Abstract
Background: Understanding the pathophysiological process of calvarial bones development is important for the treatments on relative diseases such as craniosynostosis. While, the role of fibroblast growth factor (FGF) and bone morphogenetic protein (BMP) and how they interacted in osteoblast differentiation remain unclear. Methods: we digested bone fragments around the coronal and sagittal sutures from newborn rats to harvest suture cells. Markers expression at different osteoblast differentiation stage was analyzed by increasing FGF2 concentration and BMP2 blocking in these cells. Results: BMP2 expression could be stimulated by FGF2 in a dose and time dependent manner. FGF2 stimulation may decrease early marker of osteoblast differentiation (collagen type-1, COL-1) and increase the expression of continuously-expressed or late markers (alkaline phosphatase, ALP; osteocalcin, OC and bone sialoprotein, BSP) to accelerate mineralization. Inhibition of BMP2 signaling by Noggin weakens the effect of FGF2 on induction of later-stage osteoblastic differentiation of cranial suture cells. Conclusion: Our data suggest that BMP2 signaling is required for FGF2-dependent induction of later-stage of cranial suture cell osteoblastic differentiation.
Keywords: Fibroblast growth factor 2 (FGF2), bone morphogenetic protein 2 (BMP2), suture cells, osteoblast differentiation
Introduction
The majority of calvarial bones are formed by intramembranous ossification, which occurs without previous cartilage formation [1]. Mesenchymal cells enter the osteoblastic lineage and undergo spatial and temporal progression; early preosteoblasts proliferate and then mature to osteoblasts that can differentiate and produce a collagenous matrix. Subsequent mineralization of the matrix results in the formation of the calvarial flat bones [1]. Cranial sutures are a soft connective-tissue interface between mineralized calvarial bones that primarily function as an articulation to allow expansion of bone and brain growth. Sutures consist of cells with different functions, such as mesenchymal cells and fibroblast-like cells, which are sandwiched between two osteoblast-lined bone formation fronts [2]. Any perturbation in these processes induces premature or delayed fusion of the sutures, and abnormal cranial bone formation [1,2].
Craniosynostosis (CS), the premature fusion of cranial sutures, is the second most common craniofacial development abnormality that occurs in one in 2,500 live births [3]. Because the skull fails to expand normally, CS can have serious consequences for affected children, particularly when more than one suture is affected, including raised intracranial pressure, impaired vision and hearing, airway obstruction, intellectual disabilities, and long-term psychological effects associated with abnormal head shape [4]. Children with CS require long-term management and complex transcranial surgery, which may need to be repeated as children develop. Due to difficulties with airway patency, these children are prone to higher incidences of postoperative complications [5]. It is therefore highly desirable to develop non-surgical therapies based on a better understanding of the molecular regulation of skull bone growth and suture fusion.
CS may be sporadic or occur within over 150 syndromes, including Saethre-Chotzen, Apert, Pfeiffer, and Crouzon syndromes [3-5]. These syndromes are commonly inherited as autosomal dominant traits and are characterized by high genetic and clinical heterogeneity [3-5]. The molecular etiology consist of gene mutations, including gain-of-function mutations in the fibroblast growth factor receptors (FGFRs), FGFR2 and FGFR3, and a bone morphogenetic protein (BMP) effector transcription factor, MSX2 [6,7]. Haploinsufficiency of the transcription factor TWIST1 is associated with Saethre-Chotzen Syndrome and is manifested by craniosynostosis, which is the premature closure of the calvaria sutures [8]. Reports showed that twist1 homodimers enhanced FGF responsiveness of the cranial sutures and promoted suture closure [8]. These studies suggest that FGFs, FGFRs as well as BMP signaling pathway participated in the process of osteoblastic differentiation of calvarial bones. Recent studies have shown that, FGF/FGFR signaling enhances the intrinsic osteogenic potential by selectively expanding committed osteogenic cell populations as well as inversely regulating BMP-2 and noggin gene expression [9]; low-dose FGF-2 may facilitate BMP-2-induced ectopic bone formation by altering the expression of BMPRs on the surface of bone forming progenitor cells [10]. However, what the role of FGF and BMP and how they interacted in calvarial bone formation and cranial suture fusion is still unclear.
