Abstract
Overexposure to prenatal dexamethasone (Dex) leads to small placental and fetal size and the alteration of fetal programming. Folate plays important roles in processes associated with successful pregnancy, including angiogenesis and trophoblast invasion. Placental folate transport is altered with prenatal Dex administration. The purpose of this study was to investigate the protective role of maternal folate administration in placentas exposed to Dex. In vitro, four groups of C57BL/6J pregnant mice were utilized: 1) normal drinking water + Saline injection group (NN); 2) normal drinking water + Dex injection group (ND); 3) drinking water with folate + Saline injection group (FN); and 4) drinking water with folate + Dex injection group (FD). In vivo, four treatment groups of the human extravillous trophoblast HTR-8/SVneo cells were classified: 1) control (NN); 2) Dex treatment (ND); 3) folate treatment (FN); and 4) folate and Dex treatment (FD). The results showed the maternal folate increases the placental size, birth weight, and expression of matrix metalloproteinases 9 (MMP9) in a mice model of Dex overexposure. In human extravillous trophoblast HTR8/SVneo, folate ameliorated the Dex-induced supress of cell migration and improved the expression/activity of MMP2 and MMP9. In conclusion, folate might be a potential therapy intervention to reduce the adverse effects of prenatal Dex exposure partially via improved trophoblast migration.
Keywords: Placenta, fetal programming, dexamethasone, folate
Introduction
Dexamethasone (Dex) is widely used in the world to prevent respiratory distress syndrome (RDS) with threatened preterm labour [1]. However, several studies have indicated the prenatal overexposure to Dex leads to small placental and fetal size, substantially increasing the morbidity of metabolic, cardiovascular and mental disorders in later life of offspring, which is called the ‘fetal programming’ [2]. This deterioration might be explained by the role of Dex in inhibiting the migration and invasion of trophoblast [3]. Unfortunately, how to prevent from this deterioration remains unknown.
Placenta is well accepted as the frontline of fetal exposure of insults causing adverse intrauterine environment [2]. However, placenta, per se, is exposed to maternal adverse insults. Placental dysfunction is associated with pregnancy complications such as fetal growth restriction (FGR) and preeclampsia. In pregnancies, placental extravillous trophoblast (EVT) is responsible for the erosion of uterine spiral arteries and secretion of angiogenic and vasodilator signals to remodel uterine vasculature [4]. Meanwhile, matrix metalloproteinases (MMPs) involve in the decomposition of basement membranes and the extracellular matrix (ECM) during EVT invasion [5].
Within gestation, folate is crucial in series of physiological processes associated with successful placental and fetal development, including angiogenesis and trophoblast invasion [6-8]. Recently, Wyrwoll et al have demonstrated the increasing transport of folate in the placenta with prenatal Dex administration [9], suggesting a potential interaction between folate and Dex through an, as yet, unknown mechanism. The purpose of this study was to explore the effects of folate on the placenta exposed to Dex. We hypothesize folate might attenuate the Dex-induced growth restriction of placentas and fetuses, which might partially through enhanced trophoblast migration.
Materials and methods
Animal treatment
All procedures involving animals were approved by the Laboratory Animal Care Committee of Tongji University under the Guide for the Care and Use of Laboratory Animals (NIH Guide). All efforts were made for minimizing the number of animals used and their suffering.
C57BL/6J mice (20-25 g; SLAC, Shanghai, China) were maintained under controlled lighting and temperature (22.8°C). Virgin females (8-10 weeks of age) were mated overnight with experienced males (1:1). The morning when a vaginal plug was found was designated embryonic day 1 (E1). The following four experimental groups were utilized in this study: 1) normal drinking water + Saline injection group (NN); 2) normal drinking water + Dex injection group (ND); 3) drinking water with folate + Saline injection group (FN); and 4) drinking water with folate + Dex injection group (FD). Folate supplement (8 μg/kg•d, dissolved in drinking water, 100 μg/L; Sigma) started from 2 weeks before mating till E18. Dex (100 μg/kg•d; Sigma) injection was performed at 8:00 am from E13 to E17. Six dams from each group were sacrificed on the morning of E18. Placental and fetal weight, as well as placental largest diameter on the chorionic disc was calculated as litter average (8.59 ± 1.37 placentas/pups per litter).
