Abstract
Background/Aim: Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors. It has been reported that Wnt signaling pathway plays an important role in Esophageal Cancer progression, metastasis and invasion. However the clinicopathological significance of Wnt2, GSK3β, and β-catenin in ESCC has been little reported. In the present study, the aim of this study was to investigate the clinicopathologic and prognosis roles of Wnt2, GSK3β, and β-catenin in ESCC tissue. Methods: 265 ESCC samples were analyzed by immunohistochemistry using Wnt2, GSK3β, and β-catenin antibodies. Then, correlation of Wnt2, GSK3β, and β-catenin expression with clinicopathological features and prognosis of ESCC patients was statistically analyzed. Results: Cytoplasmic Wnt2 overexpression was detected in 55.5% (147 of 265) ESCCs, which was significantly correlated with the degree of differentiation (P = 0.031). Cytoplasmic GSK3β overexpression was detected in 7.2% (19 of 265) ESCCs, and aberrant β-catenin expression was identified in 54.3% (144 of 265) of ESCCs. The positive rate of Wnt2 significantly increased with the malignant degree of Kazak ESCC patients. The aberrant β-catenin expression in GSK3β-negative ESCC was significantly associated with the ethnic, tumor size, tumor location, degree of differentiation, AJCC stage, lymph node status. Furthermore, the expression of β-catenin implicated the ethnic difference (P = 0.019). In Kaplan-Meier curve analysis, no significant correlation was observed between the expression of Wnt2, GSK3β, β-catenin and the poor prognosis of ESCCs. Conclusion: The aberrant β-catenin expression could be an adverse underlying factor in carcinogenesis and progression of ESCC. There was a different statistical signification for β-catenin in Kazakhs to compare with Hans.
Keywords: Esophageal squamous cell carcinoma, immunohistochemistry, Wnt2, GSK3β, β-catenin, clinicopathology
Introduction
Esophageal cancer (EC) is one of the most common malignant tumors, and is the sixth most common cause of cancer death worldwide [1]. Despite progress in the multimodality treatment of esophageal cancer in the past several decades, the prognosis for esophageal cancer remains poor [2]. More than 90% of esophageal cancer is squamous cell carcinoma (ESCC) [3]. Xinjiang is one of the higher incidence areas of esophagus cancer in China. The highest incidence of ESCC is Kazakhs in Xinjiang [4]. The previous study shows that Wnt signaling pathway plays an important role in esophageal cancer progression, metastasis and invasion [5]. In addition, in our previous study, LC-ESI-MS was used in fresh tumor samples to detect the gene expression profiles in ESCC tissues matched adjacent non-cancerous samples. It was found that the protein expression of Wnt2 and β-catenin was high expression in ESCC compared with normal esophageal tissue, while the protein expression of GSK3β was low expression in ESCC compared with normal esophageal tissue. However, the clinicopathological significance of Wnt2, GSK3β, and β-catenin in ESCC has been little reported. Thus, further study was designed to investigate the impacts on the clinicopathological features and prognosis of patients with ESCC.
Wnt signaling pathway not only plays an important role in embryonic development but also in cancer biology [6,7]. It is consisted of the canonical Wnt pathway, the planar cell polarity pathway and the Wnt/Ca2+ pathway [8]. the canonical Wnt pathway was considered as a central mechanism in cancer biology [8].Canonical Wnt signaling activation is multistep complex process involving in Wnt2, GSK3β, Axin, APC, β-catenin, TCF, c-myc and cyclin D1 [6].
