Abstract
Objective: To determine the association between the expression of p16 and Ki-67 and cervical lesions, and to evaluate the role of p16 and Ki-67 as prognostic markers for persistent high risk human papillomavirus (hr-HPV) infection. Methods: Totally 1,154 cases of cervical biopsies were enrolled, 331 cases with negative for dysplasia (NEG), 462 with cervical intraepithelial neoplasia 1 (CIN1), 176 with CIN2, 163 with CIN3 and 22 with cervical squamous cell carcinoma (SCC). Furthermore, 283 women with CIN1 were recruited into 12-month follow-up, and HPV specific gene detection by polymerase chain reaction was used to detect hr-HPV of cervical secretions at 6-month-interval for 12-month follow-up period. 40 women were infected with persistent hr-HPV, 182 with transient infection and 61 unfected with hr-HPV. The expression of p16 and Ki-67 were evaluated by immunohistochemical method. The immunostaining results of p16 and Ki-67 were classified into four categories: negative, 1+, 2+ and 3+. Results: There was significant increase in the expression of p16 (P < 0.001) and Ki-67 (P < 0.001) from NEG to SCC. The expression of Ki-67 (P < 0.001) but not p16 (P = 0.254) significantly increased in CIN2, CIN3. Ratio of p16 (P = 0.215) and Ki-67 (P = 0.495) positivity were not correlated with persistent hr-HPV infection. Conclusion: P16 and Ki-67 can improve the diagnostic accuracy of cervical lesions but can not predict persistent hr-HPV infection with CIN1.
Keywords: Cervical intraepithelial neoplasia (CIN), immunohistochemistry, p16, Ki-67, human papillomavirus (HPV)
Introduction
Cervical cancer, the second most common malignancy in women worldwide [1], emerges from cervical intraepithelial neoplasia (CIN). CIN can be treated effectively to prevent progression to cervical cancer. Case of CIN1 is usually recommended to a strict follow-up without treatment [2]. CIN2 and CIN3 are usually treated actively, including cervial conization or cervical loop electrosurgical excision procedure. The histological diagnosis of cervical biopsies that is often considered as the “gold standard” can be significantly hampered by intra- and inter-observer variability [3,4]. It is difficult to distinguish CINs reliably from non-neoplastic lesions, and CIN1 from CIN2/3, resulting in either overtreatment or undertreatment [5,6]. Therefore, accurate diagnosis of cervical lesions is important to clincians’ decision and patients’ treatment.
Majority of high risk human papillomavirus (hr-HPV) genotypes regress spontaneously, and only a small subset persist. Persistent hr-HPV infection is necessary for development of CIN and cervical cancer [7]. Therefore, using a suitable marker to predict persistent hr-HPV infection would be of considerable clinical value. In several published studies, the duration of hr-HPV has been defined as ranging from 2 to 14 months, with a median of 6 months [8-10]. Syrjänen K et al reported that more than 6-month hr-HPV persistence gave the most powerful estimate of a progressive disease [11]. P16INK4a (p16) is a cyclin-dependent kinase inhibitor that regulates transition from the G1 phase to the S phase of the cell cycle [12,13]. Ki-67 has been recently found to be the most reliable indicator of cellular proliferation [14,15]. The value of p16 and Ki-67 in diagnosis of CIN has already been shown. However, inter-observer variation exists in interpretation of p16 and Ki-67 immunostaining results and sample size also affects the results. In regard to the relation of p16 and Ki-67 with persistent infection of hr-HPV, no study was found.
The aim of this study was to evaluate the clinical value of p16 and Ki-67 in diagnosis of cervical lesions in a large population-based cohort study and to investigate the association between persistent hr-HPV infection diagnosed as CIN1 and p16 and Ki-67 expression.
