Abstract
Objective: This study sought to investigate the role of the forkhead transcription factor FOXO3a in the prognosis of stage II/III gastric cancer patients. Materials and methods: A single-institution cohort of 289 patients with stage II/III gastric cancer was studied. Formalin-fixed paraffin-embedded tumor and adjacent normal specimens were used for tissue microarray construction. Tissue sections were immunostained with FOXO3a. Microscopic evaluation to assess the presence and localization of FOXO3a in tumor and adjacent normal tissues was performed. Results were analyzed for association with clinicopathological characters and overall survival (OS). Results: FOXO3a expression was significantly higher in tumor tissues compared with adjacent normal tissues, and nuclear FOXO3a staining was observed to be more common in tumor samples than adjacent normal tissues. Poorer prognosis was seen in patients with tumors harboring lower expression of FOXO3a and also patients with adjacent normal tissues harboring higher expression of FOXO3a. High expression of FOXO3a in tumor tissues served as a good prognostic marker with multivariate hazard ratio (HR) of 0.737 (95% CI, 0.574 to 0.947; P = 0.017) for OS. Conclusion: The expression of FOXO3a was upregulated and activated in gastric cancer tissues, and was significantly associated with a favorable prognosis in stage II/III gastric cancer patients.
Keywords: FOXO3a, gastric cancer, prognosis
Introduction
Gastric cancer is one of the most common human malignant diseases and remains the second leading cause of cancer-related death worldwide [1]. Despite improvements in surgical approaches and chemoradiotherapy, survival of patients with advanced stage remains poor, with only around 15% of all patients reaching 5 years [2,3]. After the success of trastuzumab in patients with HER2-positive gastric cancer [4], there is an increasing interest in identifying new molecular targets for treatment. However, an adequate knowledge of the target and the potential clinical effects from its inhibition are required before considering systemic therapy.
FOXO3a, a member of the Forkhead box O (FoxO) transcription factor family, is a downstream target of the PI3K/AKT pathway. When FOXO3a is unphosphorylated, it locates in the nuclear and can activate or repress multiple target genes throughout its transcriptional activity. Once phosphorylated by Akt, FOXO3a rapidly relocates from the nucleus to the cytoplasm, where the phosphorylated FOXO3a is then be ubiquitylated, undergoing degradation [5]. FOXO3a can also be phosphorylated by IKK and ERK in response to growth factor and insulin stimulation, leading to suppression of its activity [6].
FOXO3a has been demonstrated to be a tumor suppressor in various cancers. FOXO3a overexpression has been shown to inhibit breast tumor growth and tumor size [7,8]. Furthermore, the patients with FOXO3a-high breast tumors showed significantly increased survival compared with patients with FOXO3a-low tumors [9]. Downregulation of FOXO3a is reported to promote tumor metastasis and is associated with metastasis-free survival of patients with clear cell renal cell carcinoma [10], while upregulation of FOXO3a results in suppression of bladder cancer proliferation [11]. These studies suggest that FOXO3a function as a tumor suppressor and, therefore, may serve as a direct or indirect therapeutic target. However, emerging evidence also has shown that FOXO3a promotes tumor cell survival under stress conditions, such as oxidative stress [12] and serum deprivation [13]. A recent publication has revealed FOXO3a, with a nuclear interaction of β-catenin, as a promoter of metastatic progression in colon cancer [14]. Therefore, the role of FOXO3a has yet to be definitively elucidated in a specific cancer before it is employed as a target for cancer therapy.
In the present study, we investigated the expression of FOXO3a in both gastric carcinomas and its adjacent normal tissues, and analyzed correlations of the protein expression with patient characteristics, clinical and pathological variables as well as patient outcome.
Methods
Clinical samples
289 patients with histologically confirmed gastric cancer seen at Zhongshan Hospital, Fudan University between January 2004 and December 2008 were recruited for this study. All patients underwent D2 radical resection without preoperative chemotherapy or radiotherapy. Their diagnoses were independently re-reviewed by two gastrointestinal pathologists, classified by using WHO criteria, and were staged II or III according to updated 2011 American Joint Committee on Cancer/TNM criteria post-operatively. All patients’ tumor, along with adjacent normal tissue samples were collected after radical resection and fixed in 10% buffered formalin and routinely processed for paraffin embedding.
For each patient recruited for this study, information was collected through reading medical charts and following up patients. This study was approved by the Research Ethics Committee of Zhongshan Hospital, Fudan University.
