Rezakhaniha R, Houdayer C, Stergiopulos N, André C, Guillaume Y, Berthelot A, Demougeot C. Cardiovascular effects of arginase inhibition in spontaneously hypertensive rats with fully developed hypertension. Cardiovasc Res. 2010;87:569–77. doi: 10.1093/cvr/cvq081. [DOI] [PubMed] [Google Scholar]
- 10.Bagnost T, Berthelot A, Bouhaddi M, Laurant P, André C, Guillaume Y, Demougeot C. Treatment with the arginase inhibitor N(omega)-hydroxy-nor-L-arginine improves vascular function and lowers blood pressure in adult spontaneously hypertensive rat. J Hypertens. 2008;26:1110–8. doi: 10.1097/HJH.0b013e3282fcc357. [DOI] [PubMed] [Google Scholar]
- 11.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol. 2007;581:863–72. doi: 10.1113/jphysiol.2007.128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demougeot C, Prigent-Tessier A, Marie C, Berthelot A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J Hypertens. 2005;23:971–8. doi: 10.1097/01.hjh.0000166837.78559.93. [DOI] [PubMed] [Google Scholar]
- 13.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heusch P, Aker S, Boengler K, Deindl E, van de Sand A, Klein K, Rassaf T, Konietzka I, Sewell A, Menazza S, Canton M, Heusch G, Di Lisa F, Schulz R. Increased inducible nitric oxide synthase and arginase II expression in heart failure: no net nitrite/nitrate production and protein S-nitrosylation. Heart Circ Physiol. 2010;1:446–453. doi: 10.1152/ajpheart.01034.2009. [DOI] [PubMed] [Google Scholar]
- 15.Cuervo H, Pineda MA, Aoki MP, Gea S, Fresno M, Gironès N. Inducible nitric oxide synthase and arginase expression in heart tissue during acute trypanosoma cruzi infection in mice: arginase I is expressed in infiltrating CD68+ macrophages. J Infect Dis. 2008;197:1772–82. doi: 10.1086/529527. [DOI] [PubMed] [Google Scholar]
- 16.Jung C, Gonon AT, Sjöquist PO, Lundberg JO, Pernow J. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res. 2010;85:147–54. doi: 10.1093/cvr/cvp303. [DOI] [PubMed] [Google Scholar]
- 17.Jung AS, Kubo H, Wilson R, Houser SR, Margulies KB. Modulation of contractility by myocyte-derived arginase in normal and hypertrophied feline myocardium. Am J Physiol Heart Circ Physiol. 2006;290:H1756–62. doi: 10.1152/ajpheart.01104.2005. [DOI] [PubMed] [Google Scholar]
- 18.Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, Bugaj LJ, Khan M, Santhanam L, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103:4759–64. doi: 10.1073/pnas.0506589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashyap SR, Lara A, Zhang R, Park YM, DeFronzo RA. Insulin reduces plasma arginase activity in type 2 diabetic patients. Diabetes Care. 2008;31:134–9. doi: 10.2337/dc07-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjelakovic G, Sokolovic D, Ljiljana S, Kocic G, Jevtovic T, Stojanovic I, Ilic M, Bjelakovic LJ, Zivic S, Pavlovic D, Nikolić J, Basic J. Arginase activity and magnesium levels in blood of children with diabetes mellitus. J Basic Clin Physiol Pharmacol. 2009;20:319–34. doi: 10.1515/jbcpp.2009.20.4.319. [DOI] [PubMed] [Google Scholar]
- 21.Ogino K, Takahashi N, Takigawa T, Obase Y, Wang DH. Association of serum arginase I with oxidative stress in a healthy population. Free Radic Res. 2011;45:147–55. doi: 10.3109/10715762.2010.520318. [DOI] [PubMed] [Google Scholar]
- 22.Barinov EF, Sulaeva ON, Barinova ME. Blood monocytic L-arginine metabolic changes in diabetic foot syndrome. Klin Lab Diagn. 2010;5:16–9. [PubMed] [Google Scholar]
