Abstract
Alzheimer’s disease (AD) is one of the most common forms of neurodegenerative disease. There is a growing interest in the amyloid precursor protein (APP) over the years due to its involvement in AD. Besides its role in pathological mechanisms of AD, APP participates in many signaling pathways as well. APP functions through protein-protein interactions, and in this report staufen 1 (STAU1) is demonstrated to have interaction with APP, using yeast two-hybrid screening and co-immunoprecipitation in mammalian system. STAU1 belongs to the double-stranded RNA binding protein family and can mediate mRNA degradation in mammalian system, implicating that APP may be involved in the regulation of mRNA as well.
Keywords: Amyloid precursor protein, Alzheimer’s disease, yeast two-hybrid screening, staufen 1, cell death
Introduction
Alzheimer’s disease is the most typical age-related dementia that is characterized by symptoms of memory loss and cognitive deficiency [1]. There is no cure for the disease at the moment, which worsens when progressing and finally leads to death. Prior studies have discovered two hallmarks that characterize AD: the accumulation of intracellular aggregated hyper-phosphorylated Tau and extracellular amyloid plaques consisting mainly of amyloid-β (Aβ) [2]. However, the generation of neurofibrillary tangles (NFTs) composed of Tau is not restricted to AD but is observed in other neurodegenerative diseases as well, for example Parkinson disease [3]. Therefore, researches in this field mainly focus on the role of Aβ.
Aβ is generated from sequential cleavage of amyloid precursor protein (APP) by β- and γ-secretases [4]. APP is involved in a number of biological processes in the cell, implicating functions in signaling pathways. So far, however, the physiological functions of APP remain unclear. Thus studying the role of APP in cellular signaling pathways will give us insights into the mechanisms of many physiological processes as well as possible means of regulation that leads to the cleavage of APP which generates Aβ.
APP functions through interactions with other proteins, thus studies of APP interacting proteins will reveal the role of APP in many biological reactions. Prior studies have reported several APP interacting proteins, such as ApoE [5] and Fe65 [6] which function as surface receptor and in gene transcription respectively. However, there are a large number of proteins that can have interactions with APP remain to be discovered. Since APP is a transmembrane protein, it is possible that it functions as a receptor in physiological processes, and its structure supports this hypothesis. Thus far, however, no APP ligand has been reported. The screen for APP interacting proteins and ligands can not only help us understand the physiological function of APP but also give us insights into the mechanism of AD pathology. Therefore, possible pharmacological targets of AD may be available in the future.
Yeast two-hybrid system is a useful molecular tool for testing protein-protein interactions. DUALmembrane system was adopted in our study. The premise behind this technique is that APP serves as the bait which is fused with the C-terminal half of ubiquitin (Cub) and an artificial transcription factor (LexA-VP6), while the interacting protein candidates are fused with the N-terminal half of ubiquitin (NubG). Only when the interaction is realized will Cub and NubG get into close proximity, leading to the reconstitution of the “split-ubiquitin”. The split-ubiquitin can be recognized by ubiquitin-specific proteases, which cleave the peptide chain between Cub and LexA-VP6 therefore releasing LexA-VP6. The released transcription factor can activate a set of reporter genes [7]. Above all, no reporter gene will be activated without the interaction between APP and protein candidates.
Herein, a protein in human that is encoded by staufen 1 (STAU1) gene is demonstrated to have interaction with APP. STAU1 belongs to the family of double-stranded RNA (dsRNA) binding proteins, which are characterized by several dsRNA-binding domains [8]. STAU1 has four dsRNA binding domains (dsRBD), a tubulin binding domain and a staufen-swapping motif. Our screen product contains dsRBD4 and a part of dsRBD3 which are responsible for dsRNA binding, and a tubulin binding domain. Prior studies have reported that STAU1 can mediate mRNA degradation in mammalian cells by binding to a STAU1-binding site (SBS) which locates at the 3’-untranslated region (3’-UTR) of target mRNAs.