Histological and immunohistochemical analysis
20 blocks of tissue microarray containing gastric cancers and adjacent noncancerous tissues were constructed using a microarrayer. Serial 4-μm sections were obtained from each block, with the first resultant slide being stained for HE to confirm pathologic diagnosis, and the subsequent slides stained for further immunohistochemistry.
Tissue microarray slides were routine deparaffinization and rehydrated; The rabbit polyclonal antibody against FOXO3a (CST 12829), diluted 1:100, was used as primary antibody. For antigen retrieval, the slides were heated at 98°C in an ethylene diamine tetra-acetic acid buffer (pH 9.0) for a total of 25 min and cooled naturally to room temperature. The slides were incubated with the primary antibody overnight at 4°C, then stained using a highly sensitive strepavidin-biotin-peroxidase detection system and counterstained with hematoxylin. A negative control was also incorporated using pre-immune IgG instead of the primary antibody.
Immunoreactive staining was characterized quantitatively according to the percentage of positive cells and staining intensity without prior knowledge of any of the clinicopathological information. We assigned the following proportion scores: 0 if 0% of the tumor cells showed positive staining, 1 if 0% to 10% stained, 2 if 11% to 50% stained, 3 if 51% to 75% stained, and 4 if 75% to 100% stained. We rated the intensity of staining on a scale of 0 to 3: 0, negative; 1, weak; 2, moderate; and 3, strong. We then combined the proportion and intensity scores to obtain a total score (range 1-6). All patients were designated into low (score 1-2), moderate (score 3-4) and high (score 5-6) groups based on FOXO3a expression. The test χ2 was used to determine the association of FOXO3a staining with tumor and adjacent normal tissues.
Statistical analysis
Survival was calculated starting from the date of surgery to date of death or last follow-up. Survival curves for FOXO3a were plotted using the Kaplan-Meier and compared using the log-rank test. Cox proportional hazard models were used for univariate and multivariate analysis to test clinical and genetic features for their associations with overall survival (OS). In the multivariate Cox model, variables with P < 0.1 from the univariate model were included. In addition to FOXO3a expression, the following variables were considered: age, sex, grading, histologic subtype according to Lauren’s classification, tumor location, American Joint Committee on Cancer tumor stage (7th edition), and presence of lymphovascular invasion. All statistical analyses were performed using SPSS for Windows v.17.0 (SPSS, Chicago, IL). All results were considered significant at two-sided P < 0.05 value.
Results
FOXO3a immunohistochemistry in gastric cancer tissues and adjacent normal tissues
We studied the expression pattern of FOXO3a using immunohistochemical staining on a panel of gastric cancer samples and their adjacent normal tissues. Representative expression patterns in both cancer and noncancerous samples were shown in Figure 1. The staining of FOXO3a revealed both nuclear and cytoplasmic localization in tumor and adjacent normal tissues. FOXO3a expression was significantly higher in tumor tissues compared with adjacent normal tissues (P < 0.01), and nuclear FOXO3a staining was observed to be more common in tumor samples than adjacent normal tissues (P < 0.01, Table 1).
Figure 1.
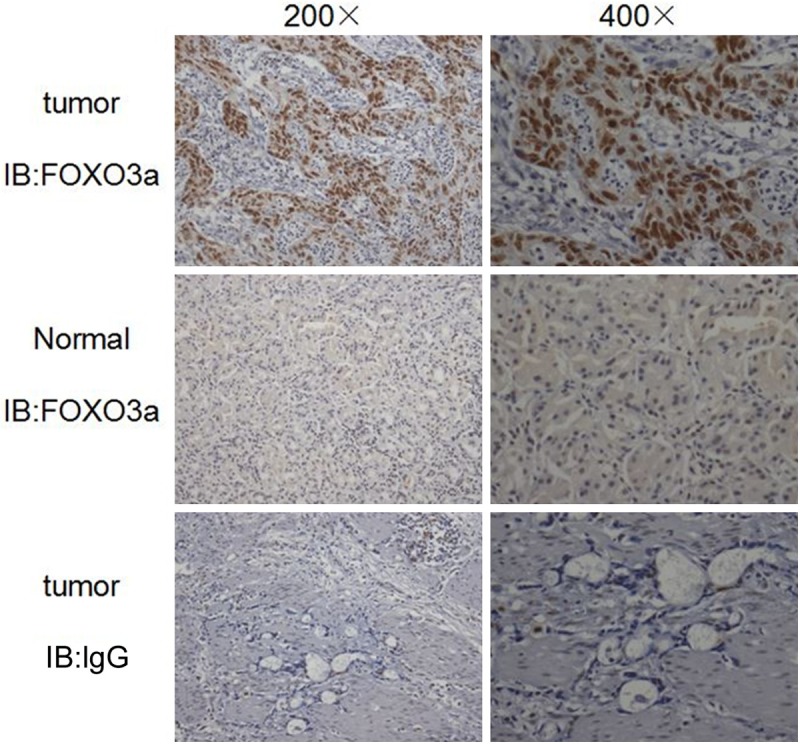
Expression of Foxo3a in gastric cancer tissues and adjacent normal tissues. Immunohistochemical (IHC) staining with antibody to Foxo3a was performed on 289 gastric cancer specimens. Images of representative staining are shown. IgG was control. Magnification, ×200, ×400.