In this study, we digested bone fragments around the coronal and sagittal sutures from newborn rats to harvest suture cells. We increased the FGF2 concentration in cultures to mimic the activated signaling that occurs in vivo setting to investigate whether FGF2 can induce BMP2 expression in cranial suture cells or not. Additionally, we measured the markers expression of different osteoblast differentiation stage to analyze what the role of FGF and BMP and how they interacted in osteoblast differentiation of cranial suture cells.
Materials and methods
Suture samples and cell culture
All experimental protocols involving animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University School of Medicine. All animal experiments have been conducted according to the Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research released by the American Psychological Association.
Calvarial bones from newborn rats were dissected from surrounding muscle and soft tissue, including dura mater and periosteum, and washed in PBS containing penicillin and streptomycin. Next, we used a dissecting microscope and cut the bone and harvested the suture complex (suture mesenchyme plus 3mm of bone on either side of the patency coronal suture or sagittal suture). Suture complexes were digested in 0.1% collagenase (Type IV; SERVA, Germany) for 3 h at 37°C. Collagenase-digested suture complexes were washed extensively with basic media [low-glucose Dulbecco’s modified essential media (L-DMEM) supplemented with glutamine (292 mg/l), 10% heat inactivated fetal bovine serum (FBS), and 1% antibiotic suspension (100 IU/ml of penicillin and 100 µg/ml of streptomycin)]. After several washes, cells were collected by centrifugation, suspended in DMEM, plated in 10 cm culture plates, and cultured in basic media.
To study osteoblast differentiation, cells were cultured for up to 21 d in growth media containing ascorbic acid (AA; 50 µg/ml) and β-glycerophosphate (BGP; 10 mM). Media were changed every 3 days.
Cells treatment
To study the role of FGF and BMP and how they interacted in osteoblast differentiation of cranial suture cells, the isolated cells were treated by various concentrations of FGF2 (Sino Biological Inc, Beijing, China), BMP2 (Sino Biological Inc, Beijing, China) and/or Noggin (Sino Biological Inc, Beijing, China).
Alkaline phosphatase staining and in vitro mineralization assays
Cells (1 × 104) were seeded in 24-well plates in basic media. After 24 h, the cells were induced to differentiate with AA and BGP. Histochemical alkaline phosphatase staining was performed according to the manufacturer’s instructions (Takara, Japan). To detect mineralized nodules formed in vitro, cultures were fixed in 4% paraformaldehyde for 10 min and rinsed with water. Cells were stained with 10% alizarin red solution and incubated at 37°C for 30 min. Mineralized nodules were identified as red spots on the culture dish.
Total RNA isolation and quantitative reverse-transcription PCR
Total cellular RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA (1 µg) was used to synthesize cDNA using M-MLV reverse transcriptase (Promega, Madison, WI) according to the manufacturer’s protocol. Quantitative real-time PCR was performed on a Light-Cycler machine (ABI, Foster City, CA, USA) using specific primers sets. The primers for rat genes are as follows: rat BMP2, Forward: 5’ CGT CAA GCC AAA CAC AAA CA 3’, Reverse: 5’ AGT CAT TCC ACC CCA CAT CA 3’; rat COL1, Forward: 5’ CGT GGA AAC CTG ATG TAT GCT 3’, Reverse: 5’ ACT CCT ATG ACT TCT GCG TCT G 3’; rat OC, Forward: 5’ GAA CAG ACA AGT CCC ACA CAG 3’, Reverse: 5’ CAG GTC AGA GAG GCA GAA TG 3’; rat BSP, Forward: 5’ TGA AGG GTC ATC GTA AAT CAG 3’; Reverse: 5’ CAG GGT ATG TTA GGG TGG TTA G 3’. GAPDH was used as internal control for reverse transcription. A series of quantified GAPDH plasmids (Biovisualab, Shanghai, China) were used as external controls and qualitative standards.