Materials and methods
Constructs and materials
NMY51 yeast strain was a gift of Dr. Lijia Qu (Peking University) and the plasmid pBT3-C bait vector was from Dr. Dean Jiang (Zhejiang University). Human Brain cDNA Library was from DUALsystems Biotech, AG (Switzerland). TIANprep Yeast Plasmid DNA Kits (Cat#DP11202) were from TIANGEN Biotech Co., Ltd. (Beijing, China). PhosSTOP (#04906845001) and cOmplete ULTRA Tablets, Mini, EASYpack (#05892970001) were from Roche Pharmaceutical, Ltd. (Switzerland). Cell Lysis Buffer for Western and IP (#P0013) and Protein A+G Agarose (#P2012) was from Beyotime Institute of Biotechnology Ltd. (Haimen, China). Normal Mouse IgG (#sc-2025) was from Santa Cruz Biotechnology (Shanghai) Co., Ltd. (Shanghai, China). Anti-HA, anti-mCherry and anti-EGFP primary antibody were from EASYBIO (Beijing, China). Clean-Blot IP Detection Reagent (HRP, 21230) was from Thermo Fisher Scientific (Beijing, China).
Transformation of plasmids into NMY51 (small scale transformation)
Several colonies of NMY51 were taken from YPAD plates and then inoculated into 50 ml YPAD and grew overnight at 30°C with shaking. Upon the OD546 of the culture reaching 0.6-0.8, the culture was centrifuged at 700 g for 5 minutes and resuspended in 2.5 ml sterile water afterwards. PEG/LiOAc (1.2 ml 50% PEG, 180 μl 1M LiOAc, 125 μl ssDNA) was added into 1.5 ml Eppendorf tubes with 300 μl in each tube and later was mixed with plasmids (2 μg for each transformation). Resuspended cells of 100 μl were added into each tube and the tubes were incubated in a 42°C water bath for 45 minutes after vortex mixing. The solutions were centrifuged at 700 g for 5 minutes and then the pellets were dissolved in 150 μl 0.9% NaCl. 50 μl suspension was taken from each transformation and plated onto one SD-leu/trp plate, one SD-leu/trp/his plate and one SD-leu/trp/his/ade plate respectively. All plates were then incubated for 4 days at 30°C.
Transformation of plasmids into NMY51 (large scale transformation)
Several colonies of NMY51 were taken from YPAD plates and then inoculated into 10 ml YPAD and grew 8 hours at 30°C with shaking. The culture was then inoculated into 50 ml YPAD and grew overnight at 30°C with shaking. The OD546 of the culture was measured with YPAD only as blank. Then the amount of culture needed for 15 OD units was calculated and added into a 50 ml centrifugal tube before centrifuged at 700 g for 5 minutes. The yeasts were resuspended in 100 ml 2×YPAD (preheated 30°C) and placed in a 1 L Erlenmeyer flask. The yeasts grew at 30°C with shaking until the OD546 reached 0.6. The culture was aliquoted into two 50 ml centrifugal tube and centrifuged at 700 g for 5 minutes. The pellet was collected and dissolved in 30 ml sterile water then again centrifuged at 700 g for 5 minutes. The supernatant was discarded and the pellet was dissolved with 1 ml LiOAc/TE (0.55 ml 1 M LiOAc, 0.55 ml 10×TE pH 7.5, 3.9 ml sterile water). After that the cells were transferred into Eppendorf tubes and centrifuged at 700 g for 5 minutes. Later the supernatant was discarded and the cells were resuspended in 600 μl LiOAc/TE. The yeast cells were ready for transformation. Single-stranded DNA (ssDNA) was prepared by heating at 99°C for 5 minutes then cooling on ice for 5 minutes. This was repeated one more time. Two centrifugal tubes were prepared and 100 μl ssDNA, 600 μl readily prepared yeast cells, 7 μg cDNA library or library vector pPR3-N and finally 2.5 ml PEG/ LiOAc (0.6 ml 1 M LiOAc, 0.6 ml 10×TE pH 7.5, 4.8 ml 50% PEG) were added in each tube. The solutions were incubated in a 30°C water bath for 45 minutes, note that overturn and blending were needed every 15 minutes. Later 160 μl DMSO was added into each tube and the solutions were incubated in a 42°C water bath for 20 minutes. Then the solutions were centrifuged at 700 g for 5 minutes and the cells were resuspended in 3 ml 2×YPAD before repeating the centrifuging. Finally the cells were resuspended in 1.5 ml 0.9% NaCl and each transformation (300 μl) was plated onto five 150 mm diameter plates. All plates were then incubated for 5 days at 30°C.