Table 1.
Expression pattern of FOXO3a in tumor and adjacent normal tissues
| Tumor tissues (%) | Adjacent normal tissues (%) | P value | |
|---|---|---|---|
| FOXO3a expression | |||
| Low | 176 (60.9) | 224 (77.5) | < 0.01 |
| Moderate | 65 (22.5) | 58 (20.1) | |
| High | 48 (16.6) | 7 (2.4) | |
| FOXO3a location | |||
| Nuclear | 78 (27.0) | 30 (10.4) | < 0.01 |
| Cytoplasm | 49 (17.0) | 50 (17.3) | |
| None | 162 (56.0) | 209 (72.3) |
Relationship between FOXO3a expression and the clinicopathological features of gastric cancer patients
According to the expression of FOXO3a in cancer samples, all cases of stage II and III gastric cancer were divided into low FOXO3a expression group (n = 176), moderate FOXO3a expression group (n = 65), and high FOXO3a expression group (n = 48). The expression of FOXO3a in cancer tissues showed strong negative correlation with tumor invasion (T stage, P < 0.05), although no associations were found between FOXO3a expression and other clinicopathological features (Table 2).
Table 2.
Association between FOXO3a expression in tumor tissues and clinicopathological variables of the studied gastric cancer patients
| Variables | FOXO3a expression in tumor tissues | P | ||
|---|---|---|---|---|
|
| ||||
| Low (n=176) | Moderate (n=65) | High (n=48) | ||
| Age (years) | > 0.05 | |||
| < 65 | 113 | 39 | 22 | |
| ≥ 65 | 63 | 26 | 26 | |
| Sex | > 0.05 | |||
| Male | 121 | 47 | 35 | |
| Female | 55 | 18 | 13 | |
| Primary tumor | > 0.05 | |||
| Noncardia | 151 | 55 | 42 | |
| Cardia | 24 | 10 | 6 | |
| Histologic subtype | > 0.05 | |||
| Intestinal | 112 | 41 | 36 | |
| Nonintestinal | 64 | 24 | 12 | |
| Grading | > 0.05 | |||
| Well | 11 | 4 | 2 | |
| Moderate | 30 | 12 | 13 | |
| Poor | 134 | 49 | 33 | |
| Tumor invasion | < 0.05 | |||
| T1/2 | 10 | 4 | 10 | |
| T3 | 71 | 22 | 17 | |
| T4 | 95 | 39 | 21 | |
| Lymph nodes | > 0.05 | |||
| pN0 | 28 | 12 | 9 | |
| pN1 | 33 | 10 | 15 | |
| pN2 | 36 | 17 | 11 | |
| pN3 | 79 | 26 | 13 | |
| stage | >0.05 | |||
| IIA | 20 | 9 | 10 | |
| IIB | 28 | 9 | 14 | |
| IIIA | 36 | 14 | 7 | |
| IIIB | 42 | 12 | 9 | |
| IIIC | 50 | 21 | 8 | |
| Lymph vascular invasion | > 0.05 | |||
| Absence | 121 | 40 | 33 | |
| Presence | 55 | 25 | 15 | |
Univariate analysis of prognostic factors in stage II and III CRC patients
The median follow-up period for the patients studied was 47 months, with a range of 2 to 91 months. FOXO3a expression in both tumor and adjacent normal tissues, lymph vascular invasion and TNM stage were significantly correlated with OS (Table 3). In particular, patients with a low level of FOXO3a expression in tumor tissues showed significantly shorter OS (P = 0.006, Figure 2) than patients with high FOXO3a expression, while patients with a high level of FOXO3a expression in adjacent normal tissues showed significantly shorter OS (P = 0.011, Figure 3) than patients with low FOXO3a expression.
