Abstract
To explore the effects of resveratrol in a human myelogenous leukemia cell line K562 and its potential molecular mechanisms. The anti-proliferation effect of resveratrol-induced apoptosis on K562 cells were detected using MTT assay. Western blotting was performed for detecting changes of SphK1 expression in total cell protein and membrane/cytosol protein in K562 cells respectively after exposure to resveratrol. A biochemical assay was used to measure the activity of SphK after treatment of resveratrol, and then S1P and ceramide levels were examined using ELISA kits. Hochest 33258 staining and flow cytometry were applied to detect the apoptosis condition of K562 cells treated with resveratrol. Resveratrol inhibited the proliferation and induced apoptosis in K562 cells in a dose and time-dependent manner. Western blotting revealed that resveratrol did not affect total SphK1 expression level in K562 cells, but significantly changed the translocation of SphK1, the membrane SphK1 was decreased while cytosol SphK1 level was elevated. The activity of SphK1 in resveratrol treated groups was decreased compared to control group with a significant decrease of S1P and increase of ceramide level. Furthermore, Hoechst 33258 staining and Annexin V-FITC analysis confirmed the notable apoptotic effect of resveratrol in its anti-leukemia process. Resveratrol-induced proliferation inhibition of K562 cells might be mediated through its modulation activity of SphK1 pathway by regulating S1P and ceramide levels, which then affected the proliferation and apoptosis process of leukemia cells. SphK1/S1P pathway represents a target of resveratrol in human leukemia.
Keywords: Resveratrol, leukemia, SphK1, S1P, apoptosis
Introduction
Chronic myelogenous leukemia (CML), a cancer of the white blood cells, is a myeloproliferative syndrome linked to a hematopoietic stem cell disorder leading to the increased production of granulocytes at all stages of differentiation. CML has an incidence 1-1.5 per 100,000 inhabitants and approximately 15% of all leukaemias diagnosed in adults with an onset at 40-60 years of age characterized by high levels of white blood cell counts, splenomegaly, weight loss, lethargy and anaemia [1,2]. TKI (tyrosine kinase inhibitors) are the treatment modality for patients with chronic phase CML. The development of tyrosine kinase inhibitors (TKIs) has led to extended lifespans for many patients with chronic myelogenous leukemia (CML). However, 20% to 30% of patients fail to respond, respond suboptimally, or experience disease relapse after treatment with imatinib. A key factor is drug resistance [3]. Herbal medicines such as Chinese medicines are able to relieve many different human diseases. A wide variety of natural compounds derived from medicinal plants have been extensively studied for the treatment of cancer. And it is believed and epidemiological data also support that naturally occurring compounds in the human diet may have lower toxicity and less possibility of drug resistance and have long lasting beneficial effects on human health [4-6].
Resveratrol (trans-3,4’,5-trihydroxystilbene; RSV) is a compound obtained primarily from root extracts of the oriental plant, Polygonum cuspidatum and from red grapes [7]. It has been identified that resveratrol has a strong chemopreventive effect against the development of several cancers, prevention of cardiovascular diseases, and is currently being used in phase I studies to treat obese and diabetic patients [8-10].
Although resveratrol has been reported to either protect CML cell lines from stress-induced apoptosis mainly through its antioxidant properties [11], or to induce cell death when used alone [12], the molecular signaling mechanisms by which resveratrol exerts its anti-leukemic effects in CML cell lines remains incompletely understood. In this study, we aimed to investigate whether resveratrol has potential antitumor effects in human CML K562 cells and to determine whether resveratrol could modulate the SphK1 activity for its anticancer effects in the human CML K562 cell line.
Materials and methods
Chemicals
Resveratrol (Figure 1A, Sigma-Aldrich, Inc., St. Louis, Mo, USA) was dissolved in DMSO at 40 mM as a stock solution. The dilutions of all reagents were freshly prepared before experiment.
Figure 1.