Materials and methods
Samples
Totally 1,154 consecutive patients with abnormal cytology diagnosed as ASC-US, ASC-H, LSIL, HSIL or cervical squamous cell carcinoma (SCC) between October 2010 and April 2013 of Haidian Maternal and Child Health Hospital of Beijing were enrolled in this study. The patients aged from 18 to 73 years (mean 34.59 years). Each subject underwent a colposcopy-directed biopsy excision procedure and simultaneously hr-HPV gene detection through polymerase chain reaction (PCR). The Ethics Committee of Haidian Maternal and Child Health Hospital approved all experimental procedures. An informed consent form was provided according to the Declaration of Helsinki, and written informed consent was obtained according to institutional guidelines from all subjects.
Formalin-fixed, paraffin-embedded tissue blocks were sliced in thickness of 4 um. Sections were stained with hematoxylin and eosin (H&E) for routine examination. Meanwhile, p16 and Ki-67 immunohistochemical (IHC) staining were done in all biopsy samples. All H&E stained sections were first reviewed by 2 independent pathologists. The consensus diagnosis was a gold standard. With diagnostic disagreement, the consensus diagnosis was confirmed by an expert pathology panel. All IHC stained sections were evaluated blinded to the initial diagnosis. 283 women with CIN1 were recruited into a 12-month follow-up. During the 12-month follow-up period, HPV genotyping of cervical secretion was performed at baseline and at 6-month-interval.
Techniques of IHC staining
All the specimens were immunostained for p16 and Ki-67 antigens through the indirect biotin streptoavidin method. All slides were deparaffinised in xylene, rehydrated in graded alcohols, washed in PBS buffer, boiled in EDTA solution (pH 8.0, 100°C, 2.5 minutes) for antigen retrieval and cooled down. The slides were stained using the automatic IHC staining equipment (LAB VISION AUTO-STAINER 480S). The primary antibodies were as follows: p16 (dilution at 1:80, ZSGB-BIO, ZM0205), Ki-67 (dilution at 1:200, ZSGB-BIO, ZM0166). Interpretation of IHC staining results: p16 was expressed in cytoplasm or nucleus while Ki-67 was expressed in nucleus. To determine the grade of p16 expression, a four-semiquantitative class was used to describe the percentage of positively stained tumor cells as follows: negative (below 5%), weak positive (1+, 5%-25%), moderate positive (2+, 26%-50%), intense positive (3+, greater than 50%). To determine the grade of Ki-67 expression, nuclei of 200 epithelial cells located across the whole epithelial layer were examined in a high-power field (× 400). Ki-67 index was defined as the percentage of Ki-67 positive cells: negative, 1+, 2+, 3+ was given when the Ki-67 index was below 5%, 5-25%, 26%-50% and greater than 50%, respectively.
Detection of hr-HPV types with method of PCR
Type-specific HPV DNA testing was performed by real-time PCR. The participants diagnosed as CIN1 accepted no treatment during the 12-month follow-up period. Oncogenic hr-HPV types detected were HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. A transient infection was defined as detection of specific hr-HPV types in a cervical sample at baseline, followed by a negative sample for the same hr-HPV type at the next detection. Persistent hr-HPV infection was defined as detection of the same hr-HPV type at a sequence of consecutive evaluations not interrupted by negative samples during the 12-month follow-up period, and hr-HPV uninfection was defined as hr-HPV types were negative at baseline.
Statistical analysis
Statistical analyses were performed using SPSS 11.5 for Windows. Frequency tables were analyzed by using the chi-square test. Bivariate correlations between ordered variables were analyzed by using spearman correlation analysis. A P value < 0.05 (two-sided) was considered as statistical significance.
