Abstract
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase regulating the cell cycle and protein synthesis, and is an attractive molecule for novel molecular targeting therapy in various cancers, including non-small cell lung cancer (NSCLC). In contrast with NSCLC, mTOR expression has not been fully investigated in SCLC. In this study, we evaluated the correlations between mTOR expression and clinical characteristics in SCLC. Immunohistochemical staining for phosphorylated mTOR (p-mTOR) was performed and histoscores were calculated on 115 SCLC tissue specimens. Based on the distribution of the data, a histoscore of 60 was used as a cutoff to dichotomize SCLCs into low versus high expression groups. Extended-stage SCLCs showed significantly lower p-mTOR expression than those of a limited-stage (P = 0.008). Lymph node metastasis was more frequently detected in the low than high expression group (P = 0.074). The high p-mTOR expression group had a weak tendency toward prolonged overall survival, but the difference was not statistically significant (P = 0.170). We found that there is a significant difference in p-mTOR expression between different clinical stages in SCLC. This result indicates that p-mTOR might play a more pivotal role in the biologic behavior of early SCLCs than advanced ones and the effectiveness of mTOR inhibitors might vary according to the extent of disease.
Keywords: Small cell lung cancer, mammalian target of rapamycin, immunohistochemistry, prognosis
Introduction
Small cell lung cancer (SCLC) accounts for approximately 15% to 20% of all lung cancers [1]. SCLC is one of the most aggressive cancers. Only 15% of patients with SCLC have a local disease at the time of diagnosis, whereas more than 55% have metastasized. The five year survival rate for the metastasized stage of the disease is 1-2% [2]. More recently, molecular target agents, including epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors have improved the survival rate for non-SCLC (NSCLC), especially adenocarcinoma. However, any effective target therapy for SCLC has not been developed yet.
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase located in mammalian cell lysosomes, nuclei, mitochondria, plasma membranes, and cytoplasm. Phosphorylated mTOR (p-mTOR) regulates the cell cycle and activates protein synthesis by phosphorylating eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1) and p70 S6 kinase. The mTOR signaling pathway is crucial for cell growth, metabolism, proliferation, and survival [3,4]. Several studies have suggested that a high expression of p-mTOR in tumor cells reflects their oncogenic potential and correlates with an unfavorable prognosis in different types of cancers [5-8]. In addition, mTOR inhibitors, such as everolimus or sirolimus, could have beneficial effects as treatments for various cancers, including NSCLC, breast, ovary, prostate, pancreas, and colon cancers [9].
The expression of p-mTOR and its clinical implications in SCLC have not been fully investigated. A recent study showed that the mTOR signaling pathway is associated with tumor cell growth in neuroendocrine lung tumors, and that inhibition of mTOR signaling prevents cell growth and survival of SCLC cells [10]. However, a phase II study of everolimus as a treatment for relapsed SCLC showed limited antitumor activity [11]. Moreover, the correlation between p-mTOR expression and the prognosis of patients with SCLC has not been described yet. Thus, results regarding p-mTOR expression in SCLC are controversial and limited.
In this study, therefore, we aimed to investigate the expression of p-mTOR in SCLCs and analyzed its association with a variety of clinical characteristics and patient survival.
Materials and methods
Patients and tissue samples
We retrieved from the pathology archives of 183 SCLC patients with tumor specimens obtained by surgical resection, CT-guided transthoracic lung biopsy, or bronchoscopic biopsy at Samsung Changwon Hospital from January 2002 to December 2009. We excluded patients who had a previous diagnosis of any other cancer; who had received chemotherapy or radiotherapy prior to enrollment in this study; and those whose accurate medical records were not available. In addition, we excluded specimens obtained from recurrent or metastatic tumors and those that were inadequate for immunohistochemical staining. Ultimately, 115 SCLC patients were included in this study. Clinicopathological data such as age, sex, smoking status, performance status, tumor size, lymph node metastasis, initial stage, and survival data were obtained from medical records. The duration of overall survival (OS) was defined as the time interval between the date of diagnosis and the date of last follow-up or death. The study was approved by the Institutional Review Board of our medical institution.