- 23.Xu W, Chen J, Lin J, Liu D, Mo L, Pan W, Feng J, Wu W, Zheng D. Exogenous H2S protects H9c2 cardiac cells against high glucose-induced injury and inflammation by inhibiting the activation of the NF-κB and IL-1β pathways. Int J Mol Med. 2015;35:177–86. doi: 10.3892/ijmm.2014.2007. [DOI] [PubMed] [Google Scholar]
- 24.Sankaralingam S, Xu H, Davidge ST. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc Res. 2010;85:194–203. doi: 10.1093/cvr/cvp277. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-Arginine Consumption by Macrophages Modulates the Expression of CD3 Chain in T Lymphocytes. J Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 26.Huynh NN, Harris EE, Chin-Dusting JEP, Andrews KL. The vascular effects of different arginase inhibitors in rat isolated aorta and mesenteric arteries. Br J Pharmacol. 2009;156:84–93. doi: 10.1111/j.1476-5381.2008.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjelakovic G, Sokolovic D, Ljiljana S, Kocic G, Jevtovic T, Stojanovic I, Ilic M, Bjelakovic LJ, Zivic S, Pavlovic D, Nikolić J, Basic J. Arginase activity and magnesium levels in blood of children with diabetes mellitus. J Basic Clin Physiol Pharmacol. 2009;20:319–34. doi: 10.1515/jbcpp.2009.20.4.319. [DOI] [PubMed] [Google Scholar]
- 28.Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol. 2006;26:2035–42. doi: 10.1161/01.ATV.0000233334.24805.62. [DOI] [PubMed] [Google Scholar]
- 29.Wold LE, Ceylan-Isik AF, Fang CX, Yang X, Li SY, Sreejayan N, Privratsky JR, Ren J. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly (ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med. 2006;40:1419–29. doi: 10.1016/j.freeradbiomed.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Wautier MP, Chappey O, Corda S, Stern DM, aSchmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 31.Guo Z, Qi W, Yu Y, Du S, Wu J, Liu J. Effect of exenatide on the cardiac expression of adiponectin receptor 1 and NADPH oxidase subunits and heart function in streptozotocin-induced diabetic rats. Diabetol Metab Syndr. 2014;6:29. doi: 10.1186/1758-5996-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris SM. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009;157:922–30. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible NO synthase dependent S-nitrosylation and activation of arginase contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 34.Morris SM. Arginine metabolism in vascular biology and disease. Vasc Med. 2005;10:83–87. doi: 10.1177/1358836X0501000112. [DOI] [PubMed] [Google Scholar]
- 35.Lim HK, Lim HK, Ryoo S, Benjo A, Shuleri K, Miriel V, Baraban E, Camara A, Soucy K, Nyhan D, Shoukas A, Berkowitz DE. Mitochondrial arginase II constrains endothelial NOS-3 activity. Am J Physiol Heart Circ Physiol. 2007;293:H3317–24. doi: 10.1152/ajpheart.00700.2007. [DOI] [PubMed] [Google Scholar]
- 36.Howarth FC, Qureshi MA. Effects of carbenoxolone on heart rhythm, contractility and intracellular calcium in streptozotocin-induced diabetic rat. Mol Cell Biochem. 2006;289:21–9. doi: 10.1007/s11010-006-9143-5. [DOI] [PubMed] [Google Scholar]
- 37.Jo H, Otani H, Jo F, Shimazu T, Okazaki T, Yoshioka K, Fujita M, Kosaki A, Iwasaka T. Inhibition of nitric oxide synthase uncoupling by sepiapterin improves left ventricular function in streptozotocin-induced diabetic mice. Clin Exp Pharmacol Physiol. 2011;38:485–93. doi: 10.1111/j.1440-1681.2011.05535.x. [DOI] [PubMed] [Google Scholar]