Statistics
Continuous variables are presented as the means ± standard deviations. Differences were analyzed by student’s t-test. A one-tailed P value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS ver. 17.0.0 (SPSS, Chicago, IL, USA).
Results
FGF2 induces BMP2 expression in a dose and time-dependent manner
To examine the effect of FGF2 on BMP2 gene expression, cranial suture cells were treated with various concentrations of FGF2 (Figure 1A). After 24 h, BMP2 gene expression was analyzed using qRT-PCR. As showed in Figure 1A, BMP2 expression increased in a dose-dependent manner up to 50 ng/ml. when FGF2 concentration exceeded 50 ng/ml, BMP2 expression reached a plateau (Figure 1A). To evaluate the time course of FGF2 stimulation on BMP2 expression, cranial suture cells were treated with 10 ng/ml FGF2, cell RNA was harvested at various time points after treatment to examine BMP2 expression. We found that BMP2 expression increased in a time-dependent manner (Figure 1B). In contrast, BMP2 treatment had no effect on FGF2 expression (data not shown). In conclusion, these data suggested that FGF2 might induce BMP2 expression, but BMP2 had no effect on FGF2 expression.
Figure 1.
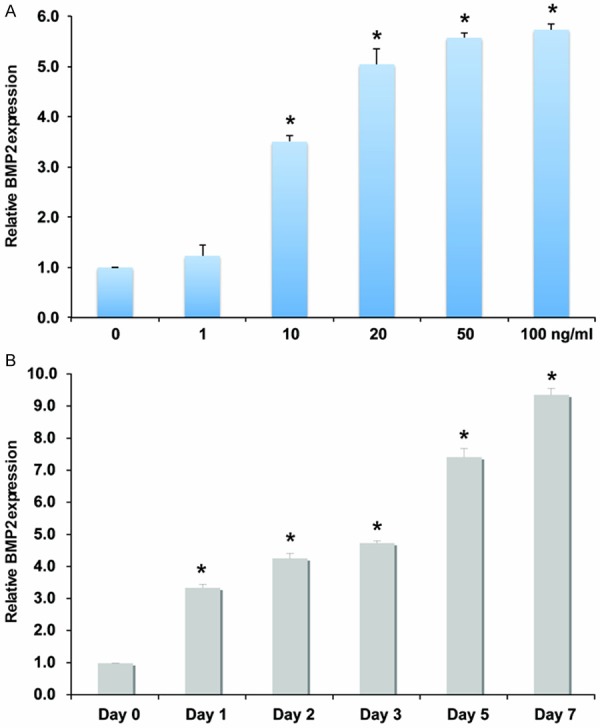
FGF2 induces BMP2 expression in cranial suture cells. A: qRT-PCR analysis of BMP2 in cranial suture cells after treatment with different doses of FGF2. *indicates the significance when compared with no FGF2. B: qRT-PCR analysis of BMP2 in cranial suture cells after treatment with 10 ng/ml FGF2 at different time points. *indicates the significance when compared with day 0.