Immunohistochemisty
Formalin-fixed, paraffin-embedded tissue samples were cut into 4 μm sections for immunohistochemical staining. All sections were deparaffinized in a series of xylene baths and rehydrated with a graded alcohol series. For antigen retrieval, sections were heated in an autoclave for 13 minutes in 10 mM citrate buffer (pH 6.0). After blocking the endogenous peroxidase activity with 3% hydrogen peroxide for 10 minutes, slides were incubated with the primary antibody was for 30 minutes at room temperature. The primary antibody was a rabbit monoclonal antibody (clone 49F9, 1:50, Cell Signaling Technology, Danvers, MA, USA) for p-mTOR. A DAKO EnVision Kit (Dako, Carpinteria, CA, USA) was used as the secondary antibody, which was applied during a 15 minute room temperature incubation period. After washing the slides in phosphate buffered saline for 10 minutes, 3, 3’-diaminobenzidine was used as a chromogen, and Mayer’s hematoxylin counterstain was applied. Colon carcinoma was used as the positive control. The negative control was stained with a buffer instead of the primary antibody.
Immunostained slides were interpreted by a single observer (HWL) who was blinded to the clinicopathological data. Histoscores were calculated by multiplying the intensity score (0 = negative; 1 = weak; 2 = moderate; 3 = strong) and proportion score (percentage of positive tumor cells; range = 0-100), and ranged from 0 to 300 [5,7]. When histoscores were classified into two groups for statistical analyses, we used a cutoff of 60 on the basis of the distribution of the data (Figure 1). Cases with a histoscore of 60 or lower were classified as low expression, while cases with a histoscore higher than 60 were classified as high expression.
Figure 1.
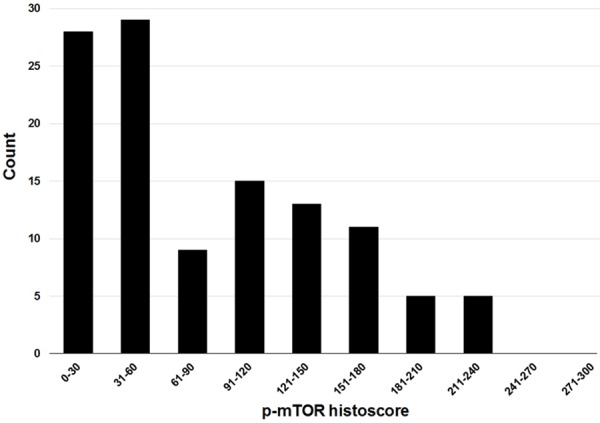
Distribution of p-mTOR histoscores.
Statistical analysis
Data are presented as median and range for continuous variables, and as numbers and percentages for categorical variables. The relationships between the categorical variables were analyzed by the chi-square test or Fisher’s exact test. The impacts of parameters on OS were analyzed by the Kaplan-Meier method, and differences were compared by the log-rank test. In all statistical analyses, a P-value of <0.05 was considered statistically significant. All data were analyzed using IBM SPSS Statistics Ver. 19 (SPSS, Chicago, IL, USA).
Results
Clinical characteristics of SCLC patients
The 115 patients with SCLC were composed of 88 males and 27 females. At the time of diagnosis, the median age of these patients was 68 years (range 49 to 93 years). Eighty-eight patients (77.4%) were current or past smokers, while 26 (22.6%) were never smokers. The performance statuses of 87 patients (75.7%) were ECOG 0-2 and 28 (24.3%) were ECOG 3-4. Seventy-five tumors (65.2%) were extended disease and 40 (34.8%) were limited disease. According to the 7th edition of the TNM staging system, 64 tumors (55.7%) were T3-T4 stage, while 51 (44.3%) were T1-T2 stage. Nodal metastasis was detected in 101 (87.8%) cases. These clinical characteristics are summarized in Table 1.
Table 1.
Demographic characteristics of 115 patients with small cell lung cancer
| Characteristics | N (%) |
|---|---|
| Median age (range) | 68 (43-93) |
| Sex | |
| Male | 88 (76.5) |
| Female | 27 (23.5) |
| Smoking status | |
| Never smoker | 26 (22.6) |
| Ex-smoker | 48 (41.7) |
| Current smoker | 41 (35.7) |
| Performance status | |
| ECOG 0-2 | 87 (75.7) |
| ECOG 3-4 | 28 (24.3) |
| Tumor size | |
| T1 (T1a-T1b) | 13 (11.3) |
| T2 (T2a-T2b) | 38 (33.0) |
| T3-T4 | 64 (55.7) |
| Lymph node metastasis | |
| Absent (N0) | 13 (11.3) |
| Present (N1-3) | 102 (88.7) |
| Initial stage | |
| Limited | 34 (29.6) |
| Extended | 81 (70.4) |
Correlation between p-mTOR expression and clinical characteristics
Of the 115 tumors, 101 (87.8%) were positive for p-mTOR with cytoplasmic immunostaining (Figure 2). Histoscores ranged from 0 to 240, with a median of 70. As previously stated, a histoscore of 60 or lower was considered low p-mTOR expression, and a histoscore higher than 60 was considered high expression. We analyzed the correlation between p-mTOR expression and clinical characteristics (Table 2).