Verification of positive interactions
Five days after the transformation of cDNA Library, white colonies from the plates were taken and inoculated with SD-leu/trp medium. The strain was permitted to grow at 30°C for 2 days with shaking. The yeast plasmids were later extracted and transformed into E. coli. Colonies of E. Coli were taken and inoculated in 2 ml LB medium (50 μg/ml Ampicillin) and then grew overnight at 37°C with shaking before the plasmids were extracted. The extracted E. coli plasmids (1 μg) were transformed together with pBT3-SUC-APP (1 μg) into NMY51 (please see Transformation of plasmids into NMY51). The transformed solution was plated onto SD-leu/trp/his/ade/7.5 mM 3AT plates. Five days after incubating at 30°C, plates with white colonies were selected and the β-galactosidase assay was performed. The E. coli plasmids during the tests in which filter paper turned blue were sent for sequencing. The results were compared with Human genomic and transcript and the Nucleotide collection in NCBI by BLAST.
β-galactosidase assay
The overlay mix was prepared to contain 100 ml/L 10×PBS, pH 7.4, 5 g/L agarose and 1 ml/L X-Gal (X-Gal stock’s concentration was 100 mg/ml, soluted in N,N-dimethylformamide) and was placed in a 50°C water bath. Whatman filter papers were placed onto agar plates with colonies and were incubated for 10 minutes at room temperature. The Whatman filter papers were then incubated in liquid nitrogen for 5 minutes. The filters were put on another plate with colony side up and thawed for 5 minutes. The filters were overlaid with the overlay mix to make sure that the entire surface of each was covered. The filters were incubated at room temperature from 30 minutes to 4 hours until blue colors developed on the filters.
Calcium phosphate transfection of HEK293T cell line
Upon the cells growing to a confluence of 80%, Hek293T cells were dissociated by trypsin for 1 minute. The trypsin was then inactivated by fresh medium and the cells were centrifuged at 500 g for 2 minutes afterwards. The supernatant was discarded and fresh medium was used to resuspend the cells. Then the cells were counted with a counting chamber and were adjusted to a density of 2 × 105 cells/ml before transferred to a 6-well plate. Each well had a volume of 2 ml, containing 4 × 105 cells. The cells grew overnight and then the medium was changed. Two Eppendorf tubes were prepared. One of them contained a mixture of 10 μl 2 M CaCl2, 4 μg plasmid and sterile water with a final volume of 100 μl, and the other 100 μl 2×HBS. The CaCl2/DNA mixture was gently mixed and slowly added into HBS, followed by 20 times of air bubbling through the mixture. The mixture was finally added to cells by dropping evenly and slowly into medium.
Co-immunoprecipitation
Hek293T cells were washed twice with pre-chilled PBS 24 hours post-transfection. A 100 μl mixture of Cell Lysis Buffer for Western and IP supplemented with PhosSTOP and cOmplete ULTRA Tablets was added into each well and the lysis lasted for 5 minutes. Later the cells were harvested and were centrifuged 13000 rpm at 4°C for 15 minutes. The supernatant was collected and taken 20 μl as positive control (input) supplemented with 4 μl 6× loading buffer. For the remaining supernatant, 20 μl Protein A+G Agarose and 2.5 μl normal mouse IgG were added and was incubated for 30 minutes at 4°C with shaking. Then the supernatant was collected and 20 μl Protein A+G Agarose and 1.5 μl primary antibody was added. The mixture was incubated at 4°C overnight before centrifuged 13000 rpm at 4°C for 1 minute the next day. The pellet was washed 5 times with 1 ml pre-chilled PBS and each time was centrifuged 13000 rpm at 4°C for 1 minute. The supernatant was finally discarded and 1× loading buffer was added before heating at 99°C for 5 minutes and then cooling on ice for 5 minutes. Then the mixture was centrifuged 13000 rpm at 4°C for 1 minute and the supernatant was proceeded Western-blot together with the positive control. Anti-mCherry or anti-EGFP was used for the detection of protein expression.
HEK293T cell viability assay
HEK293T cells were seeded in a 96-well plate and the medium was discarded 48 hours post-transfection, and 100 μl fresh medium was added into each well afterwards. The cell viability assay was proceeded according the protocol of CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega).