Treatment of FGF2 promotes later-stage osteoblast differentiation and suppresses early marker of osteoblast differentiation
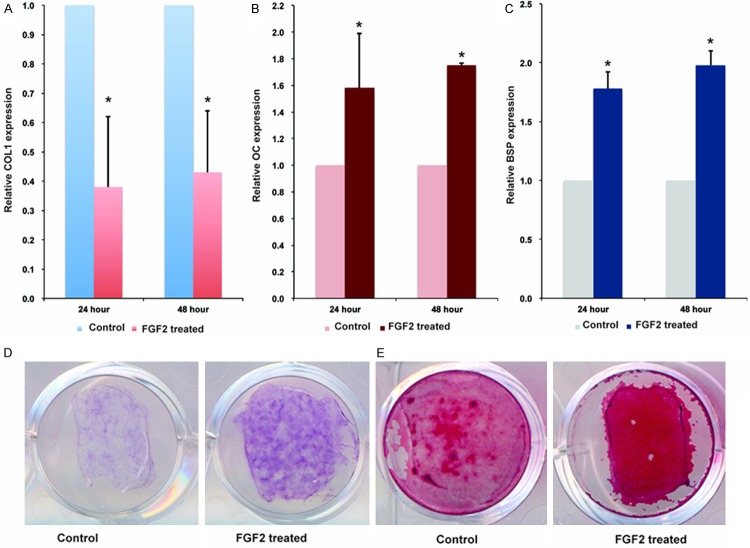
In order to determine whether FGF2 will alter osteoblast differentiation, we treated cranial suture cells with 10 ng/ml FGF2. After 24 or 48 h, the expression of osteoblast differentiation markers were analyzed. Treatment with FGF2 decreased the expression levels of collagen type-1 (COL-1), a marker that is expressed early during osteoblast differentiation, at 24 and 48 h (Figure 2A). Expression of osteocalcin (OC), a late osteogenic differentiation marker, increased upon the addition of FGF2 (Figure 2B). In addition, expression of bone sialoprotein (BSP), a marker of mature osteoblasts, was also increased in the presence of FGF2 (Figure 2C). ALP, a marker that is expressed early in osteoblast differentiation but continues to be up-regulated at later stages, was also significantly enhanced after FGF2 treatment (Figure 2D). Finally, mineralized nodule staining showed that mineralized nodule was also increased upon the addition of FGF2 (Figure 2E). Thus, we conclude that FGF2 stimulation may decrease early marker of osteoblast differentiation (COL-1) and increase the expression of continuously-expressed and late markers (ALP, OC, and BSP) to accelerate mineralization.
Figure 2.
Treatment of FGF2 promotes later-stage osteoblast differentiation and suppresses early markers of osteoblast differentiation. qRT-PCR analyses of osteoblast differentiation markers after cranial suture cells were treated with 10 ng/ml for 24 and 48 h. the mRNA level of control was defined as 1, mRNA levels of cells treated with FGF2 were converted by dividing by mRNA level of control. A: COL-1. B: OC. C: BSP. D: ALP staining. E: Mineralized nodule staining of cranial suture cells after treated with 10 ng/ml FGF2 for 7 days. *Indicates the significance when compared with controls.
BMP2 signals are required for FGF2-dependent induction of later-stage osteoblast differentiation
We have shown that FGF2 could stimulate BMP2 expression, decrease early marker of osteoblast differentiation (COL-1) and increase the expression of continuously-expressed and late markers (ALP, OC, and BSP) to accelerate mineralization. Therefore, we hypothesized that BMP2 signals are required for FGF2-dependent induction of later-stage osteoblast differentiation.
To evaluate this hypothesis, we used the natural BMP antagonist, Noggin, to inhibit BMP signals in cranial suture cells. Noggin is an extracellular BMP-binding protein that forms inactive complexes with BMP2, 4, and to a lesser extent, BMP7 [11]. As showed in Figure 3, when the cranial suture cells were treated with 10 ng/ml FGF2 and 1 µg/ml Noggin, 24 hours after the treatment, compared with the cells treated with FGF2 alone, OC and BSP expression was significantly decreased to 71 ± 9% and 68 ± 11% respectively (Figure 3A and 3B). ALP staining and mineralized nodule staining was showed that ALP and mineralized nodule were also suppressed by Noggin (Figure 3C and 3D). Taken together, these data suggest that BMP2 signals are required for FGF2-dependent induction of later-stage osteoblast differentiation in cranial suture cells.
Figure 3.