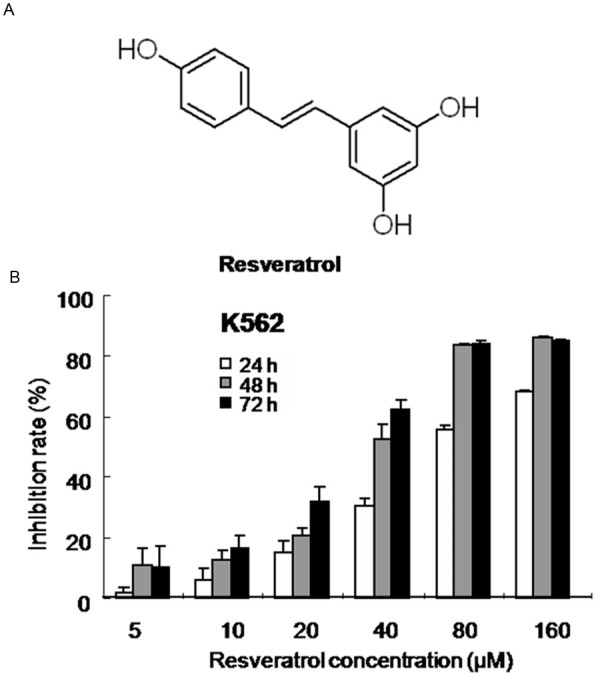
Growth inhibition effect of K562 cells induced by resveratrol. A. The chemical structure of resveratrol. B. K562 cells were exposed to increasing concentrations of resveratrol or an equal volume of the drug’s vehicle DMSO for up to 72 h. Viable cells were evaluated by MTT assay and denoted as a percentage of untreated controls at the concurrent time point. The bars indicate mean ± S.D. (n=3).
Cell lines
The human myeloid leukemia cell line K562 was obtained from the Institute of Hematology of Chinese Academy of Medical Sciences (Tianjin, China). Cancer cells were cultured in RPMI-1640 (Hyclone) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Gibco), penicillin-streptomycin (100 IU/ml to 100 μg/ml), 2 mM glutamine, and 10 mM HEPES buffer at 37°C in a humidified atmosphere (5% CO2 -95% air).
Growth and cell proliferation analysis by MTT assay
Human leukemic cells K562 of 5×103 per well seeded in 96-well plates were incubated with increasing concentrations of resveratrol (10, 20, 40, 80, 160 μM) for 24, 48 and 72 h, respectively. Control cells were treated with an equal volume of the drug’s vehicle DMSO at a concentration did not exhibit a modulating effect on cell growth. Cell growth inhibition was evaluated by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay.
Determination of biochemical SphK activity
A biochemical assay was used to measure the activity of SphK. After treatment of resveratrol (40 μM), cells were resuspended in ice-cold 0.1 M PBS (pH 7.4) containing 20% glycerol, 1 mM mercaptoethanol, 1 mM EDTA, phosphatase inhibitors (20 mM ZnCl2, 1 mM sodium orthovanadate, and 15 mM sodium fluoride), protease inhibitors (10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride), and 0.5 mM 4-deoxypyridoxine and disrupted by freeze-thawing. Lysates were assayed for sphingosine kinase activity, based on the Sphk-catalyzed transfer of the -phosphate group of ATP (using a mixture of cold ATP and [γ32P] ATP; 1 μCi/sample) to a specific substrate, and the products were separated by TLC on Silica Gel G60 and visualized by autoradiography [13].
Extraction of total protein, membrane and cytosol protein
Total cell protein of K562 cells after treatment of resveratrol for 24 h was obtained using a cell lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 0.1% Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 2 mM sodium orthovanadate, 4 mM sodium pyrophosphate, 100 mM NaF, and 1:500 protease inhibitor mixture (Sigma-Aldrich, USA).
To examine the translocation of SphK1, we extracted membrane and cytosol protein of K562 cells after treatment of resveratrol for 24 h using a Membrane and Cytosol Protein Extraction Kit according to the manufacturer’s protocols (Beyotime, China).
Western blot analysis
30 μg of protein per lane were fractionated by 10% SDS-PAGE. Then proteins were electro-transferred onto nitrocellulose membranes and then protein levels were detected using dilutions of primary antibodies. β-actin was used as the loading controls. Triplicate experiments were performed with triplicate samples [14].