Wnt2, a ligand protein, is secreted by tumor fibroblasts (TF) in a paracrine fashion [9]. Wnt2 in the different cells can activate different signaling pathways, including WNT2/β-catenin pathway, also called the canonical Wnt pathway [10]. WNT2/β-catenin pathway promotes β-catenin expression and induces β-catenin translocation from the cell membrane to the cytoplasm even to the nucleus [7,11]. β-catenin is a multifunctional protein which mediates cell-extracellular matrix adhesion and promotes tumor proliferation and metastasis [12-14]. β-catenin not only has been extensively identified and studied in embryonal development [6], but also plays a vital role in tumor progression by affecting E-cadherin linking cell adhesion and Wnt signaling pathway [13,14]. Wnt2 signaling pathway can enhance the stability of β-catenin and cause the accumulation of free β-catenin in the nucleus [15]. β-catenin, which combines with T-cell factor/lymphoid enhancer factor (TCF/LEF) family, stimulates the transcription of specific genes which include in oncogenic transformation [16,17]. GSK3β is one of the few signaling mediators that play central roles in a diverse range of signaling pathways, including Wnt signaling pathway [18]. GSK3β mediates phosphorylation triggers β-catenin destabilization. However, Wnt signal inhibits GSK3β activity and increases free β-catenin level [19].
The purpose of this study was to investigate the clinicopathological significance of Wnt2, GSK3β, and β-catenin and to analyze their correlations with the prognosis.
Materials and methods
Patients and tissue samples
Informed consent was obtained from every patient prior surgery. The study was approved by Ethical Committee of the First Affiliated Hospital of Xinjiang Medical University. Between 2007 and 2014, 265 specimens of human ESCC and paired adjacent normal esophageal squamous epithelium were obtained from patients of different grades who underwent esophagectomy at First Affiliated Hospital of Xinjiang Medical University. At the same time, all patients never received any radiotherapy or chemotherapy prior to surgery. The patients consisted of 115 Kazakhs who was defined as experimental group and 150 Hans who was defined as control group. Diagnosis was performed at the Department of Pathology, First Affiliated Hospital of Xinjiang Medical University. All of the samples were histopathologically diagnosed as ESCC, and the following information was recorded for each patient: age, gender, ethnic, tumor location, tumor size, degree of differentiation, clinicopathological stage based on the seventh edition of the American Joint Committee on Cancer staging system (AJCC) [20], and lymph node status.
Immunohistochemistry
Formalin-fixed, paraffin-embedded primary tissues were cut 3 um thick. Tissue slides were blocked in xylene for 30 minutes and in graded ethanol for 5 minutes. To enhance antigen retrieval, the slides were autoclaved for 20 minutes in 1% sodium citrate buffer (PH 6.0) and then left at room temperature. Then the slides was incubated with 3% H2O2 in methanol for 15 min to quench the endogenous peroxidase activity .Slides were incubated with the primary antibodies at 4°C overnight against Wnt2 (1:200, rabbit monocolonal antibody, Abcam, UK), β-catenin (1:300, rabbit monocolonal antibody, Abcam, UK), and GSK3β (1:150, rabbit monocolonal antibody, Abcam, UK). The slides were incubated with the corresponding secondary antibodies (ZSGB, China) for 30 minutes at 37°C. The slides were immersed in the prepared DAB solution. Finally, the slides were counterstained with hematoxylin, dehydrated through increasing graded alcohol, coverslipped with mounting.
Immunohistochemical scoring
The expression of Wnt2, GSK3β, β-catenin was assessed by two independent pathologists who had no previous knowledge of clinical data. Positive expression of Wnt2, primarily a cytoplasmic pattern, was defined when five or more Wnt2-positive tumor fibroblasts (TF) in the stromal compartment were detected per microscopy field (20 × in magnification) [9]. Regarding the β-catenin expression, we used the classification of staining patterns as follows: (1) preserved expression pattern, if > 70% cancer epithelial cells were stained in the cell membranes; (2) reduced expression pattern, if the cancer cells stained were ≤ 70%; (3) translocation expression pattern, if immunoreactivity was present in the cytoplasm and/or nuclear in more than 10% of the cancer cells [21]. Reduced expression pattern and translocation expression pattern were defined as the aberrant expression of β-catenin. The intensity score of cytoplasmic GSK3β was evaluated as follows: 0, negative; 1, weak staining; 2, moderate staining; 3, strong staining. The score of 0 or 1 was considered low-expression of GSK3β, and the score of 2 or 3 was considered high-expression of GSK3β.