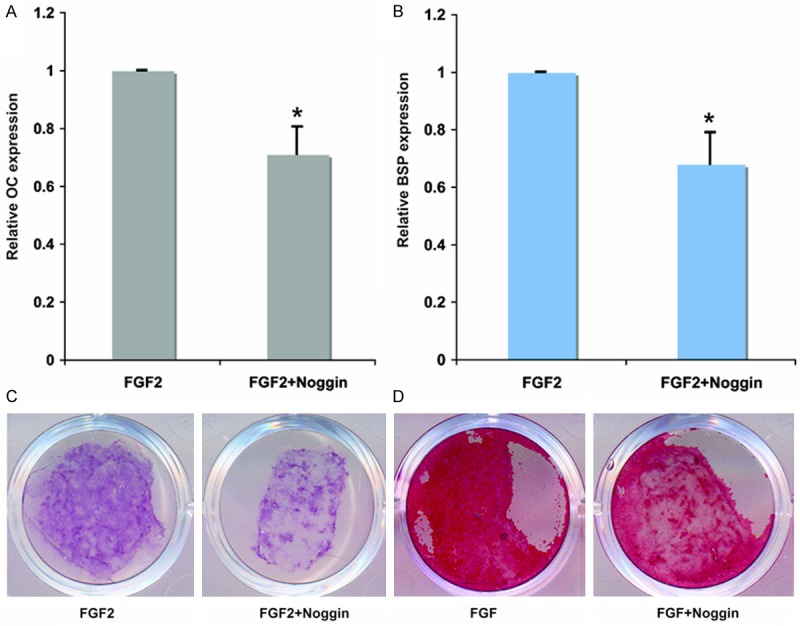
Inhibition BMP2 signaling by Noggin weakens the effect of FGF2 on inducing later-stage cranial suture cell osteoblastic differentiation. qRT-PCR analyses of later-stage osteoblastic differentiation markers, the mRNA level of cells treated with FGF2 only was defined as 1; mRNA levels of cells treated with FGF2 plus Noggin were converted by dividing by mRNA level of cells treated with FGF2 only. A: The changes of OC expression after 24 hours treated with 10 ng/ml FGF2 or co-treated with 10 ng/ml FGF2 and 1 μg/ml Noggin. B: The changes of BSP expression after 24 hours treated with 10 ng/ml FGF2 or co-treated with 10 ng/ml FGF2 and 1 μg/ml Noggin. C: ALP staining of cranial suture cells treated for 7 days with 10 ng/ml FGF2 or co-treated with 10 ng/ml FGF2 and 1 μg/ml Noggin. D: Mineralized nodule staining of cranial suture cells treated for 7 days with 10 ng/ml FGF2 or co-treated with 10 ng/ml FGF2 and 1 μg/ml Noggin. *Indicates the significance when compared with cells treated with FGF2 alone.
Discussion
Calvarial bone is formed by an intramembranous bone-forming process that involves many signaling molecules [1,2]. Constitutive activation of the FGF signaling pathway accelerates osteoblast differentiation and results in premature cranial suture closure [1,2]. BMP signaling pathways are also involved in the bone formation process [1-3]. However, the relationship between these two main signaling cascades is still unclear. In this study, we found that FGF2 treatment of cranial suture cells stimulated BMP2 gene expression. However, BMP2 treatment did not alter FGF2 expression. These observations indicate that BMP2 is downstream of FGF2 signaling. We further found that FGF2 stimulated BMP2 expression in a dose- and time-dependent manner. These findings are similar to the results of Choi et al. [12], but disagree with the data from Biver et al. [13]. Biver et al. found expression of BMPs and their receptors are up-regulated during human mesenchymal stem cell (HMSC) osteogenic differentiation, but FGF2 completely blocked the increase of BMP2, BMP4, BMPR1A, and BMPR1B. These differences may be caused by different cell types or different culture conditions. BMP2 has several roles in calvarial bone formation, acting at different stages to drive differentiation of mesenchymal osteoprogenitors into committed osteoblasts and promote mineralization of osteoblasts [14-17]. Recent studies have suggested that dysregulation of BMP2 activity is associated with suture fusion and craniosynostosis. Notably, Boston-type craniosynostosis appears to result from a gain-of-function mutation in MSX2 [18,19], a downstream transcriptional effector of BMP signaling. Animal models with high levels of exogenous BMP2 [20], or with constitutively active BMP type I receptors, cause premature suture fusion and craniosynostosis [21]. Therefore, we hypothesized that increased BMP2 expression would enhance BMP signaling pathways and accelerate cranial suture cell osteoblast differentiation, resulting in premature cranial suture closure. This may be how constitutively activated FGFR mutations cause craniosynostosis.