Table 3.
Uni- and multi-variate analysis of OS for the studied gastric cancer patients
| Variables | Univariate analysis | Multivariate analysis |
|---|---|---|
|
|
|
|
| P (Log-rank) | P (Log-rank) | |
| FOXO3a expression in tumor tissues | ||
| Low | 0.006 | 0.017 |
| Moderate | ||
| High | ||
| FOXO3a expression in adjacent normal tissues | ||
| Low | 0.011 | 0.475 |
| Moderate | ||
| High | ||
| Age (years) | ||
| < 65 | 0.451 | - |
| ≥ 65 | ||
| Sex | ||
| Male | 0.742 | - |
| Female | ||
| Tumour location | ||
| Noncardia | 0.114 | - |
| Cardia | ||
| Lauren’s classification | ||
| Intestinal | 0.173 | - |
| Nonintestinal | ||
| Tumour differentiation | ||
| Well | 0.165 | - |
| Moderate | ||
| Poor | ||
| TNM stage | ||
| II | 0.000 | 0.000 |
| III | ||
| Lymph vascular invasion | ||
| Absence | 0.001 | 0.017 |
| Presence |
Figure 2.
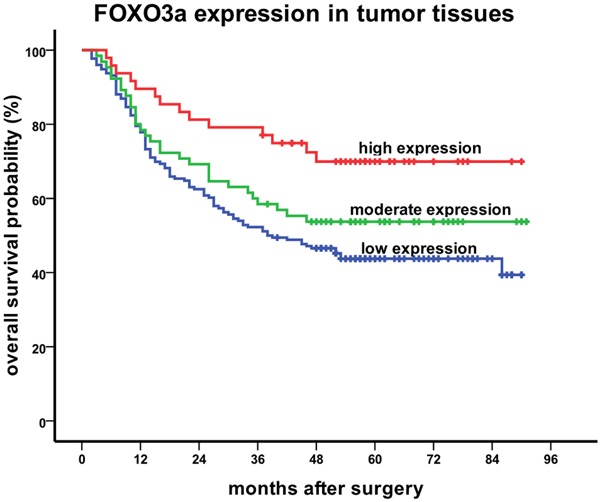
Kaplan-Meier curves of FOXO3a expression in tumor tissues for stage II/III gastric cancer patients in relation to OS (P = 0.006).
Figure 3.

Kaplan-Meier curves of FOXO3a expression in adjacent normal tissues for stage II/III gastric cancer patients in relation to OS (P = 0.011).
Multivariate analysis of prognostic factors in stage II and III CRC patients
Further multivariate COX regression analysis indicated that FOXO3a expression in tumor tissues served as a predictor of good prognosis regarding OS (HR = 0.737, 95% CI: 0.574-0.947, P = 0.017) in stage II and III gastric cancer patients, while TNM stage and lymph vascular invasion served as poor prognostic marker regarding OS (TNM stage: HR = 3.197, 95% CI: 1.990-5.137, P = 0.000; lymph vascular invasion: HR = 1.509, 95% CI: 1.075-2.118, P = 0.017) in these gastric cancer patients (Table 3).
Discussion
A number of receptors and downstream pathways are known to be aberrantly activated in gastric cancer. One of the important pathways that have been implicated in the pathogenesis of gastric cancer is the PI3K/Akt pathway and its related genes [15-18]. FOXO3a is a key downstream target in this pathway which prompted us to study its expression and associations in gastric cancer. Our data reveals that the expression of FOXO3a is up-regulated in gastric cancer tissues as compared with the adjacent normal tissues. In addition, as the presence of nuclear FOXO3a expression in a tumor indicates unphosphorylation by Akt, our data also suggests that the transcriptional activity of FOXO3a is more common in tumor tissues than adjacent normal tissues.
As a transcription factor, FOXO3a regulates multiple genes involved in cell cycle regulation, apoptosis, stress response, and DNA damage repair in normal tissues [12,19-23]. In cancer, FOXO3a has been shown to be deregulated in several tumor types and defined as a tumor suppressor in most studies [24,25]. However, the expression pattern of FOXO3a in cancer and normal tissues is not clear. On one hand, it is reported that the expression of FOXO3a mRNA and protein was lower in gastric cancer tissues compared with their adjacent non-tumorous tissues [9]. On the other hand, FOXO3a has been revealed to have a stronger immunostaining in the nucleus of breast cancer than benign breast tissues [27]. Our results are in agreement with the second notion. We speculate that in normal tissues, FOXO3a conducts genetic transcription in a minimum quantity of expression and is degraded before long. While in cancerous tissues, as a crucial tumor suppressor, either the expression of FOXO3a is up-regulated or the degradation is slowed down so as to gather more activated FOXO3a inside the nucleus to induce cell cycle arrest and apoptosis and eventually restrain tumor progression.