Statistical analysis
All statistical analyses were performed using the software package from SPSS version 16.0 for Windows (SPSS Inc, IL, USA). The continuous variables were expressed as means ± SEM. The associations between canonical Wnt signaling pathway proteins expression and different clinical characteristics were estimated using Fisher’s exact test or χ2 test. The patients were routinely followed-up clinically Overall survival (OS) was defined as time between date of surgery and date of death or the date of last follow-up. Overall survival was calculated using the Kaplan-Meier method. Overall survival was defined as the time from the date of surgical resection to the date of death. Differences were indicated statistically significant when P was less than 0.05 and all P values were two-tailed.
Results
Clinical characteristics of ESCC patients
The patients consisted of 189 (71.1%) males and 76 (28.6%) females with the median age at diagnosis were 62.15 years. The median age of Hans at diagnosis was 64.41. However, the median age of Kazakhs at diagnosis was 59.2, which was smaller than the median age of Hans. Most of the cases were moderately differentiated, included 67 Kazakhs and 96 Hans. The 265 patients were classified according to AJCC as follow: pathological stage T0, 17 (6.4%); pathological stage T1, 122 (46%); pathological stage T2, 35 (13.2%); pathological stage T3, 91 (34.2%). In addition, the rate of lymph node metastasis in the Kazakhs (34.8%) was higher than the rate of lymph node metastasis in the Hans (20.0%). Patients detailed characteristic included in this study were summarized in Table 1. The patients were routinely followed-up clinically after surgery for a median period of 15 months.
Table 1.
Characteristics of 265 ESCC patients included in this study n (%)
| Characteristic | Kazakh | Han | Total |
|---|---|---|---|
| Age | |||
| Median | 59.2 | 64.41 | 62.15 |
| Range | 60.00 ± 9.019 | 65 ± 8.712 | 63.00 ± 9.201 |
| Sex | |||
| Male | 80 (69.6) | 109 (72.2) | 189 (71.1) |
| Female | 35 (30.4) | 41 (27.2) | 76 (28.6) |
| Tumor size | |||
| ≤ 3 cm | 36 (31.7) | 37 (24.5) | 78 (29.7) |
| > 3 cm | 79 (68.3) | 97 (64.2) | 187 (70.3) |
| Tumor location | |||
| upper | 3 (2.6) | 10 (6.6) | 13 (5.0) |
| middle | 33 (27) | 70 (46.7) | 103 (38.9) |
| lower | 79 (68.6) | 70 (46.7) | 149 (56.1) |
| Degree of differentiation | |||
| Well-differentiated | 23 (20) | 19 (12.7) | 42 (15.8) |
| moderately differentiated | 67 (58.3) | 96 (64.0) | 163 (61.6) |
| poorly differentiated | 25 (21.7) | 35 (23.3) | 60 (22.6) |
| AJCC stage | |||
| T0 | 4 (3.5) | 13 (8.6) | 17 (6.4) |
| T1 | 58 (50.4) | 64 (42.4) | 122 (46.0) |
| T2 | 18 (15.7) | 17 (11.3) | 35 (13.2) |
| T3 | 35 (30.4) | 56 (37.1) | 91 (34.2) |
| Lymph node status | |||
| No | 75 (65.2) | 120 (80.0) | 195 (70.7) |
| Yes | 40 (34.8) | 30 (20.0) | 70 (25.6) |
ESCC: esophageal squamous cell carcinoma; AJCC: American joint committee on cancer.
Wnt pathway proteins expression in ESCCs
Wnt2 expression appeared in the form of a cytoplasmic staining pattern (Figure 1A). In most (147/265) ESCC cases, the Wnt2 expression was positive in tumor fibroblasts. However, the Wnt2 expression was negative in normal esophageal epithelial tissues (Figure 1B). The expression of Wnt2 in the tumors in Kazakhs was 60.0% (69/115), which was higher than that in the tumors in Hans (52%, 78/150), but there was no statistical significant difference (P > 0.05). The positive rate of Wnt2 in Kazakh ESCC patients with various differentiation degree were: Well-differentiated, 52.2% (12/23); moderately differentiated, 55.2% (37/67); poorly differentiated, 80% (20/25). The result indicated the positive rate of Wnt2 significantly increased with the malignant degree of Kazak ESCC patients (P = 0.031). Furthermore, no significant differences were observed according to the tumor size, tumor location, degree of differentiation, AJCC stage, lymph node status (Table 2).