Results
Determination and optimization of APP bait protein screening stringency
We cloned wt-APP695 into pBT3-SUC bait vector. Then pBT3-SUC-APP was co-transformed with NubWT and NubG respectively into NMY51 (small scale transformation) in order to determine the type of SD- plate for the screen of interacting proteins with pBT3-SUC-APP. Each transformation was plated onto a SD-leu/trp plate, a SD-leu/trp/his plate and a SD-leu/trp/his/ade plate respectively, and the number of colonies was counted on each plate 4 days later. It turned out that pBT3-SUC-APP co-transformed with NubWT grew well on all three kinds of plates, while pBT3-SUC-APP co-transformed with NubG grew only on the SD-leu/trp plate and the SD-leu/trp/his plate and was inhibited on the SD-leu/trp/his/ade plate (Figure 1). Therefore we chose SD-leu/trp/his/ade plate as the screening condition. Although pBT3-SUC-APP co-transformed with NubG grew better on the SD-leu/trp plate, we noted some colonies on the SD-leu/trp/his/ade plate as well (Figure 1, red circles), which revealed that further optimization was required.
Figure 1.
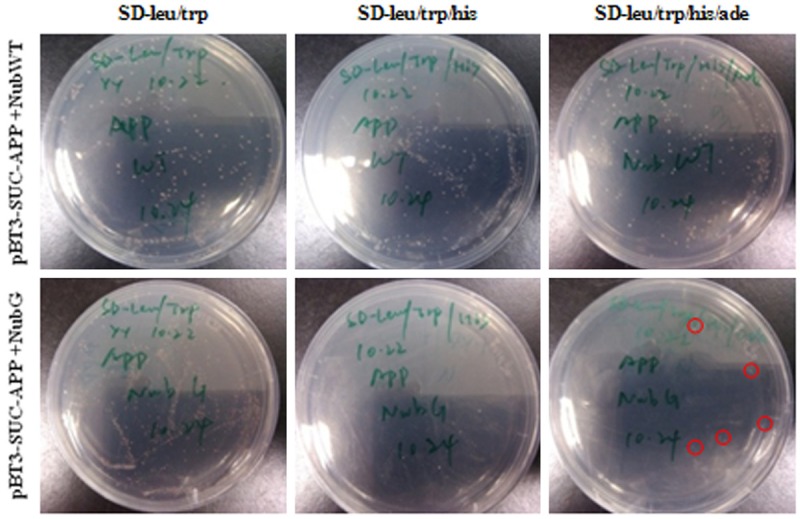
Colony numbers in SD-leu/trp, SD-leu/trp/his and SD-leu/trp/his/ade plates after 4 days cotransformation of pBT3-SUC-APP with NubWT and NubG. The red circle showed the colony in SD-leu/trp/his/ade plate cotransformed with pBT3-SUC-APP and NubG.
In order to avoid unwanted background, we transformed the cDNA library vector pPR3-N and pBT3-SUC-APP into NMY51 (large scale transformation) and added different levels of 3-aminotriazole (3-AT) to the SD-leu/trp/his/ade medium. As a competitive inhibitor of HIS3 gene product, 3-AT increased the screening stringency. We added 2.5, 5, 7.5, 10, 20 mM 3-AT respectively to the SD-leu/trp/his/ade plate for the screen of pBT3-SUC-APP. Four days after the co-transformation, the plates with 0-5 colonies are considered to have no or little background (Table 1). We therefore chose the SD-leu/trp/his/ade plate with 7.5 mM 3-AT (SD-leu/trp/his/ade/7.5 mM 3-AT) as the APP screening condition.
Table 1.
Optimizing the screening stringency by 3-AT
| Plasmids for Transformation | Concentration of 3-AT/mM | Number of colonies on the basic plate | Comment |
|---|---|---|---|
| pBT3-SUC-APP+pPR3-N | 2.5 | 8 | Strong background, unsuitable for screening |
| 5 | 7 | Strong background, unsuitable for screening | |
| 7.5 | 3 | Low background, suitable for screening | |
| 10 | 0 | No background, maybe a little stringent for screening | |
| 20 | 0 | No background, too stringent for screening |
cDNA Library transformation and selection of interactors with APP
After the co-transformation of pBT3-SUC-APP and cDNA Library, 15 positive clones grew on the SD-leu/trp/his/ade plate with 7.5 mM 3-AT. After the co-transformation of pBT3-SUC-APP and its screening products, we observed 8 SD-leu/trp/his/ade/7.5 mM 3-AT plates with white colonies of which the Whatman filter papers turned blue during the β-galactosidase assay, indicating these 8 proteins had interactions with APP in the yeast two hybrids experiment (Figure 2). The BLAST results of these 8 proteins are presented in Table 2. The DNA sequences of 8 proteins are shown in supplementary materials.
Figure 2.