Assay for S1P and ceramide levels
After treatment, cells and the culture medium were centrifuged at 3,000 g for 10 min, and then the supernatants were aliquoted for analysis. The levels of S1P and ceramide were measured using ELISA kit (1-sohingosine 1-phosphate (S1P) ELISA kit and Human- Ceramide ELISA kit, Shanghai Biovol Biotech, China) according to manufacturer’s protocol.
Hochest 33258 staining
Apoptotic cells were evaluated by morphologic observation using Hochest 33258 staining. Specifically, K562 cells at logarithmic growth were seeded in 96-well plates by density of 1×104/well. After K562 cells were treated with resveratrol (20 μM and 40 μM), cells in the plates were centrifuged and fixed with 3.7% paraformaldelyde for 30 min at room temperature, and then washed and stained with Hoechst 33258 at 37°C for 30 min. Cells were observed under a fluorescence microscope (Nikon, Tokyo, Japan) equipped with a UV filter.
Annexin V/FITC and PI staining analysis
Leukemic cells seeded in 6-well plates (2.0×105 per well) were treated with increasing concentrations of resveratrol for 24 h. Cells were harvested and washed with cold PBS. The cell surface phosphatidylserine in apoptotic cells was quantitatively estimated using an Annexin V/FITC and 7-AAD apoptosis detection kit according to manufacturer’s instructions (Roche, USA). Cell apoptosis condition was analyzed on the FACScan flow cytometry after each treatment (Becton Dickinson, USA). Triplicate experiments with triplicate samples were performed.
Statistical analysis
All of the experiments were repeated at least three times and data were expressed as mean ± SD (standard deviation). Statistical software SPSS15.0 was used for the assessment. The Student’s t test was used to compare means of two groups and One-Way ANOVA was used for comparing means of multiple samples. P < 0.05 was considered as statistically significant.
Results
Inhibition of leukemic cell proliferation
Human leukemia cells were treated with resveratrol for up to 72 h and then subjected to the MTT assay. Resveratrol effectively inhibited proliferation of the human leukemia cell K562. As shown in Figure 1B, resveratrol in the concentrations range of 5-160 µM had a dose- and time-dependent antiproliferative effect on K562 cells for up to 72 h of exposure (5 μM for 24 h exposure, P > 0.05; 10-160 μM, P < 0.05 vs. drug’s vehicle control). The maximum inhibition rate of 83.9% was observed with use of 160 μM resveratrol for 72 h exposure.
Resveratrol affected the membrane translocation of SphK1 without decreasing its total expression level
SphK1 is mainly cytosolic and migrates to the plasma membrane to generate S1P. Results show that treatment of resveratrol resulted in translocation of SphK1 to the cytoplasm (Figure 2A and 2B), while in the mean time, total protein analysis showed that there was no significant difference of total SphK1 expression among control group and resveratrol treatment groups (P > 0.05, Figure 2A).
Figure 2.

Resveratrol induced the redistribution of SphK1 in K562 cells as estimated by Western blot assay. A. K562 cells were exposed to various concentrations of resveratrol and the levels of SphK1 in membrane and cytosol were measured by western blot analysis. B. Quantitative analysis of the expression of SphK1 in membrane and cytoplasm. The bars indicated mean ± S.D. (n=3). *P < 0.05, **P < 0.01 vs. vehicle control. Triplicate experiments were performed with triplicate samples.
Resveratrol inhibits SphK activity
Treatment of resveratrol significantly inhibited (P < 0.01) SphK activity (Figure 3A). The activity of SphK of cells exposed to 40 μM Resveratrol treatment was decreased to 56.3% of control group (P < 0.05). S1P levels in different groups were then detected by an ELISA kit. Our data showed that the S1P levels of 20 and 40 μM Resveratrol treatment group were reduced by 17.4% and 36.3% compared to control group (Figure 3B, P < 0.05).
Figure 3.

Resveratrol induced the decrease of SphK activity in K562 cells and changes of S1P and ceramide levels. A. SphK activity in K562 cells was decreased by treatment of resveratrol. B. Quantitative analysis of the secretion of S1P in K562 cells estimated by ELISA assay. C. Quantitative analysis of the secretion of ceramide in K562 cells estimated by ELISA assay. The bars indicated mean ± S.D. (n=3). *P < 0.05, **P < 0.01 vs. vehicle control. Triplicate experiments were performed with triplicate samples.