Figure 2.
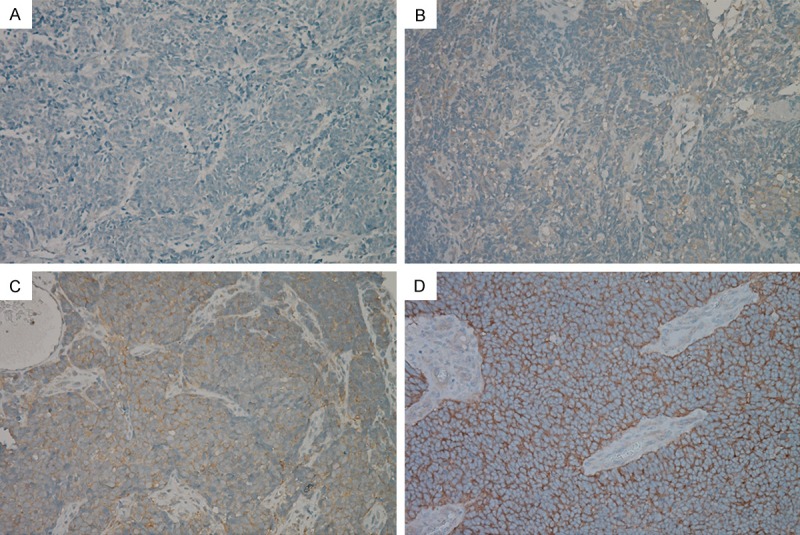
Immunohistochemical staining for p-mTOR in small cell lung cancer: negative expression for p-mTOR (A); weakly positive expression (B); moderately positive expression (C); and strongly positive expression (D).
Table 2.
Correlation between p-mTOR expression and clinical characteristics in 115 patients with small cell lung cancer
| Characteristics | p-mTOR expression | ||
|---|---|---|---|
|
| |||
| Low (%) | High (%) | P-value | |
| Total | 57 (100) | 58 (100) | |
| Age (years) | |||
| ≤65 | 19 (33.3) | 13 (22.4) | 0.193 |
| >65 | 38 (66.7) | 45 (77.6) | |
| Sex | |||
| Male | 46 (80.7) | 42 (72.4) | 0.380 |
| Female | 11 (19.3) | 16 (27.6) | |
| Smoking status | |||
| Never smoker | 9 (15.8) | 17 (29.3) | 0.161 |
| Ex-smoker | 24 (42.1) | 24 (41.4) | |
| Current smoker | 24 (42.1) | 17 (29.3) | |
| Performance status | |||
| ECOG 0-2 | 46 (80.7) | 41 (70.7) | 0.278 |
| ECOG 3-4 | 11 (19.3) | 17 (29.3) | |
| Tumor size | |||
| T1 (T1a-T1b) | 6 (10.5) | 7 (12.1) | 0.393 |
| T2 (T2a-T2b) | 17 (29.8) | 21 (36.2) | |
| T3-T4 | 34 (59.7) | 30 (51.7) | |
| Lymph node metastasis | |||
| Absent (N0) | 3 (5.3) | 10 (17.2) | 0.074 |
| Present (N1-3) | 54 (94.7) | 48 (82.8) | |
| Initial stage | |||
| Limited | 10 (17.5) | 24 (41.4) | 0.008 |
| Extended | 47 (82.5) | 34 (58.6) | |
The level of p-mTOR expression inversely correlated with the tumor stage, and p-mTOR expression was significantly higher in limited-stage than in extended-stage SCLCs (P = 0.008). In addition, p-mTOR had higher expression in SCLCs without nodal metastasis compared with those with nodal metastasis, although the difference was not statistically significant (P = 0.074). No significant association was observed between p-mTOR expression and other clinical characteristics.