Cell culture and treatment
The human extravillous trophoblast HTR-8/SVneo cells were obtained from ATCC® and cultured in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12, Gibco), containing 10% FBS (Gibco) and 1% penicillin/streptomycin (Gibco). Every test was repeated thrice. Four treatment groups were utilized: 1) control (NN); 2) Dex treatment (ND); 3) folate treatment (FN); and 4) folate and Dex treatment (FD). Cells were plated on 6 cm dishes with Dex (20 μM) and/or folate (10-6 M). After 48 h, cells and culture media were frozen at -80°C before further analyses.
Migration assay
Migration assay was performed using BD FalconTM cell culture inserts with uncoated porous filters (8-μm pore size) [10]. Firstly, cells were prepared with Dex (20 μM) and/or folate (10-6 M) for 24 h. 10 × 104 cells were then seeded into the upper chamber in 500 μl of FBS free DMEM/F-12 containing Dex (20 μM) and/or folate (10-6 M). Meanwhile, 800 μl of DMEM/F12 + 10% FBS containing Dex (20 μM) and/or folate (10-6 M) was added into the lower chamber. For the control cells, 500 μl of FBS free DMEM/F-12 was added into the upper chamber, while 800 μl of DMEM/F12 + 10% FBS were added into the lower chamber. Cells were allowed to migrate for 24 h. Migrated cells were dyed with Calcein-AM (Invitrogen) before observed. For each chamber, five fields of view (FOV) were randomly selected for cell counting. The optimal cell counting time in this study is at 20 h of migration, so the cell density of the migrated cells was calculated at 20 h.
Quantification of mRNA by real-time PCR
Total RNA was extracted from 6 placentas of 6 dams in each group or triplicate HTR-8/SVneo cells following the manufacturer’s protocol (RNAsimple Total RNA kit, Tiangen Biotech, Beijing, China). The mRNA (1.0 μg) was reverse-transcribed to cDNA using PrimeScript® RT reagent Kit (Takara BIO Inc., Dalian, China). Real-time RT-PCR was performed using power SYBR Green PCR master mix (Takara BIO Inc., Dalian, China) to measure mouse MMP2 and MMP9, and human MMP9 mRNA. Housekeeping gene GAPDH was routinely performed to control sampling errors. The 2-ΔΔCT method was utilized to calculate the ratio of the target gene over the control group in each sample. The sequences of primers adopted were as follows: mouse-MMP2 (forward) 5’-AACTTTGAGAAGGATGGCAAGT-3’, (reverse) 5’-TGCCAC CCATGGTAAACAA-3’; mouse-MMP9 (forward) 5’-CTGGACAGCCAGACA CTAAAG-3’, (reverse) 5’-CTCGCGGCAAGTCTTCAGAG-3’; human-MMP9 (forward) 5’-GGGACGCAGACATCGTCATC-3’, (reverse) 5’-TCGTCATCG TCGAAATGGTGGGC-3’; and GAPDH (forward) 5’-GCACCGTCAAGGCTG AGAAC-3’, (reverse) 5’-TGGTGAAGACGCCAGTGGA-3’.
Gelatin zymography
The biologic activity of MMP2 and MMP9 in HTR-8/SVneo cells were analyzed by gelatin zymography [11]. Total protein in conditioned culture media was extracted and 50 μg protein was electrophoresed in 10% polyacrylamide gel containing 0.1 mg/ml gelatin (Sigma) under nonreducing condition. After electrophoresis the gel was rinsed, incubated, stained and destained as Al-Raawi et al described [11]. The MMP bands were scanned and semi-quantified by a quantity system (FR-200, Furi, Shanghai, China).