Results
Demographic characteristics of subjects
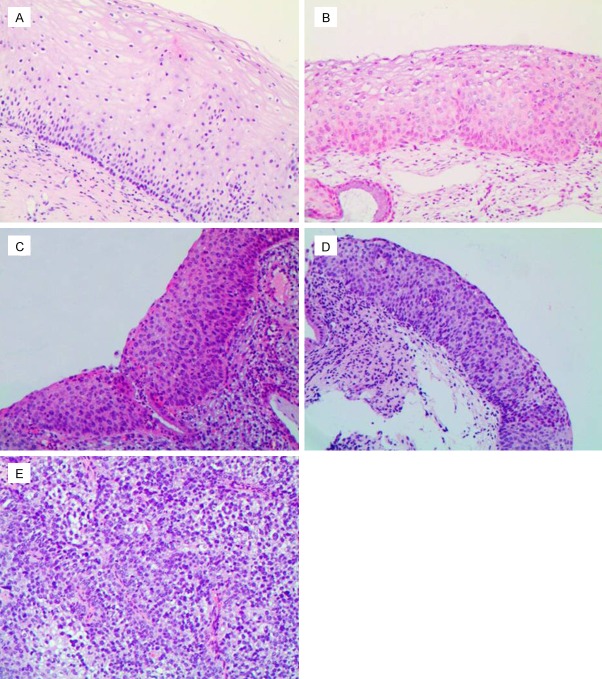
Totally 1,154 patients, 331 patients with NEG (28.7%) (Figure 1A), 462 patients with CIN1 (40.0%) (Figure 1B), 176 patients with CIN2 (15.3%) (Figure 1C), 163 patients with CIN3 (14.1%) (Figure 1D) and 22 patients with SCC (1.9%) (Figure 1E) were included in this study.
Figure 1.
Hematoxylin and eosin staining (100 ×). A: Negative for dysplasia; B: Cervical intraepithelial neoplasia1 (CIN1); C: CIN2; D: CIN3; E: Squamous cell carcinoma.
Expression of p16 and Ki-67 increased with the severity of cervical lesions
Table 1 presented the expression of p16 and Ki-67 in relation to the grade of cervical lesions. The positive rate of p16 in NEG (Figure 2A), CIN1 (Figure 2B), CIN2 (Figure 2C), CIN3 (Figure 2D) and SCC (Figure 2E) was 21.88%, 67.32%, 98.85%, 99.38%, and 100% respectively. P16 and Ki-67 (Figure 3A-E) expression significantly increased with disease progression (p16, P < 0.001; Ki-67, P < 0.001). In addition, the expression of Ki-67 (P < 0.001) but not p16 (P = 0.254) significantly increased in CIN2 and CIN3.
Table 1.
Expression of p16 and Ki-67 in relation to grade of cervical lesions (n)
| p16 | Ki-67 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Diagnosis | Negative | 1+ | 2+ | 3+ | N.S | Negative | 1+ | 2+ | 3+ | N.S |
| NEG | 257 | 56 | 14 | 2 | 2 | 230 | 98 | 2 | 1 | 0 |
| CIN1 | 149 | 95 | 97 | 115 | 6 | 97 | 271 | 73 | 15 | 6 |
| CIN2 | 2 | 11 | 8 | 153 | 2 | 4 | 38 | 64 | 68 | 2 |
| CIN3 | 1 | 4 | 2 | 156 | 0 | 2 | 9 | 33 | 118 | 1 |
| SCC | 0 | 1 | 1 | 20 | 0 | 0 | 0 | 3 | 19 | 0 |
| Total | 407 | 167 | 122 | 446 | 10 | 333 | 416 | 175 | 221 | 9 |
N.S: not satisfactory. NEG: negative for dysplasia. CIN: cervical intraepithelial neoplasia. SCC: cervical squamous cell carcinoma.
Figure 2.