Correlation between p-mTOR expression and patient survival
The median follow-up period was 69.5 days (range 4 to 3304 days). During follow-up, 113 (98.3%) of the 115 patients died from disease progression. The median duration of diagnosis to death was 5.8 months. The 2-year cumulative survival rate was 7.9%. SCLC patients with low p-mTOR expression had worse 2-year cumulative survival rates compared to those with high p-mTOR expression, although the difference was not statistically significant (3.6% vs. 12.1%; P = 0.170) (Figure 3).
Figure 3.
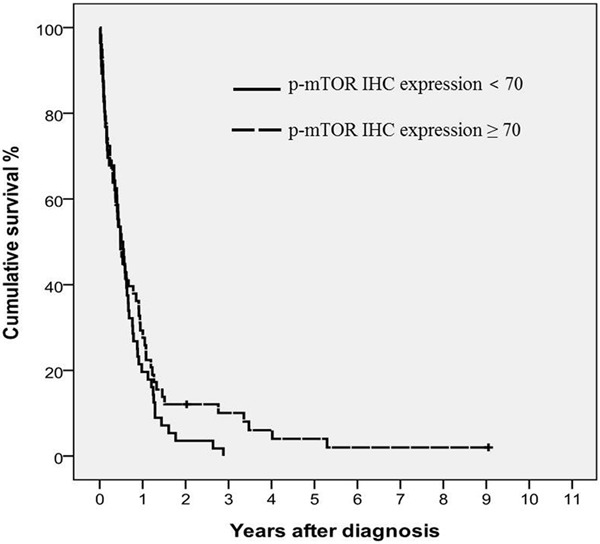
Survival analysis using the Kaplan-Meier method. Cumulative survival rates according to the expression levels of p-mTOR (P = 0.170).
Discussion
SCLC is the second most common primary lung cancer, accounting for approximately 15-20% of all lung cancers [1]. Because of its aggressive growth and extensive metastasis, the main therapy for SCLC has been chemoradiation [1,12]. SCLC shows a better response to initial chemotherapy, as compared to NSCLC. However, SCLC has a higher recurrence rate and worse prognosis than NSCLC. In recent years, molecular targeting agents, especially for EGFR and ALK-EML4, have been developed for the treatment of adenocarcinoma, but not SCLC [13]. New therapeutic strategies that can improve the survival of SCLC patients have not been developed yet.
mTOR is involved in various cellular processes including cell growth, metabolism, proliferation, and survival by regulating protein synthesis, and is activated by numerous molecules such as growth factors, hormones, cytokines, and other signaling molecules. mTOR is one of the key molecules of the PI3K/AKT/mTOR pathway that contributes to the development of various human cancers [3,4]. For this reason, mTOR has been regarded as an attractive potential target of anticancer agents.
Recent studies have shown the influences of the PI3K/AKT/mTOR pathway on SCLC pathogenesis. Tsurutani et al. [14] reported that activation of PI3K/AKT/mTOR pathway plays a critical role in cellular survival and induces resistance to imatinib, and Krystal et al. [15] reported that PI3K/AKT signaling induces a resistance to etoposide-mediated cell death. In both studies, mTOR inhibitors showed inhibition of growth and survival of human SCLC cells. However, two phase II clinical trials have shown conflicting results. The study using temsirolimus (CCL-779), a novel mTOR inhibitor, did not show any significant increase in progression-free survivals in patients with extensive-stage SCLC [16]. Similarly, the other phase II study with everolimus (RAD001) failed to show a significantly improved survival rate in previously treated and relapsed SCLCs [11]. These results suggest that all SCLCs do not respond well to mTOR inhibitors, and that there might be a specific SCLC group for which mTOR inhibitors would be more effective. We speculated that p-mTOR expression in tumor tissues could provide a clue to identify the specific SCLC group that is more sensitive to mTOR inhibitors. However, the association between p-mTOR expression and the clinical features of SCLC still remains unclear.
We found that p-mTOR expression is significantly higher in limited-stage than extended-stage SCLCs, and that lymph node metastasis was more frequently detected in the low p-mTOR expression group, as compared to the high expression group, although the difference was not statistically significant. In our study, only two patients were alive after the study period. They both had a limited-stage tumor with high p-mTOR expression. SCLC patients with high p-mTOR expression showed better survival rates than those with low p-mTOR expression, but it was not statistically significant. Our results are in accordance with previous studies on neuroendocrine tumors of the lung [10,17]. They demonstrated that high-grade neuroendocrine carcinomas, such as large cell neuroendocrine carcinoma (LCNEC) or SCLC, have lower levels of p-mTOR expression compared with low-grade neuroendocrine tumors such as typical or atypical carcinoid, and that p-mTOR expression inversely correlates with tumor size in high-grade neuroendocrine carcinomas.