Our subsequent analysis results confirmed our suspicions. High expression of FOXO3a in cancer tissues was significantly correlated with light tumor invasion, and patients with a low level of FOXO3a expression in tumor tissues showed evidently shorter OS than patients with high FOXO3a expression. Previous publications have shown that FOXO3a nuclear localization is associated with good prognosis in luminal-like breast cancer [9], while decreased expression of FOXO3a gene is associated with poor prognosis in primary gastric adenocarcinoma [26]. Our results are consistent with the previous reports and give a strong support to the notion that FOXO3a functions as a tumor suppressor in gastric cancer.
The reasons why patients harboring higher expression of FOXO3a in gastric cancer tissues showed longer OS may be explained as follows: firstly, as a tumor suppressor, FOXO3a induces tumor apoptosis as well as inhibits tumor progression and angiogenesis [7,24,27,28], which controls the development of primary tumor. Secondly, FOXO3a may serve as a therapeutic target by mediating the cytostatic and cytotoxic effects of chemotherapeutic drugs. For example, the chemotherapeutic drugs cisplatin and paclitaxel, currently used in adjuvant or palliative treatment of gastric cancer, induce FOXO3a dephosphorylation and activation, which ultimately dictate the overall cellular response to chemotherapy in breast and colon cancer cells [29,30]. To date, however, there are few studies regarding FOXO3a as a mediator of chemotherapeutic drugs in gastric cancer. Further researches are required to reveal the underlying mechanisms by which FOXO3a prolongs the overall survival of gastric cancer patients.
We also evaluated the relationship between FOXO3a expression in adjacent normal tissues and OS. Interestingly, patients harboring higher expression of FOXO3a in adjacent normal tissues, though only 7 cases, showed significantly poorer OS than those with low and moderate FOXO3a expression. As we have speculated before, the expression of FOXO3a ought to be extremely low in normal tissues and over expression may disturb regular cell functions. Besides, high expression of FOXO3a in normal tissues enhances sensitivity to chemotherapeutics and may lead to severe side effects which reduce patients’ tolerance to treatments. However, the concrete mechanisms still need to be verified.
In conclusion, strong FOXO3a staining in nuclear is found to be more common in gastric cancer tissues compared with adjacent normal tissues. In addition, FOXO3a expression in the cancer specimens negatively correlates with tumor invasion. High expression of FOXO3a in tumor tissues and low expression of FOXO3a in adjacent normal tissues predict longer OS of gastric cancer patients. FOXO3a expression in gastric cancer is an independent risk factor of OS. While the exact mechanisms underlying gastric caner still await more research, our study seems to suggest that FOXO3a may be a good prognostic marker in gastric caner. More importantly, therapeutics based on targeting FOXO3a may hold promises in the management of gastric cancer.