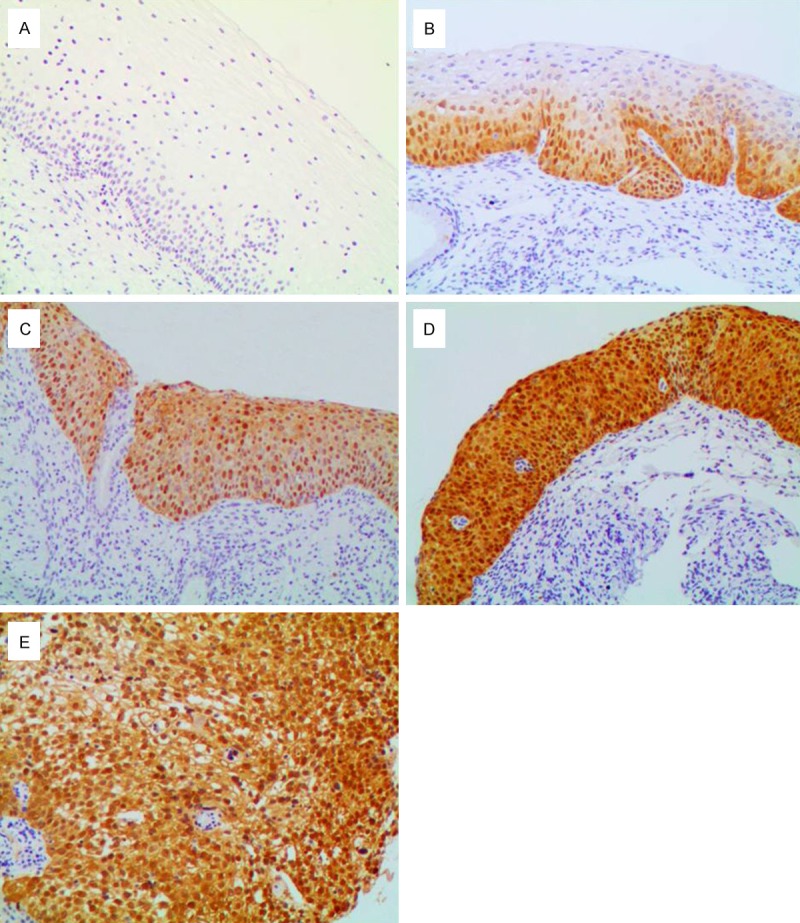
Immunohistochemical staining of p16 (100 ×). A: In negative for dysplasia, negative staining; B: In cervical intraepithelial neoplasia1 (CIN1), diffuse p16 immunostaining restricted to the lower third of the cervical epithelium; C: In CIN2, diffuse p16 immunostaining of full thickness positivity of the cervical epithelium; D: In CIN3, diffuse immunostaining of full thickness positivity of the dysplastic epithelium; E: In squamous cell carcinoma, diffuse strong positive.
Figure 3.
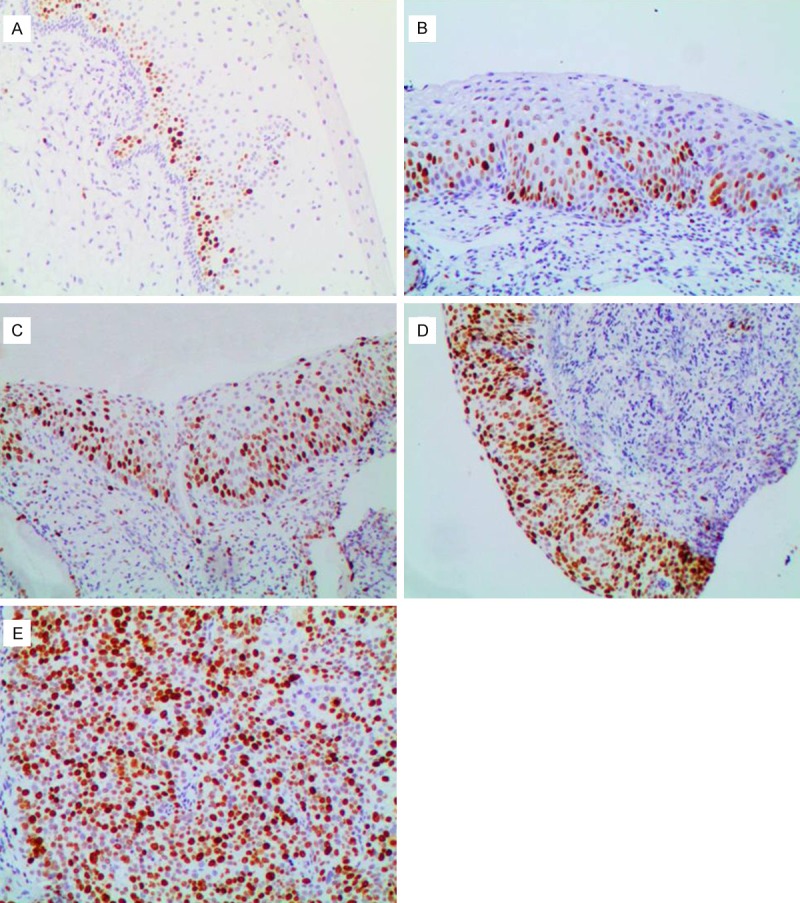
Immunohistochemical staining of Ki-67 (100 ×). A: In negative for dysplasia, negative staining in normal cervical tissue except in the basal and parabasal cells; B: In cervical intraepithelial neoplasia1 (CIN1): diffuse Ki-67 immunostaining restricted to the lower third of the cervical epithelium; C: In CIN2, diffuse Ki-67 immunostaining of two-thirds of the cervical epithelium; D: In CIN3, diffuse Ki-67 immunostaining of full thickness positivity of the dysplastic epithelium; E: In squamous cell carcinoma, diffuse strong positive.