Interestingly, the above results in SCLC contradict previous data regarding other types of cancers. In various cancers, it is well-established that activated AKT/mTOR pathway promotes growth, proliferation, and survival of tumor cells, leading to carcionogenesis and chemoresistance [18-20], and that high p-mTOR expression is associated with unfavorable prognostic factors [5-8]. A possible hypothesis to explain our unexpected results is that activated mTOR might have a crucial role for initial SCLC cell transformation, but thereafter downstream molecules of mTOR signaling process or another signaling pathways, including fatty acid synthase (Fas) and Ras/Raf/MEK/ERK pathway, might dominate in their contribution to tumor proliferation and progression, and negatively regulate mTOR in advanced SCLCs [21,22]. Further molecular studies are needed to identify cross-talk between intracellular pathways that could alter the oncogenic potential of mTOR in SCLC.
On the basis of our results, mTOR inhibitors might be more effective for limited-stage SCLCs with high p-mTOR expression, but do not have a definite advantage in treatment for extended-stage SCLCs with low p-mTOR expression. This may be why previous phase II clinical trials with mTOR inhibitors failed to show improved survival rates in advanced or relapsed SCLC patients. In SCLC, the therapeutic value of mTOR inhibitors seems to be limited because novel target therapies are far more necessary for advanced or chemoresistant SCLCs than for early ones.
Our study has some limitations. First, this is a retrospective study with a small number of enrolled cases. Second, we could not evaluate the correlation between mTOR and related candidate molecules. Finally, our study does not provide any information about the response to mTOR inhibitors according to the expression level of p-mTOR in SCLCs. Despite the limitations, however, our study showed that p-mTOR is expressed higher in a specific SCLC group with a limited stage and without lymph node metastasis. This result indicates that p-mTOR might play a more pivotal role in the biologic behavior of early SCLCs than advanced ones and the effectiveness of mTOR inhibitors might vary according to the extent of disease. New trials incorporating the response to mTOR inhibitors need to consider the preceding evaluation of the expression level of p-mTOR in SCLC.
Acknowledgements
This study was supported by a grant from the Dong-A ST.
Disclosure of conflict of interest
None.
References
- 1.Amini A, Byers LA, Welsh JW, Komaki RU. Progress in the management of limited-stage small cell lung cancer. Cancer. 2014;120:790–798. doi: 10.1002/cncr.28505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argiris A, Murren JR. Staging and clinical prognostic factors for small-cell lung cancer. Cancer J. 2001;7:437–447. [PubMed] [Google Scholar]
- 3.Marinov M, Fischer B, Arcaro A. Targeting mTOR signaling in lung cancer. Crit Rev Oncol Hematol. 2007;63:172–182. doi: 10.1016/j.critrevonc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Sun SY, Fu H, Khuri FR. Targeting mTOR signaling for lung cancer therapy. J Thorac Oncol. 2006;1:109–111. [PubMed] [Google Scholar]
- 5.Dhillon T, Mauri FA, Bellezza G, Cagini L, Barbareschi M, North BV, Seckl MJ. Overexpression of the mammalian target of rapamycin: a novel biomarker for poor survival in resected early stage non-small cell lung cancer. J Thorac Oncol. 2010;5:314–319. doi: 10.1097/JTO.0b013e3181ce6604. [DOI] [PubMed] [Google Scholar]
- 6.An JY, Kim KM, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Prognostic role of p-mTOR expression in cancer tissues and metastatic lymph nodes in pT2b gastric cancer. Int J Cancer. 2010;126:2904–2913. doi: 10.1002/ijc.24872. [DOI] [PubMed] [Google Scholar]
- 7.Zhang YJ, Dai Q, Sun DF, Xiong H, Tian XQ, Gao FH, Xu MH, Chen GQ, Han ZG, Fang JY. mTOR signaling pathway is a target for the treatment of colorectal cancer. Ann Surg Oncol. 2009;16:2617–2628. doi: 10.1245/s10434-009-0555-9. [DOI] [PubMed] [Google Scholar]