Statistics
Data was shown as mean ± S.E.M. Two-way ANOVA followed by least significance difference (LSD) was used to compare continuous variable among groups. Values of P < 0.05 were considered significant differences.
Results
In this study, a prenatal Dex-exposed mice model with or without perinatal folate supplementation was established to assess variability of placental and fetal growth. Dex exposure resulted in the reduction of placental weight and diameter (P < 0.05, Figure 1A), as well as that of fetal weight at E18 (P < 0.01, Figure 1B). With the administration of folate, a significant increase in placental and fetal weight in the FD group was observed when comparing with the ND group (P < 0.01, Figure 1A). No significant difference of placental diameter was presented between the ND and FD group (Figure 1B). The placental mRNA expression of MMP9 was decreased by excess Dex (NN vs. ND, P < 0.05, Figure 1C). This reduction was ameliorated by the maternal administration of folate (ND vs. FD, P < 0.05, Figure 1C).
Figure 1.
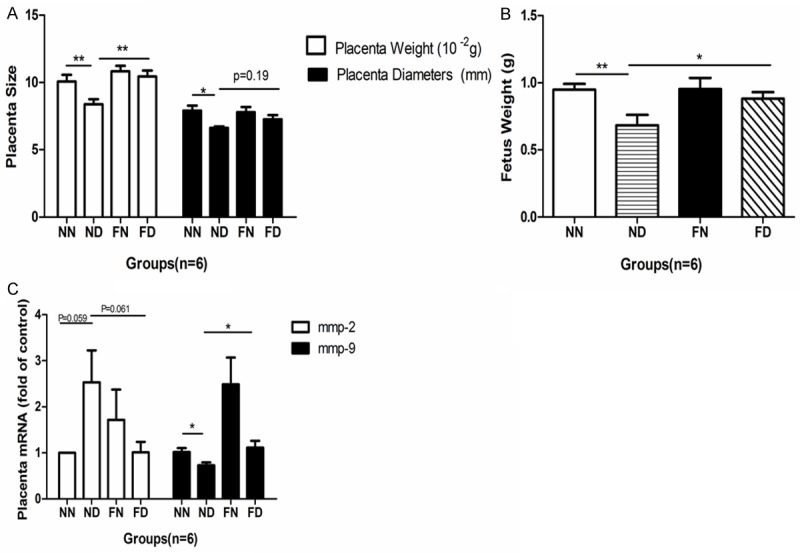
Effects of prenatal dexamethasone and/or folic acid on the mice placental size (A), fetal weight (B), and mRNA expression of MMP2 and MMP9 (C). The placental and fetal measurements were calculated as litter average. The n number represents number of dams. Data represents mean ± S.E.M in each group, *P < 0.05, **P < 0.01. NN, normal drinking water + Saline injection group; ND, normal drinking water + Dex injection group; FN, drinking water with folate + Saline injection group; FD, drinking water with folate + Dex injection group.
To further investigate the potential impacts of folate on the placenta exposed to Dex, human EVT cells HTR-8/SVneo were subject to Dex and/or folate. The migration of HTR-8/SVneo cells with the 20 μM Dex treatment was reduced by around 45.6% (P < 0.01, Figure 2A, 2B), consistent with a 60% reduction in the MMP9 mRNA expression (P < 0.01; Figure 2C). The migration of cells with the 10-6 M folate and 20 μM Dex (FD) treatment was significantly enhanced by 216% compared to that of cells with the ND treatment (P < 0.001, Figure 2A, 2B), accompanied by a 120% increase in the MMP9 mRNA expression (P < 0.05, Figure 2C).
Figure 2.