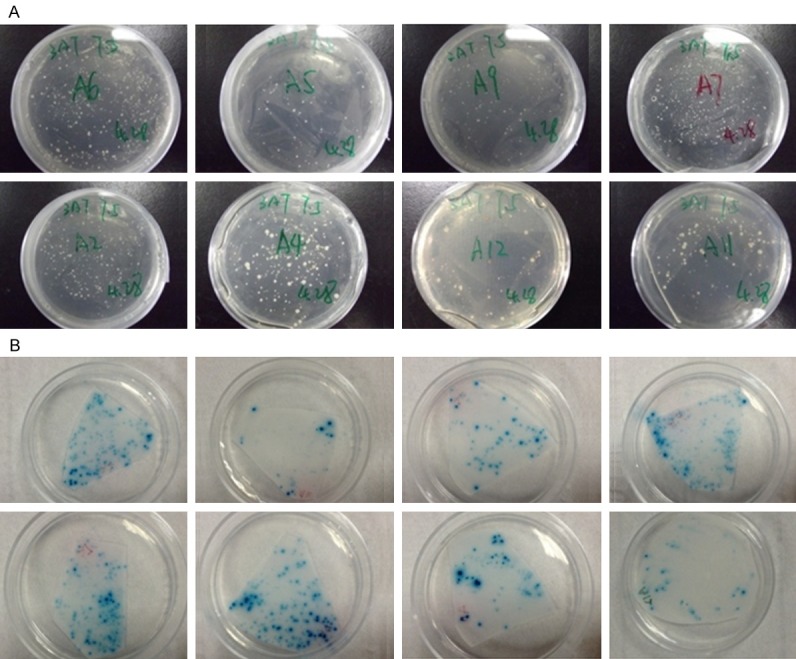
Confirmation of positive interactors of APP. A. Co-transformation of APP and its binding candidates in SD-leu/trp/his/ade/7.5 mM 3-AT plates. B. X-gal detection of APP and its binding candidates.
Table 2.
Candidates of APP interaction proteins
| Protein Name (Gene Name) |
|---|
| ATP synthase subunit a (MT-ATP6) |
| Vesicle-associated membrane protein-associated protein B/C (VAPB) |
| Stomatin-like protein 2, mitochondrial (STOML2) |
| Amyloid beta A4 precursor protein-binding family B member 1 (APBB1) |
| Emopamil binding protein (EBP) |
| Human DNA sequence from clone RP11-258C19 on chromosomeXp11.21-11.23, complete sequence |
| Double-stranded RNA-binding protein Staufen homolog 1 (STAU1) |
| Lanosterol synthase (LSS) |
Confirmation of interactions in mammalian system
In order to confirm the interaction between APP intracellular domain (APPin) and STAU1 in the mammalian system by Co-IP, we fused C-terminal of APP intracellular domain with mCherry, while STAU1 N-terminal of STAU1 with HA tag and C-terminal of it with EGFP. Then both plasmids were transfected into Hek293T cells and the cells were harvested for Co-IP experiment 24 hours later. EGFP was detected in the precipitation complex of mCherry (Figure 3A, red arrow). However, mCherry was not detected in the precipitation complex of EGFP (Figure 3B).
Figure 3.
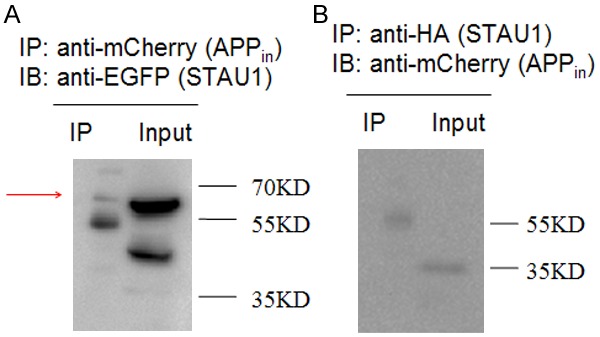
Co-immunoprecipitation of STAU1 with APP intracellular domain in HEK293T cells cotransfected with APPin-mCherry and HA-STAU1-EGFP. A. Cell lysates were immunoprecipitated with an anti-mCherry antibody for APP intracellular domain and probed with an anti-EGFP antibody for STAU1 (56 KD). B. Cell lysates were immunoprecipitated with an anti-HA antibody for STAU1 and probed with an anti-mCherry antibody for APP intracellular domain (35 KD).