In the mean time, we also measured the content of ceramide by ELISA after treatment. As shown in Figure 3C, ceramide levels in resveratrol treatment group were elevated 1.5-fold and 2.3-fold by 20 and 40 μM Resveratrol treatment compared with control group.
Resveratrol induces leukemic cell apoptosis
The induction of apoptosis was employed to evaluate the inhibitory effect of resveratrol following SphK inhibition. The morphologic changes were first examined by Hoechst 33258 staining (Figure 4). After K562 cells were treated with 20 μM and 40 μM resveratrol for 24 h, the apoptotic morphologic changes were observed as compared with the vehicle control. In vehicle control group, the nuclei of K562 cells were round and homogeneously stained (Figure 4A) while resveratrol-treated cells exhibited evident apoptosis characteristics including cell shrinkage and membrane integrity loss or deformation, nuclear fragmentation and chromatin compaction of late apoptotic appearance (Figure 4A).
Figure 4.
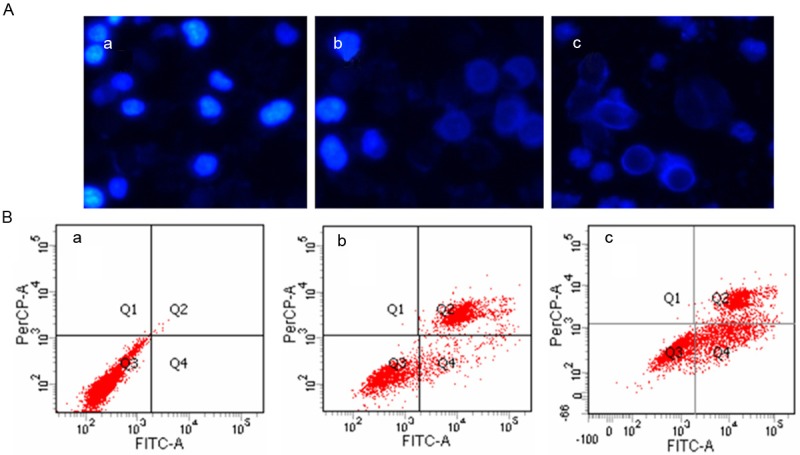
Resveratrol induced the morphologic changes and apoptosis of K562 cells in vitro. A. Cells treated with resveratrol were subjected to Hoechst 33258 staining and viewed under microscope. B. Detection of apoptotic cells by flow cytometric analysis. a. Vehicle control; b. 20 μM; c. 40 μM.
The apoptosis induction of resveratrol in K562 cells were then analyzed by flow cytometry assay. Our data showed significant increase of apoptotic cells after exposure to resveratrol for 24 h (Figure 4B; Table 1). In the vehicle group, there were barely a few apoptotic cells (0.98%), while in groups of concentrations of 20 μM and 40 μM of resveratrol, the percentage of apoptotic cells was 33.17% and 58.28%, respectively, in K562 cells.
Table 1.
Detection of apoptotic cells by flow cytometric analysis
| Concentration of resveratrol | Vehicle | 20 μM | 40 μM |
|---|---|---|---|
| Percentage of apoptotic cells | 0.98% | 33.17% | 58.28% |
Discussion
A number of studies have revealed that resveratrol hits a variety of target molecules and cellular signaling pathways pertinent to normal human physiology and directly applicable to pathological disease states, it exerts its anti-proliferative and anti-carcinogenic effects involved various signaling mechanisms such as apoptosis induction, suppression of invasion and metastasis, increased antioxidant capacity, and sensitization to chemotherapy-triggered apoptosis [7,15]. In this research, we confirmed that resveratrol inhibited the proliferation of K562 cells in a concentration- and time-dependent manner. However, the accurate pro-apoptotic and molecular signaling pathways mediated by resveratrol to induce its complex anti-leukemic effects in cancer cells remain incompletely understood.