Expression of p16 immunostaining was positively associated with that of Ki-67 immunostaining
Expression level of p16 was in positive relation with that of Ki-67 (P < 0.001). It is worth mentioning that 268 cases with p16 and Ki-67 negative expression were diagnosed as NEG or CIN1. 199 out of 209 cases with strong positive expression (3+) in p16 and Ki-67 had high grade CIN (including CIN2 and CIN3) or SCC (Table 2). 3 patients with high grade CIN whose p16 expression was negative had positive expression of Ki-67. 6 patients with high grade CIN whose Ki-67 expression was negative had positive expression of p16.
Table 2.
Correlation between expression of p16 and Ki-67 immunostaining (n)
| p16 | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Ki-67 | Negative | 1+ | 2+ | 3+ | NS | Total |
| Negative | 268 | 49 | 7 | 5 | 3 | 332 |
| 1+ | 129 | 89 | 84 | 110 | 4 | 416 |
| 2+ | 5 | 21 | 25 | 120 | 3 | 174 |
| 3+ | 3 | 6 | 5 | 209 | 0 | 223 |
| NS | 3 | 2 | 1 | 3 | 0 | 9 |
| Total | 408 | 167 | 122 | 447 | 10 | 1154 |
Note: 3 cases of p16 staining were not satisfactory (NS).
The expression of p16 and Ki-67 did not correlate with hr-HPV infection status
Among 283 participants with CIN1, 40 cases had persistent infection of hr-HPV, 182 cases had transient hr-HPV infection. There was poor correlation between p16 as well as Ki-67 positive ratio and HPV infected states (p16, r = -0.097; Ki-67, r = -0.010). P16 as well as Ki-67 positivity ratio had no significant difference in persistent hr-HPV infection, transient hr-HPV infection and hr-HPV uninfection with CIN1 (p16, P = 0.215; Ki-67, P = 0.495) (Table 3).
Table 3.
Expression of p16 and Ki-67 in relation to Hr-HPV infection status (n)
| Result | Persistent infection | Transient infection | Hr-HPV negative | P value |
|---|---|---|---|---|
| P16 negative | 12 | 60 | 27 | 0.215 |
| P16 positive | 28 | 122 | 34 | |
| Ki-67 negative | 18 | 68 | 27 | 0.495 |
| Ki-67 positive | 22 | 114 | 34 |
Hr-HPV: high risk human papillomavirus.
Discussion
We previously reported expression of p53 was similar in CINs infected with different hr-HPV types. Here we demonstrate that there is significant increase in the expression of p16 and Ki-67 from NCT to SCC and that the two biomarkers are of no prognostic value in predicting persistent hr-HPV infection.
There are only few studies available for biomarkers predicting persistent hr-HPV infection. Persistent infection of HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 has been found to cause the great majority of CINs and cervical cancer, and the hr-HPV genotypes upon have been defined as “carcinogenic” viral types [16,17]. Numerous studies on the value of p16 and Ki-67 immunostaining in cervical lesions mainly focus on reliable diagnosis of high-grade CIN.