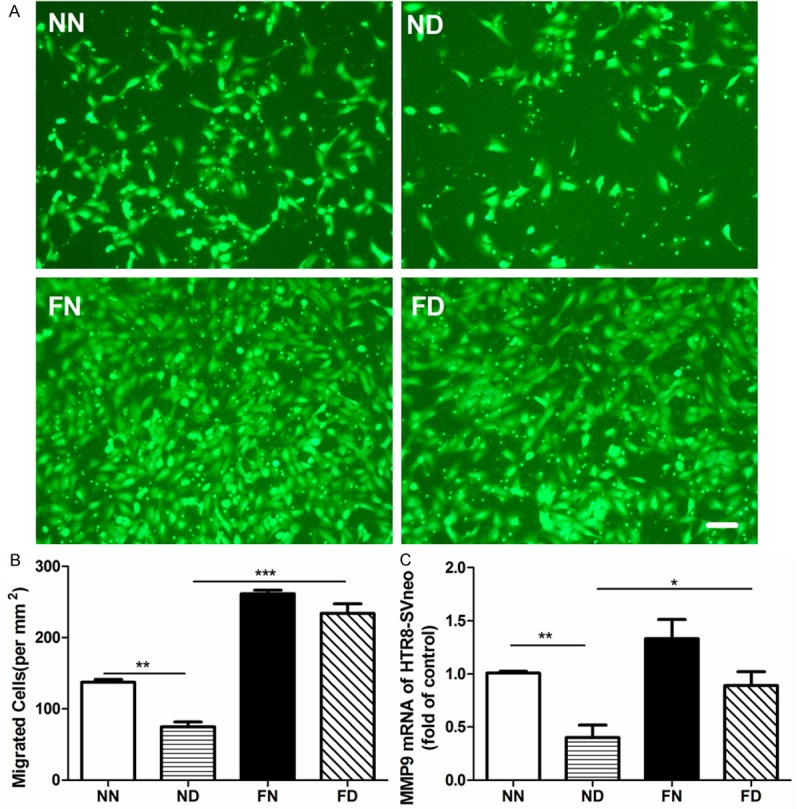
Effects of prenatal dexamethasone and/or folic acid on the migration and the MMPs expression in HTR-8/SVneo cells. A. HTR-8/SVneo cells migrating from the upper chamber (green), magnification ×100, Bar = 100 μm. B. Cell density of migrated HTR-8/SVneo cells. Each chamber, cells were calculated from five randomly collected visions under fluorescence microscopy. C. The MMP9 mRNA level of HTR-8/SVneo cells. Data represents mean ± S.E.M from three assays, *P < 0.05, **P < 0.01, ***P < 0.001. NN, control; ND, Dex treatment; FN, folate treatment; FD, folate and Dex treatment.
Gelatin zymography was performed to assess the biological activity of MMP2 and MMP9 in human EVT cells HTR-8/SVneo. With the 20 μM Dex treatment, the activity of MMP2 was reduced though without significant difference (P = 0.073, Figure 3A, 3B), while that of MMP9 was decreased significantly by 32% (P < 0.001; Figure 3A, 3B). The activity of MMP2 in the cells with the folate supplementation (FD) increased by 37% when compared to that in the cells with the ND treatment (P < 0.05, Figure 3A, 3B). Although different MMP9 activity of cells was not presented between ND and FD (P < 0.01, Figure 3A, 3B), that was observed between ND and FN (P < 0.01, Figure 3A, 3B).
Figure 3.