There is evidence indicating that mutations in FGFR1-FGFR3 cause craniosynostosis syndromes via ligand-independent constitutive activation of receptors [22]. In addition, analyses of the Bulgy-eye mouse, which is generated by an insertional mutation at the FGF3/FGF4 locus, indicate that ligand overexpression may also cause a craniosynototic phenotype [22-24]. There is considerable evidence indicating that FGF2 overexpression could also result in a craniosynototic phenotype. Kim et al. applied FGF2 via beads to the osteogenic front and reported that suture closure is accelerated [22]. Greenwald et al. used a dominant-negative adenoviral FGFR1 construct injected into rats in utero. They found physiological posterior frontal suture fusion was inhibited, and injection of a construct that increased FGF2 biological activity resulted in the fusion of normal patent coronal suture [23]. Similarly, FGF2-treated posterior frontal sutures in rats show enhanced suture fusion [24].
BMPs were originally identified by their ability to induce ectopic bone formation [25]. They have subsequently been shown to be important signaling molecules in many developmental processes, including cranial suture fusion and patency [21]. A mutation in MSX2, a BMP effector transcription factor, accounts for Boston-type craniosynostosis. A recent study in a rabbit model system found that high levels of BMP2 causes premature suture fusion [20]. There is also considerable data suggesting that FGFs-FGFRs and BMPs interact during bone formation. When endogenous FGF signaling was blocked using a virally-transduced dominant-negative FGFR, BMP-2 gene expression was drastically reduced, demonstrating that endogenous FGF/FGFR signaling is a positive upstream regulator of BMP-2 in calvarial osteoblasts. Expression of the BMP antagonist Noggin was inhibited by FGF2 and FGF9 [9]. Nakamura et al. reported that FGF2 facilitates BMP-2-induced ectopic bone formation by altering BMP receptor expression on the surface of bone-forming progenitor cells [10].
We harvested primary cranial suture cells from the suture mesenchyme plus 3 mm of bone on either side of the patency coronal suture or sagittal suture. Thus, we harvested cells at all stages of osteoblast differentiation [13]. Bone was dissected free of dura mater to avoid the influence of growth factors secreted from dura mater [26]. We found that FGF2 stimulation decreased expression of early markers of osteoblast differentiation (COL-1) but increased expression of later markers (OC and BSP) and continuously-expressed markers (ALP). FGF2 stimulation also accelerated mineralization. Our data suggest that FGF2 and BMP2 cooperate during later-stage osteoblast differentiation. It has been shown that FGF-FGFR induces osteoblast differentiation through PKC signaling and ERK-MAPK signaling [27,28]. Because previous research has shown that FGF2 could induce BMP2 expression and co-treatment of FGF2 and BMP2 could induce later-stage osteoblast differentiation, we hypothesize that FGF2 induce osteoblast differentiation through BMP signaling. Our data showed that when BMP2 signaling was inhibited by Noggin, it weakened the effect of FGF2 on OC and BSP expression, suggesting that BMP2 is required for FGF-induction of later-stage osteoblast differentiation. Our work suggests that the FGF2/BMP2 axis controls osteoblast differentiation, with FGF2 enhancing BMP2 expression and BMP2 providing signals that enhance FGF2-dependent induction of later-stage differentiation.
This is an in vitro study, and we did not examine other influence factors (such as intracranial pressure) that are very important for osteoblast differentiation. Therefore, our results should be verified in vivo.