Acknowledgements
This research is supported by the project 81273187 from National Natural Science Foundation of China (www.nsfc.gov.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA. Gastroesophageal cancers: Progress and problems. J Nat Compr Canc Netw. 2008;6:813–4. [PubMed] [Google Scholar]
- 3.Goscinski MA, Larsen SG, Warloe T, Stoldt S, Nesland JM, Suo ZH, Giercksky KE. Adenocarcinomas on the rise-does it influence survival from oesophageal cancer? Scand J Surg. 2009;98:214–20. doi: 10.1177/145749690909800404. [DOI] [PubMed] [Google Scholar]
- 4.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomized controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 5.Brunet A, Bonni A, Zigmond MJ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang JY, Hung MC. Deciphering the Role of Forkhead Transcription Factors in Cancer Therapy. Curr Drug Targets. 2011;12:1284–1290. doi: 10.2174/138945011796150299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 8.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, Huang H, Kuo HP, Lee DF, Li LY, Lien HC, Cheng X, Chang KJ, Hsiao CD, Tsai FJ, Tsai CH, Sahin AA, Muller WJ, Mills GB, Yu D, Hortobagyi GN, Hung MC. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Zou L, Lu WQ, Zhang Y, Shen AG. Foxo3a expression is a prognostic marker in breast cancer. PLos One. 2013;8:e70746. doi: 10.1371/journal.pone.0070746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y, Ai Q, Zhang P, Song EL, Huang QB, Fan Y, Zhang X. Downregulation of FOXO3a promotes tumor metastasis and is associated with metastasis-free survival of patients with clear cell renal cell carcinoma. Clin Cancer Res. 2014;20:1779–90. doi: 10.1158/1078-0432.CCR-13-1687. [DOI] [PubMed] [Google Scholar]
- 11.Yu C, Zhang Z, Liao W, Zhao X, Liu L, Wu Y, Liu Z, Li Y, Zhong Y, Chen K, Li J, Zhou F, Song L. The tumor-suppressor gene Nkx2.8 suppresses bladder cancer proliferation through upregulation of FOXO3a and inhibition of the MEK/ERK signaling pathway. Carcinogenesis. 2012;33:678–86. doi: 10.1093/carcin/bgr321. [DOI] [PubMed] [Google Scholar]
- 12.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Zhang H, Chen Y, Fan L, Fang J. Forkhead Transcription Factor FOXO3a Protein Activates Nuclear Factor κB through B-cell lymphoma/leukemia 10 (BCL10) Protein and Promotes Tumor Cell Survival in Serum Deprivation. J Biol Chem. 2012;287:17737–17745. doi: 10.1074/jbc.M111.291708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenbaum SP, Ordóñez-Morán P, Puig I, Chicote I, Arqués O, Landolfi S, Fernández Y, Herance JR, Gispert JD, Mendizabal L, Aguilar S, Ramón Y, Cajal S, Schwartz S Jr, Vivancos A, Espín E, Rojas S, Baselga J, Tabernero J, Muñoz A, Palmer HG. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 15.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 16.Osaki M, Kase S, Adachi K, Takeda A, Hash-imoto K, Ito H. Inhibition of the PI3K-Akt signaling pathway enhances the sensitivity of Fas-mediated apoptosis in human gastriccarcinoma cell line, MKN-45. J Cancer Res Clin Oncol. 2004;130:8–14. doi: 10.1007/s00432-003-0505-z. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Go HY, Jin DH, Kim HP, Hong MH, Chung WY, Park JH, Jang JB, Jung H, Shin YC, Kim SH, Ko SG. Inhibition of the PI3K-Akt/PKB survival pathway enhanced an ethanol extract of Rhus verniciflua Stokes-inducedapoptosis via a mitochondrial pathway in AGS gastric cancer cell lines. Cancer Lett. 2008;265:197–205. doi: 10.1016/j.canlet.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 18.Byun DS, Cho K, Ryu BK, Lee MG, Park JI, Chae KS, Kim HJ, Chi SG. Frequent monoallelic deletion of PTEN and its reciprocal associatioin with PIK3CA amplification in gastric carcinoma. Int J Cancer. 2003;104:318–327. doi: 10.1002/ijc.10962. [DOI] [PubMed] [Google Scholar]
- 19.Finnberg N, El-Deiry WS. Activating FOXO3a, NF-kappaB and p53 by targeting IKKs: an effective multi-faceted targeting of the tumor-cell phenotype? Cancer Biol Ther. 2004;3:614–616. doi: 10.4161/cbt.3.7.1057. [DOI] [PubMed] [Google Scholar]
- 20.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO’s road. Sci STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 21.Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L, Coffer PJ. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27 (KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JY, Xia W, Hu MC. Ionizing radiation activates expression of FOXO3a, Fas ligand, and Bim, and induces cell apoptosis. Int J Oncol. 2006;29:643–648. [PMC free article] [PubMed] [Google Scholar]
- 24.Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol. 2006;41:709–717. doi: 10.1016/j.exger.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 26.Yang XB, Zhao JJ, Huang CY, Wang QJ, Pan K, Wang DD, Pan QZ, Jiang SS, Lv L, Gao X, Chen HW, Yao JY, Zhi M, Xia JC. Decreased expression of the FOXO3a gene is associated with poor prognosis in primary gastric adenocarcinoma patients. PLoS One. 2013;8:e78158. doi: 10.1371/journal.pone.0078158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 28.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 30.Fernández de Mattos S, Villalonga P, Clardy J, Lam EW. FOXO3a mediates the cytotoxic effects of cisplatin in colon cancer cells. Mol Cancer Ther. 2008;7:3237–3246. doi: 10.1158/1535-7163.MCT-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]