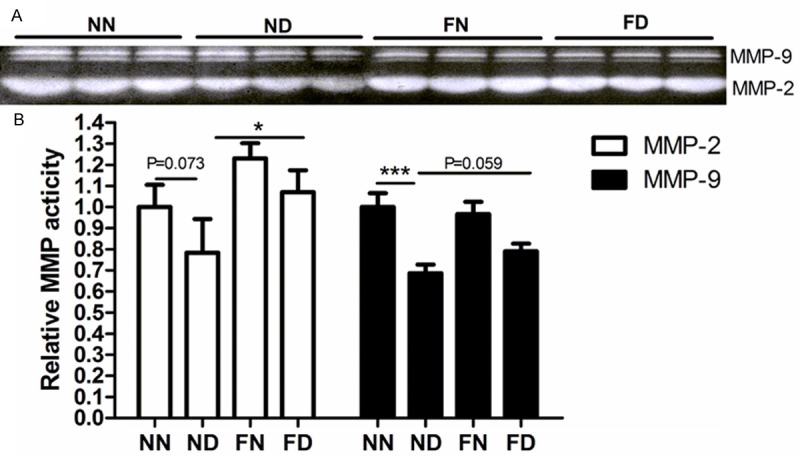
The activity of MMPs in conditioned media of HTR-8/SVneo cells. A. MMP2 and MMP9 bands analysed by gelatin zymography. B. Densitometric analysis of the MMP activity. Data represents mean ± S.E.M from three assays, *P < 0.05, **P < 0.01, ***P < 0.001. NN, control; ND, Dex treatment; FN, folate treatment; FD, folate and Dex treatment.
Discussion
In this study, we show the folate supplementation might improve the growth of the placenta and fetus exposed to Dex. In vitro, folate might ameliorate the Dex-induced supress of migration and improve the expression/activity of MMP2 and 9 in HTR8/SVneo cells. Our study for the first time reports that folate probably protects the placentas from overexposure to Dex, which might partially be through improving the migration of trophoblast.
In accordance with previous studies, our study confirmed the prenatal Dex exposure restricts placental and fetal growth [12,13]. Prenatal glucocorticoids (GCs) and FGR are both strongly predictive to the long-term cardiovascular and metabolic disorders in adulthood of offspring [2,14]. Our results might suggest a potential role of folate in improvement of fetal growth in adverse intrauterine environment.
In placenta, impaired extravillous trophoblast erosion of underlying decidua and walls of uterine spiral arteries is the cause of impaired remodelling of uterine vasculature. Failure of uterine artery remodelling could lead to failure of maternal blood perfusion of the placenta that could be the underlying basis for FGR [4,15]. Early studies documented the glucocorticoids inhibited the ability of trophoblast to invade through a Matrigel matrix [3,16], which was hypothesized as the underlying cause of FGR. On the contrary, Williams et al [8] reported folate improved the trophoblast invasion, correlating with increased placental secretion of MMP2, MMP3 and MMP9. For the first time, we demonstrates folate might ameliorate the Dex-induced suppress of the trophoblast migration in present study.
MMPs are important factors on cell invasion. MMP2 and MMP9 involve in digestion of collagen type IV and denatured collagen. MMP9 plays a more important role than MMP2 in trophoblast invasion [17]. The expression and protein activity of MMP2 and MMP9 in HTR8/SVneo cells with the Dex and/or folate treatment was partially in accordance with the ability of cell migration in present study. Although the folate supplementation failed to reverse the Dex-induced inhibition of MMP9 activity, different activity of MMP9 between the Dex and folate treatment might indicate the opposite impacts of Dex and folate on MMP9 modulation.
In conclusion, folate might ameliorate the Dex-induced fetal and placental growth restriction. This protective impact might be associated with the role of folate in improving trophoblast migration. Thus, the data presented here serves to suggest folate as a potential therapy intervention to reduce the adverse effects of prenatal Dex exposure. However, our data should be interpreted with care. Although folate induced no obvious placental disturbance in present study, accumulating evidence suggests this traditionally-regarded harmless nutrient, may be a double-edged sword especially for its potential to enhance proliferation of malignant cell [18]. Further studies are still necessary to determine the safety of perinatal folate intervention.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81270759) and the Major State Basic Research Development Program of China (973 Program) No. 2012CB966300.
Disclosure of conflict of interest
None.