Disclosure of conflict of interest
None.
Abbreviations
- ALP
Alkaline phosphatase
- BMP2
Bone morphogenetic protein 2
- BSP
Bone sialoprotein
- COL-1
Collagen-1
- FGF2
Fibroblast growth factor 2
- OC
Osteocalcin
- RT-PCR
Real-time quantitative polymerase chain reaction
References
- 1.Mansukhani A, Bellosta P, Sahni M, Basilico C. Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol. 2000;149:1297–1308. doi: 10.1083/jcb.149.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong L, Mao JJ. Tissue-engineered rabbit cranial suture from autologous fibroblasts and BMP2. J Dent Res. 2004;83:751–756. doi: 10.1177/154405910408301003. [DOI] [PubMed] [Google Scholar]
- 3.Wilkie AO. Epidemiology and genetics of craniosynostosis. Am J Med Gene. 2000;90:82–84. doi: 10.1002/(sici)1096-8628(20000103)90:1<82::aid-ajmg15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Dwivedi PP, Grose RH, Filmus J, Hii CS, Xian CJ, Anderson PJ, Powell BC. Regulation of bone morphogenetic protein signalling and cranial osteogenesis by Gpc1 and Gpc3. Bone. 2013;55:367–376. doi: 10.1016/j.bone.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Sinkueakunkit A, Chowchuen B, Kantanabat C, Sriraj W, Wongswadiwat M, Bunsangjaroen P, Chantawong S, Wittayapairoj A. Outcome of anesthetic management for children with craniofacial deformities. Pediatr Int. 2013;55:360–365. doi: 10.1111/ped.12080. [DOI] [PubMed] [Google Scholar]
- 6.Muenke M, Gripp KW, McDonald-McGinn DM, Gaudenz K, Whitaker LA, Bartlett SP, Markowitz RI, Robin NH, Nwokoro N, Mulvihill JJ, Losken HW, Mulliken JB, Guttmacher AE, Wilroy RS, Clarke LA, Hollway G, Adès LC, Haan EA, Mulley JC, Cohen MM Jr, Bellus GA, Francomano CA, Moloney DM, Wall SA, Wilkie AO. A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. Am J Hum Genet. 1997;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkie AO. Craniosynostosis: genes and mechanisms. Hum Mol Genet. 1997;6:1647–1656. doi: 10.1093/hmg/6.10.1647. [DOI] [PubMed] [Google Scholar]
- 8.Connerney J, Andreeva V, Leshem Y, Mercado MA, Dowell K, Yang X, Lindner V, Friesel RE, Spicer DB. Twist1 homodimers enhance FGF responsiveness of the cranial sutures and promote suture closure. Dev Biol. 2008;318:323–334. doi: 10.1016/j.ydbio.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakhry A, Ratisoontorn C, Vedhachalam C, Salhab I, Koyama E, Leboy P, Pacifici M, Kirschner RE, Nah HD. Effects of FGF-2/-9 in calvarial bone cell cultures: differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone. 2005;36:254–266. doi: 10.1016/j.bone.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y, Tensho K, Nakaya H, Nawata M, Okabe T, Wakitani S. Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone. 2005;36:399–407. doi: 10.1016/j.bone.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 12.Choi KY, Kim HJ, Lee MH, Kwon TG, Nah HD, Furuichi T, Komori T, Nam SH, Kim YJ, Kim HJ, Ryoo HM. Runx2 regulates FGF2-induced Bmp2 expression during cranial bone development. Dev Dyn. 2005;233:115–121. doi: 10.1002/dvdy.20323. [DOI] [PubMed] [Google Scholar]