Multiple studies have demonstrated that sphingolipids, in addition to being structural constituents of cell membranes, play key roles as signaling molecules. The sphingolipid metabolites and the kinases that produce them have emerged as critical players in a number of fundamental biological processes important for health and disease. The key players among the sphingolipid metabolites include ceramide (N-acyl sphingosine), sphingosine, and sphingosine-1-phosphate (S1P), and particularly, ceramide and sphingosine 1-phosphate (S1P), have been investigated as important effecter molecules that regulate cell proliferation, differentiation, angiogenesis, and survival in opposite directions [16,17]. S1P is a pleiotropic phospholipid with many biological functions mainly produced intracellularly by two closely related sphingosine kinase isoenzymes, SphK1 and SphK2. SphK1 and its function have been well studied, which is overexpressed in various types of cancers including acute leukemia patients, and upregulation of SphK1 has been associated with tumor angiogenesis and resistance to radiation and chemotherapy [18,19].
SphK1 is mainly cytosolic and when migrates to the plasma membrane to generate S1P, here in this research we also found a relatively high expression of SphK1 in human leukemia K562 cells. It has been previously reported that resveratrol could inhibit SphK1 activity in C5a-induced inflammatory responses [13]. And although resveratrol treatment did not reduce the total SphK1 level in our present study, it did affect the translocation of SphK1 (Figure 2). The following kinase activity analysis also showed that resveratrol could significantly inhibit SphK activity (Figure 3A).
S1P and ceramide are closely involved in regulating cell responses such as proliferation and apoptosis, the balance between ceramide and S1P is important to determine faith of cancer cells to undergo growth inhibition or survival [20-23]. In particular, the sphingolipid sphingosine-1-phosphate (S1P) elicits a plethora of cellular responses in many physiological and pathological processes including proliferation, survival, movement, angiogenesis, and chemoresistance, while ceramide potentiates signaling events which drive apoptosis, cell cycle arrest, and autophagic responses [14,24]. Sphingosine kinase 1 (SphK1) can prevent ceramide accumulation, in part, by promoting its metabolism into S1P [25]. In the present study, our data showed that resveratrol increase S1P level and decrease ceramide level (Figure 3B, 3C).
Precious researches have clearly demonstrated that SphK1 acts as an oncogene and over-expression of SphK1/S1P can induce tumor growth and inhibit ceramide-mediated apoptosis, resulting in drug resistance in multiple cancers including leukemias [26-28]. Based on the results of Hoechst 33258 staining and Annexin V-FITC analysis, we confirmed that resveratrol significantly induced apoptosis in K562 cells while restore the balance of S1P and ceramide. All of the results suggested that resveratrol may regulate leukemia cell proliferation and apoptosis via its modulation of SphK1/S1P signaling.
In conclusion, our results provide novel insights into the modes of action of resveratrol in its anti-leukemia activity in human leukemia K562 cells. Resveratrol-induced proliferation inhibition of K562 cells might be mediated through its modulation activity of SphK1 pathway by regulating S1P and ceramide levels, which then affected the proliferation and apoptosis process of leukemia cells. Taken together, the results support the potential of resveratrol to be researched as an attractive and promising compound for treatment of cancers.
Disclosure of conflict of interest
None.