STAU1 reduced Hek293T cell viability
The phenomenon that Hek293T cells displayed a poor cell status when transfected STAU1 alone during the Co-IP experiment was observed. Therefore we studied the effect of APP and STAU1 on Hek293T cell viability. C-terminal of full length APP was fused with mCherry (APPfl-mCherry). Compared with transfection of empty vector EGFP alone, either transfection of APPfl-mCherry alone or transfection of STAU1-EGFP alone resulted in the reduced cell viability of Hek293T cells (Figure 4).
Figure 4.
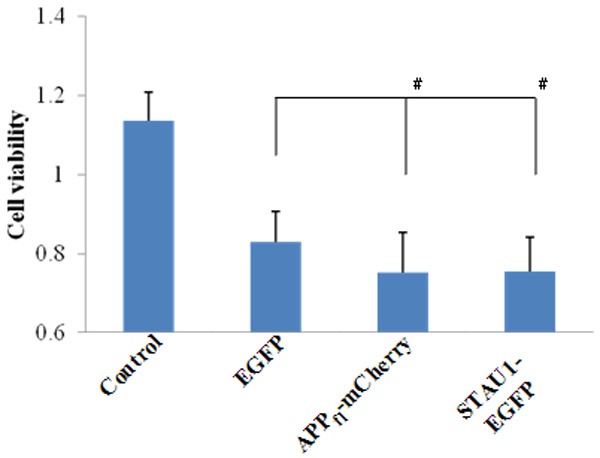
STAU1 decreased the cell viability in Hek293T cells. Data represent Mean ± SME. #P < 0.05.
Discussion
APP is mostly known for its generation of Aβ, which characterize the accumulation of amyloid plaque in AD mouse models. However, abundant evidence has shown that it participates in numerous signaling pathways in cells. It is understandable that an insight into these APP related biological process may help us reveal the mechanism of the generation of Aβ. As APP functions through interactions with other proteins, screen for its interacting proteins would be of significant importance. Using the DUALmembrane system, the yeast two-hybrid screen was performed and several genes in the Human Brain cDNA Library were obtained whose protein products have interaction with APP. Among them, STAU1 was demonstrated again in the mammalian system by Co-IP. STAU1 was previously reported to mediate mRNA degradation in mammalian cells. The results of our experiments showed that both APP and STAU1 can reduce the viability of mammalian cells. Therefore, it is possible that there is a link between this reduction in cell viability and the mRNA degradation process in which we hypothesize that APP is involved. However, limited by conditions, we did not demonstrate this hypothesis. In fact, a number of diseases are due to abnormalities in the regulation of mRNA, thus studies on this STAU1-mediated mRNA decay in the future may help us understand these diseases. So far, there is still much to be elucidated on how APP and STAU1 interact and whether this interaction has any regulation on APP that leads to the pathology of AD.
Acknowledgements
This work was supported by the National Science Foundation of China (NSFC) Fund for Distinguished Young Scholars (81425009), Beijing Natural Science Foundation (7142085) and Peking University Collaborative Fund (464-10606-00416).
Disclosure of conflict of interest
None.
References
- 1.Adlard PA, Cummings BJ. Alzheimer’s disease-a sum greater than its parts? Neurobiol Aging. 2004;25:725–733. doi: 10.1016/j.neurobiolaging.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Trans Med. 2011;3:77sr71–77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perl DP. Neuropathology of Alzheimer’s disease and related disorders. Neurol Clin. 2000;18:847–864. doi: 10.1016/s0733-8619(05)70229-2. [DOI] [PubMed] [Google Scholar]
- 4.Steiner H, Haass C. Intramembrane proteolysis by presenilins. Nat Rev Mol Cell Biol. 2000;1:217–224. doi: 10.1038/35043065. [DOI] [PubMed] [Google Scholar]
- 5.Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarr PE, Contursi C, Roncarati R, Noviello C, Ghersi E, Scheinfeld MH, Zambrano N, Russo T, D’Adamio L. Evidence for a role of the nerve growth factor receptor TrkA in tyrosine phosphorylation and processing of β-APP. Biochem Biophys Res Comm. 2002;295:324–329. doi: 10.1016/s0006-291x(02)00678-2. [DOI] [PubMed] [Google Scholar]
- 7.Johnsson N, Varshavsky A. Split ubiquitin as a sensor of protein interactions in vivo. Proc Natl Acad Sci U S A. 1994;91:10340–10344. doi: 10.1073/pnas.91.22.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park E, Maquat LE. Staufen-mediated mRNA decay. Wiley Interdiscip Rev RNA. 2013;4:423–435. doi: 10.1002/wrna.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]