References
- 1.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013;34:841–845. doi: 10.1016/j.placenta.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 3.Mandl M, Ghaffari-Tabrizi N, Haas J, Nohammer G, Desoye G. Differential glucocorticoid effects on proliferation and invasion of human trophoblast cell lines. Reproduction. 2006;132:159–167. doi: 10.1530/rep.1.00976. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 5.Huppertz B, Kertschanska S, Demir AY, Frank HG, Kaufmann P. Immunohistochemistry of matrix metalloproteinases (MMP), their substrates, and their inhibitors (TIMP) during trophoblast invasion in the human placenta. Cell Tissue Res. 1998;291:133–148. doi: 10.1007/s004410050987. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg G, Colla M, Endres M. Folic acid, neurodegenerative and neuropsychiatric disease. Curr Mol Med. 2009;9:315–323. doi: 10.2174/156652409787847146. [DOI] [PubMed] [Google Scholar]
- 7.Salihu HM, Salinas-Miranda A, de la Cruz C, Alio AP. The role of folic acid in fetal programming of birth phenotypes and early human development: a biopsychosocial perspective. J Dev Orig Health Dis. 2013;4:442–457. doi: 10.1017/S2040174413000317. [DOI] [PubMed] [Google Scholar]
- 8.Williams PJ, Bulmer JN, Innes BA, Broughton Pipkin F. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biol Reprod. 2011;84:1148–1153. doi: 10.1095/biolreprod.110.088351. [DOI] [PubMed] [Google Scholar]
- 9.Wyrwoll CS, Kerrigan D, Holmes MC, Seckl JR, Drake AJ. Altered placental methyl donor transport in the dexamethasone programmed rat. Placenta. 2012;33:220–223. doi: 10.1016/j.placenta.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Gu W, Gao T, Shen J, Sun Y, Zheng X, Wang J, Ma J, Hu XY, Li J, Hu MJ. MicroRNA-183 inhibits apoptosis and promotes proliferation and invasion of gastric cancer cells by targeting PDCD4. Int J Clin Exp Med. 2014;7:2519–2529. [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Raawi D, Abu-El-Zahab H, El-Shinawi M, Mohamed MM. Membrane type-1 matrix metalloproteinase (MT1-MMP) correlates with the expression and activation of matrix metalloproteinase-2 (MMP-2) in infl ammatory breast cancer. Int J Clin Exp Med. 2011;4:265–275. [PMC free article] [PubMed] [Google Scholar]
- 12.Baisden B, Sonne S, Joshi RM, Ganapathy V, Shekhawat PS. Antenatal dexamethasone treatment leads to changes in gene expression in a murine late placenta. Placenta. 2007;28:1082–1090. doi: 10.1016/j.placenta.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuffe JS, O’Sullivan L, Simmons DG, Anderson ST, Moritz KM. Maternal corticosterone exposure in the mouse has sex-specific effects on placental growth and mRNA expression. Endocrinology. 2012;153:5500–5511. doi: 10.1210/en.2012-1479. [DOI] [PubMed] [Google Scholar]
- 14.Fukami T, Sun X, Li T, Yamada M, Desai M, Ross MG. Mechanism of programmed obesity: altered central insulin sensitivity in growth-restricted juvenile female rats. J Dev Orig Health Dis. 2013;4:239–248. doi: 10.1017/S2040174413000019. [DOI] [PubMed] [Google Scholar]
- 15.Chaddha V, Viero S, Huppertz B, Kingdom J. Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med. 2004;9:357–369. doi: 10.1016/j.siny.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Librach CL, Feigenbaum SL, Bass KE, Cui TY, Verastas N, Sadovsky Y, Quigley JP, French DL, Fisher SJ. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem. 1994;269:17125–17131. [PubMed] [Google Scholar]
- 17.Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 18.Strickland KC, Krupenko NI, Krupenko SA. Molecular mechanisms underlying the potentially adverse effects of folate. Clin Chem Lab Med. 2013;51:607–616. doi: 10.1515/cclm-2012-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]