In present study, we collected a considerable amount of samples and used four-category interpretation criteria to evaluate the value of p16 and Ki-67 immunostaining in cervical lesions. The present study is the first to investigate the prognostic value of p16 and Ki-67 immunostaining in persistent hr-HPV infection diagnosed as CIN1.
The results showed the p16 and Ki-67 expression were higher in CIN2, CIN3 and SCC than those in CIN1, and significant difference was observed between CIN1 and NEG. It fits well with recent studies [18,19]. P16 and Ki-67 expression significantly increased with disease progression. It indicates that p16 and Ki-67 immunostaining are useful in the evaluation of cervical lesions. It is worth mentioning that 3 cases of p16-negative CIN2/3 had positive expression of Ki-67 and 6 cases of Ki-67- negative CIN2/3 had positive expression of p16. It suggests p16 immunostaining combined with Ki-67 can increase the diagnostic accuracy of high grade CIN and that p16 and Ki-67 are complementary biomarkers. Not p16 but Ki-67 expression had significant difference in CIN2 and CIN3, indicating that p16 overexpression is an early event and Ki-67 works during the course of CIN progress. It needs a further study to verify.
Infection with hr-HPV results in integration of hr-HPV into the host genome. The early gene E7 is the urgent oncogene of hr-HPV. E7 binds to the tumor suppressor retinoblastoma protein (pRB) and thus inactivates pRB, resulting in G1–S transition of the cell cycle. The HPV- E7 determines the inactivation of pRb with a consequent increase of free E2F in the cell, leading to aberrant proliferation (marked by increased levels of Ki-67 expression) [20,21]. P16, a pRb regulator, underlies a negative feedback controlled by the pRB whose inactivation results in an overexpression of p16. As to the relation between hr-HPV and p16 as well as Ki-67 expression, most researchers believe that p16 is a surrogate marker for CIN induced by hr-HPV and that hr-HPV negative status accompanied by p16 positivity is often regarded as a false negative. However, our study showed that there was poor correlation between p16 as well as Ki-67 positive ratio and HPV infected status (p16: r = -0.097, P = 0.103; Ki67: r = -0.010, P = 0.867). To investigate the role of p16 and Ki-67 in persistent hr-HPV infection, we conducted a 12-month follow-up for hr-HPV detection. In our study, the studied population was homogeneous since all the enrolled cases were diagnosed as CIN1. Meanwhile, CIN1 reflects active HPV replication and is generally managed clinically by follow-up rather than immediate treatment. In some cases with HPV-negative in our study, p16 was also positive, which fits well with literature reported [22]. No significant difference of p16 and Ki-67 expression (p16, P = 0.215; Ki-67, P = 0.495) was observed in persistent hr-HPV infection, transient hr-HPV infection and hr-HPV uninfection. It reveals that p16 and Ki-67 biomarkers are useless in prediction of persistent HPV infection. Branca et al showed p16 did not seem to be of any prognostic value in predicting the clearance of hr-HPV after treatment of CIN [23]. Comparably, subjects in our study received no treatment. We also showed p16 and Ki-67 immunostaining was of no prognostic value in persistent hr-HPV infection. The reasons why p16 and Ki-67 had no significant difference regardless of hr-HPV infection status may be as follows: 1) p16 expression is closely associated with CIN grade. In our study, all the cases were in diagnosis of CIN1, the same degree of CIN. For this reason, no significant difference was observed regardless of HPV infection status. 2) P16 overexpression is likely due to non-HPV related genetic or epigenetic loss of pRb [24]. 3) Whether hr-HPV will persist or regress depends upon natural history of pathogen itself [7] and the host response to HPV infection [25,26]. From this point of this view, we put forward the hypothesis that p16 and Ki-67 have little relation to natural history of hr-HPV and cannot be indicators of the host immune response. The mechanisms of p16 and Ki-67 upon hr-HPV persistence are not clear and deserve further study.