- 13.Biver E, Soubrier AS, Thouverey C, Cortet B, Broux O, Caverzasio J, Hardouin P. Fibroblast growth factor 2 inhibits up-regulation of bone morphogenic proteins and their receptors during osteoblastic differentiation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2012;427:737–742. doi: 10.1016/j.bbrc.2012.09.129. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Harris MA, Rossini G, Dunstan CR, Dallas SL, Feng JQ, Mundy GR, Harris SE. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60:283–290. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- 15.Hay E, Hott M, Graulet AM, Lomri A, Marie PJ. Effects of bone morphogenetic protein-2 on human neonatal calvaria cell differentiation. J Cell Biochem. 1999;72:81–93. doi: 10.1002/(sici)1097-4644(19990101)72:1<81::aid-jcb9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Marie PJ, Debiais F, Hay E. Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling. Histol Histopathol. 2002;17:877–885. doi: 10.14670/HH-17.877. [DOI] [PubMed] [Google Scholar]
- 17.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 18.Jabs EW, Müller U, Li X, Ma L, Luo W, Haworth IS, Klisak I, Sparkes R, Warman ML, Mulliken JB. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell. 1993;75:443–450. doi: 10.1016/0092-8674(93)90379-5. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Golden S, Wu L, Maxson R. The molecular basis of Boston-type craniosynostosis: the Pro148->His mutation in the N-terminal arm of the MSX2 homeodomain stabilizes DNA binding without altering nucleotide sequence preferences. Hum Mol Genet. 1996;5:1915–1920. doi: 10.1093/hmg/5.12.1915. [DOI] [PubMed] [Google Scholar]
- 20.Kinsella CR Jr, Cray JJ, Durham EL, Burrows AM, Vecchione L, Smith DM, Mooney MP, Cooper GM, Losee JE. Recombinant human bone morphogenetic protein-2-induced craniosynostosis and growth restriction in the immature skeleton. Plast Reconstr Surg. 2011;127:1173–1181. doi: 10.1097/PRS.0b013e318205f2b4. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu Y, Yu PB, Kamiya N, Pan H, Fukuda T, Scott GJ, Ray MK, Yamamura K, Mishina Y. Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. J Bone Miner Res. 2013;28:1422–1433. doi: 10.1002/jbmr.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Rice DP, Kettunen PJ, Thesleff I. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 1998;125:1241–1251. doi: 10.1242/dev.125.7.1241. [DOI] [PubMed] [Google Scholar]
- 23.Greenwald JA, Mehrara BJ, Spector JA, Warren SM, Fagenholz PJ, Smith LE, Bouletreau PJ, Crisera FE, Ueno H, Longaker MT. In vivo modulation of FGF biological activity alters cranial suture fate. Am J Pathol. 2001;158:441–452. doi: 10.1016/s0002-9440(10)63987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moursi AM, Winnard PL, Winnard AV, Rubenstrunk JM, Mooney MP. Fibroblast growth factor 2 induces increased calvarial osteoblast proliferation and cranial suture fusion. Cleft Palate Craniofac J. 2002;39:487–496. doi: 10.1597/1545-1569_2002_039_0487_fgfiic_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 25.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 26.Opperman LA, Passarelli RW, Morgan EP, Reintjes M, Ogle RC. Cranial sutures require tissue interactions with dura mater to resist osseous obliteration in vitro. J Bone Miner Res. 1995;10:1978–1987. doi: 10.1002/jbmr.5650101218. [DOI] [PubMed] [Google Scholar]
- 27.Debiais F, Lemonnier J, Hay E, Delannoy P, Caverzasio J, Marie PJ. Fibroblast growth factor-2 (FGF-2) increases N-cadherin expression through protein kinase C and Src-kinase pathways in human calvaria osteoblasts. J Cell Biochem. 2001;81:68–81. doi: 10.1002/1097-4644(20010401)81:1<68::aid-jcb1024>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Park J, Park OJ, Yoon WJ, Kim HJ, Choi KY, Cho TJ, Ryoo HM. Functional characterization of a novel FGFR2 mutation, E731K, in craniosynostosis. J Cell Biochem. 2012;113:457–464. doi: 10.1002/jcb.23368. [DOI] [PubMed] [Google Scholar]