- 8.Sun CH, Chang YH, Pan CC. Activation of the PI3K/Akt/mTOR pathway correlates with tumour progression and reduced survival in patients with urothelial carcinoma of the urinary bladder. Histopathology. 2011;58:1054–1063. doi: 10.1111/j.1365-2559.2011.03856.x. [DOI] [PubMed] [Google Scholar]
- 9.Albanell J, Dalmases A, Rovira A, Rojo F. mTOR signalling in human cancer. Clin Transl Oncol. 2007;9:484–493. doi: 10.1007/s12094-007-0092-6. [DOI] [PubMed] [Google Scholar]
- 10.Righi L, Volante M, Rapa I, Tavaglione V, Inzani F, Pelosi G, Papotti M. Mammalian target of rapamycin signaling activation patterns in neuroendocrine tumors of the lung. Endocr Relat Cancer. 2010;17:977–987. doi: 10.1677/ERC-10-0157. [DOI] [PubMed] [Google Scholar]
- 11.Tarhini A, Kotsakis A, Gooding W, Shuai Y, Petro D, Friedland D, Belani CP, Dacic S, Argiris A. Phase II study of everolimus (RAD001) in previously treated small cell lung cancer. Clin Cancer Res. 2010;16:5900–5907. doi: 10.1158/1078-0432.CCR-10-0802. [DOI] [PubMed] [Google Scholar]
- 12.Califano R, Abidin AZ, Peck R, Faivre-Finn C, Lorigan P. Management of small cell lung cancer: recent developments for optimal care. Drugs. 2012;72:471–490. doi: 10.2165/11597640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Ulivi P, Zoli W, Capelli L, Chiadini E, Calistri D, Amadori D. Target therapy in NSCLC patients: Relevant clinical agents and tumour molecular characterisation. Mol Clin Oncol. 2013;1:575–581. doi: 10.3892/mco.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsurutani J, West KA, Sayyah J, Gills JJ, Dennis PA. Inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin-mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy. Cancer Res. 2005;65:8423–8432. doi: 10.1158/0008-5472.CAN-05-0058. [DOI] [PubMed] [Google Scholar]
- 15.Krystal GW, Sulanke G, Litz J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther. 2002;1:913–922. [PubMed] [Google Scholar]
- 16.Pandya KJ, Dahlberg S, Hidalgo M, Cohen RB, Lee MW, Schiller JH, Johnson DH Eastern Cooperative Oncology Group (E1500) A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the Eastern Cooperative Oncology Group (E1500) J Thorac Oncol. 2007;2:1036–1041. doi: 10.1097/JTO.0b013e318155a439. [DOI] [PubMed] [Google Scholar]
- 17.Alì G, Boldrini L, Capodanno A, Pelliccioni S, Servadio A, Crisman G, Picchi A, Davini F, Mussi A, Fontanini G. Expression of p-AKT and p-mTOR in a large series of bronchopulmonary neuroendocrine tumors. Exp Ther Med. 2011;2:787–792. doi: 10.3892/etm.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Dan HC, Park S, Yang L, Liu Q, Kaneko S, Ning J, He L, Yang H, Sun M, Nicosia SV, Cheng JQ. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–987. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 19.Oki E, Baba H, Tokunaga E, Nakamura T, Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, Maehara Y. Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer. 2005;117:376–380. doi: 10.1002/ijc.21170. [DOI] [PubMed] [Google Scholar]
- 20.Liu SQ, Yu JP, Yu HG, Lv P, Chen HL. Activation of Akt and ERK signalling pathways induced by etoposide confer chemoresistance in gastric cancer cells. Dig Liver Dis. 2006;38:310–318. doi: 10.1016/j.dld.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu M, Kondo M, Ito Y, Kume H, Suzuki R, Yamaki K. Soluble Fas and Fas ligand provide new information on metastasis and response to chemotherapy in SCLC patients. Cancer Detect Prev. 2005;29:175–180. doi: 10.1016/j.cdp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Pardo OE, Arcaro A, Salerno G, Tetley TD, Valovka T, Gout I, Seckl MJ. Novel cross talk between MEK and S6K2 in FGF-2 induced proliferation of SCLC cells. Oncogene. 2001;20:7658–7667. doi: 10.1038/sj.onc.1204994. [DOI] [PubMed] [Google Scholar]