In conclusion, p16 and Ki-67 are diagnostic markers for cervical lesions, and the expression level of p16 and Ki-67 increase as the cervical lesion is higher, p16 is an early event of CIN and Ki-67 works in all the duration of CIN. But p16 and Ki-67 are of no prognostic value in predicting persistent hr-HPV infection. The duration of our study was restricted to a 12-month follow-up, and a longer duration of follow-up might be more convincing.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81200041) and the Science and Technology Projects of Haidian District, China (No. K2008058).
Disclosure of conflict of interest
None.
References
- 1.Chih HJ, Lee AH, Colville L, Binns CW, Xu D. A review of dietary prevention of human papillomavirus-related infection of the cervix and cervical intraepithelial neoplasia. Nutr Cancer. 2013;65:317–28. doi: 10.1080/01635581.2013.757630. [DOI] [PubMed] [Google Scholar]
- 2.Rouzier R. [Management of CIN1] . J Gynecol Obstet Biol Reprod (Paris) 2008;37(Suppl 1):S114–20. doi: 10.1016/j.jgyn.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Martin CM, O’Leary JJ. Histology of cervical intraepithelial neoplasia and the role of biomarkers. Best Pract Res Clin Obstet Gynaecol. 2011;25:605–15. doi: 10.1016/j.bpobgyn.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Dascau V, Furau G, Furau C, Paiusan L, Radu A, Stanescu C. Cervical intraepithelial neoplasia in the “dr. Salvator vuia” clinical obstetrics and gynecology hospital-arad during the 2000-2009 period. Maedica (Buchar) 2012;7:138–42. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen EY, Tran A, Raho CJ, Birch CM, Crum CP, Hirsch MS. Histological ‘progression’ from low (LSIL) to high (HSIL) squamous intraepithelial lesion is an uncommon event and an indication for quality assurance review. Mod Pathol. 2010;23:1045–51. doi: 10.1038/modpathol.2010.85. [DOI] [PubMed] [Google Scholar]
- 6.Creagh T, Bridger JE, Kupek E, Fish DE, Martin-Bates E, Wilkins MJ. Pathologist variation in reporting cervical borderline epithelial abnormalities and cervical intraepithelial neoplasia. J Clin Pathol. 1995;48:59–60. doi: 10.1136/jcp.48.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2536–45. doi: 10.1158/1055-9965.EPI-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 9.Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, Basu J, Tachezy R, Lewis R, Romney S. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–71. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 10.Kulmala SM, Shabalova IP, Petrovitchev N, Syrjänen KJ, Gyllensten UB, Johansson BC, Syrjänen SM New Independent States of the former Soviet Union Cohort Study Group. Type-specific persistence of high-risk human papillomavirus infections in the New Independent States of the former Soviet Union Cohort Study. Cancer Epidemiol Biomarkers Prev. 2007;16:17–22. doi: 10.1158/1055-9965.EPI-06-0649. [DOI] [PubMed] [Google Scholar]
- 11.Syrjanen K, Shabalova I, Naud P, Kozachenko V, Derchain S, Zakharchenko S, Roteli-Martins C, Nerovjna R, Longatto-Filho A, Kljukina L, Tatti S, Branovskaja M, Hammes LS, Branca M, Grunjberga V, Erzen M, Sarian LO, Juschenko A, Costa S, Podistov J, Syrjänen S New Independent States of the Former Soviet Union and the Latin American Screening Study Research Groups. Persistent high-risk human papillomavirus infections and other end-point markers of progressive cervical disease among women prospectively followed up in the New Independent States of the Former Soviet Union and the Latin American Screening study cohorts. Int J Gynecol Cancer. 2009;19:934–42. doi: 10.1111/IGC.0b013e3181a834fe. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Li XJ, Zhu W, Liu XP. Detection and pathological value of papillomavirus DNA and p16 and p53 protein expression in cervical intraepithelial neoplasia. Oncol Lett. 2014;7:738–44. doi: 10.3892/ol.2014.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Q, Fu B, Liu J, Xu J, Zhao T. Combined detection of p16(INK4a) and IMP3 increase the concordance rate between cervical cytologic and histologic diagnosis. Int J Clin Exp Pathol. 2013;6:1549–57. [PMC free article] [PubMed] [Google Scholar]