References
- 1.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2012 update on diagnosis, monitoring, and management. Am J Hematol. 2012;87:1037–1045. doi: 10.1002/ajh.23282. [DOI] [PubMed] [Google Scholar]
- 2.Rumjanek VM, Vidal RS, Maia RC. Multidrug resistance in chronic myeloid leukaemia: how much can we learn from MDR-CML cell lines? Biosci Rep. 2013;33:e00081. doi: 10.1042/BSR20130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabbour EJ, Cortes JE, Kantarjian HM. Resistance to tyrosine kinase inhibition therapy for chronic myelogenous leukemia: a clinical perspective and emerging treatment options. Clin Lymphoma Myeloma Leuk. 2013;13:515–529. doi: 10.1016/j.clml.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damery S, Gratus C, Grieve R, Warmington S, Jones J, Routledge P, Greenfield S, Dowswell G, Sherriff J, Wilson S. The use of herbal medicines by people with cancer: a cross-sectional survey. Br J Cancer. 2011;104:927–933. doi: 10.1038/bjc.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic Applications of Herbal Medicines for Cancer Patients. Evid Based Complement Alternat Med. 2013;2013:302426. doi: 10.1155/2013/302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai S, To KK, Lin G. Circumvention of multi-drug resistance of cancer cells by Chinese herbal medicines. Chin Med. 2010;5:26. doi: 10.1186/1749-8546-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang F, Wu XN, Chen J, Wang WX, Lu ZF. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp Ther Med. 2014;7:1611–1616. doi: 10.3892/etm.2014.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 9.Shen M, Jia GL, Wang YM, Ma H. Cardioprotective effect of resvaratrol pretreatment on myocardial ischemia-reperfusion induced injury in rats. Vascul Pharmacol. 2006;45:122–126. doi: 10.1016/j.vph.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S, Auberger P. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK. Cancer Res. 2010;70:1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 11.MacCarrone M, Lorenzon T, Guerrieri P, Agro AF. Resveratrol prevents apoptosis in K562 cells by inhibiting lipoxygenase and cyclooxygenase activity. Eur J Biochem. 1999;265:27–34. doi: 10.1046/j.1432-1327.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 12.Luzi C, Brisdelli F, Cinque B, Cifone G, Bozzi A. Differential sensitivity to resveratrol-induced apoptosis of human chronic myeloid (K562) and acute lymphoblastic (HSB-2) leukemia cells. Biochem Pharmacol. 2004;68:2019–2130. doi: 10.1016/j.bcp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Issuree PD, Pushparaj PN, Pervaiz S, Melendez AJ. Resveratrol attenuates C5a-induced inflammatory responses in vitro and in vivo by inhibiting phospholipase D and sphingosine kinase activities. FASEB J. 2009;23:2412–2424. doi: 10.1096/fj.09-130542. [DOI] [PubMed] [Google Scholar]
- 14.Sun DF, Gao ZH, Liu HP, Yuan Y, Qu XJ. Sphingosine 1-phosphate antagonizes the effect of all-trans retinoic acid (ATRA) in a human colon cancer cell line by modulation of RARβ expression. Cancer Lett. 2012;319:182–189. doi: 10.1016/j.canlet.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 16.Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirai K, Kaneshiro T, Wada M, Furuya H, Bielawski J, Hannun YA, Obeid LM, Ogretmen B, Kawamori T. A role of sphingosine kinase 1 in head & neck carcinogenesis. Cancer Prev Res (Phila) 2011;4:454–462. doi: 10.1158/1940-6207.CAPR-10-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobue S, Iwasaki T, Sugisaki C, Nagata K, Kikuchi R, Murakami M, Takagi A, Kojima T, Banno Y, Akao Y, Nozawa Y, Kannagi R, Suzuki M, Abe A, Naoe T, Murate T. Quantitative RT-PCR analysis of sphingolipid metabolic enzymes in acute leukemia and myelodysplastic syndromes. Leukemia. 2006;20:2042–2046. doi: 10.1038/sj.leu.2404386. [DOI] [PubMed] [Google Scholar]
- 19.Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- 20.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemoto S, Nakamura M, Osawa Y, Kono S, Itoh Y, Okano Y, Murate T, Hara A, Ueda H, Nozawa Y, Banno Y. Sphingosine kinase isoforms regulate oxaliplatin sensitivity of human colon cancer cells through ceramide accumulation and Akt activation. J Biol Chem. 2009;284:10422–10432. doi: 10.1074/jbc.M900735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Beckman BS, Foroozesh M. A review of ceramide analogs as potential anticancer agents. Future Med Chem. 2013;5:1405–1421. doi: 10.4155/fmc.13.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruce CR, Risis S, Babb JR, Yang C, Kowalski GM, Selathurai A, Lee-Young RS, Weir JM, Yoshioka K, Takuwa Y, Meikle PJ, Pitson SM, Febbraio MA. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes. 2012;61:3148–3155. doi: 10.2337/db12-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 27.Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, Ogretmen B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- 28.Van Brocklyn J, Letterle C, Snyder P, Prior T. Sphingosine-1-phosphate stimulates human glioma cell proliferation through Gi-coupled receptors: role of ERK MAP kinase and phosphatidylinositol 3-kinase beta. Cancer Lett. 2002;181:195–204. doi: 10.1016/s0304-3835(02)00050-2. [DOI] [PubMed] [Google Scholar]