- 14.Rudolph P, Peters J, Lorenz D, Schmidt D, Parwaresch R. Correlation between mitotic and Ki-67 labeling indices in paraffin-embedded carcinoma specimens. Hum Pathol. 1998;29:1216–22. doi: 10.1016/s0046-8177(98)90248-9. [DOI] [PubMed] [Google Scholar]
- 15.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 16.Tornesello ML, Buonaguro L, Giorgi-Rossi P, Buonaguro FM. Viral and cellular biomarkers in the diagnosis of cervical intraepithelial neoplasia and cancer. Biomed Res Int. 2013;2013:519619. doi: 10.1155/2013/519619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 18.Nam EJ, Kim JW, Kim SW, Kim YT, Kim JH, Yoon BS, Cho NH, Kim S. The expressions of the Rb pathway in cervical intraepithelial neoplasia; predictive and prognostic significance. Gynecol Oncol. 2007;104:207–11. doi: 10.1016/j.ygyno.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Roncaglia MT, Fregnani JH, Tacla M, DE Campos SG, Caiaffa HH, Ab’saber A, DA Motta EV, Alves VA, Baracat EC, Longatto Filho A. Characterization of p16 and E6 HPV-related proteins in uterine cervix high-grade lesions of patients treated by conization with large loop excision. Oncol Lett. 2013;6:63–8. doi: 10.3892/ol.2013.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khleif SN, DeGregori J, Yee CL, Otterson GA, Kaye FJ, Nevins JR, Howley PM. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci U S A. 1996;93:4350–4. doi: 10.1073/pnas.93.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuschenbach M, Waterboer T, Wallin KL, Einenkel J, Dillner J, Hamsikova E, Eschenbach D, Zimmer H, Heilig B, Kopitz J, Pawlita M, Doeberitz Mv, Wentzensen N. Characterization of humoral immune responses against p16, p53, HPV16 E6 and HPV16 E7 in patients with HPV-associated cancers. Int J Cancer. 2008;123:2626–31. doi: 10.1002/ijc.23837. [DOI] [PubMed] [Google Scholar]
- 22.Lin ZH, Shen XH, Jin Z, Kim Y, Lee E, Kim H, Kim I. Human papillomavirus genotyping by oligonucleotide microarray and p16 expression in uterine cervical intraepithelial neoplasm and in invasive carcinoma in Korean women. Pathol Int. 2005;55:491–6. doi: 10.1111/j.1440-1827.2005.01858.x. [DOI] [PubMed] [Google Scholar]
- 23.Branca M, Ciotti M, Santini D, Di Bonito L, Giorgi C, Benedetto A, Paba P, Favalli C, Costa S, Agarossi A, Alderisio M, Syrjänen K. p16(INK4A) expression is related to grade of cin and high-risk human papillomavirus but does not predict virus clearance after conization or disease outcome. Int J Gynecol Pathol. 2004;23:354–65. doi: 10.1097/01.pgp.0000139639.79105.40. [DOI] [PubMed] [Google Scholar]
- 24.Compton AM, Moore-Medlin T, Herman-Ferdinandez L, Clark C, Caldito GC, Wang XI, Thomas J, Abreo FW, Nathan CO. Human papillomavirus in metastatic lymph nodes from unknown primary head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2011;145:51–7. doi: 10.1177/0194599811400385. [DOI] [PubMed] [Google Scholar]
- 25.Hibma MH. The immune response to papillomavirus during infection persistence and regression. Open Virol J. 2012;6:241–8. doi: 10.2174/1874357901206010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez AC, Garcia-Pineres AJ, Hildesheim A, Herrero R, Trivett M, Williams M, Atmella I, Ramírez M, Villegas M, Schiffman M, Burk R, Freer E, Bonilla J, Bratti C, Pinto LA. Alterations of T-cell surface markers in older women with persistent human papillomavirus infection. Int J Cancer. 2011;128:597–607. doi: 10.1002/ijc.25371. [DOI] [PMC free article] [PubMed] [Google Scholar]