Figure 1.
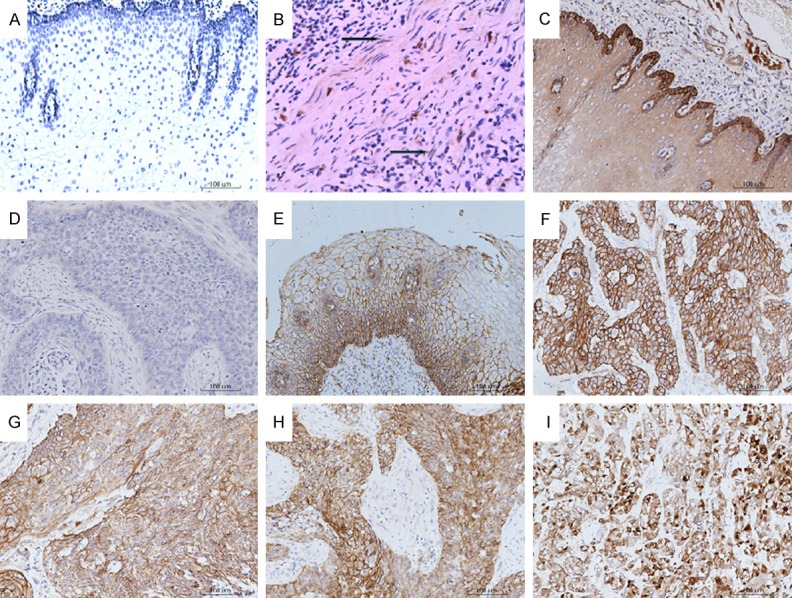
Immunohistochemical expressions of Wnt2, GSK3β, β-catenin in normal and cancer tissues of the esophagus (Original magnification ×200). A. Wnt2-negative staining in normal esophageal tissue; B. Cytoplasmic Wnt2-positive staining in ESCC tissue; C. Cytoplasmic GSK3β-positive staining in normal esophageal tissue; D. GSK3β-negative staining in ESCC tissue; E. Membranous β-catenin-positive staining in normal esophageal tissue; F. Membranous-cytoplasmic β-catenin-positive staining in ESCC tissue; G. Cytoplasmic β-catenin-positive staining in ESCC tissue; H. Cytoplasmic-nuclear β-catenin-positive staining in ESCC tissue; I. Nuclear β-catenin-positive staining in ESCC tissue.
Table 2.
Associations between Wnt2, GSK3β, β-catenin and the clinicopathological characteristic in ESCC
| Kazakh | Han | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Wnt2 | GSK3β | β-catenin | Wnt2 | GSK3β | β-catenin | Wnt2 | GSK3β | β-catenin | |
| Positive rate | 60.0% | 8.9% | 54.8% | 52.0% | 6.2% | 60% | 56.3% | 7.2% | 55.5% |
| P (Tumor location) | 0.270 | 0.109 | 0.568 | 0.681 | 0.087 | 0.701 | 0.605 | 0.002 | 0.314 |
| P (Tumor size) | 0.176 | 0.348 | 0.116 | 0.954 | 0.632 | 0.738 | 0.773 | 0.294 | 0.075 |
| P (Degree of differentiation) | 0.031 | 0.394 | 0.636 | 0.854 | 0.666 | 0.72 | 0.060 | 0.707 | 0.584 |
| P (AJCC stage) | 0.542 | 0.815 | 0.057 | 0.165 | 0.789 | 0.260 | 0.974 | 0.543 | 0.091 |
| P (Lymph node metastasis) | 0.214 | 0.666 | 0.134 | 0.590 | 0.571 | 0.212 | 0.234 | 0.858 | 0.117 |
| P (nation) | 0.659 | 0.625 | 0.401 | ||||||
The expression of GSK3β presented in the form of a cytoplasmic staining pattern (Figure 1C, 1D). Regarding the GSK3β expression, in all patients, 19 (7.2%) carcinomas were high-expression compared to 144 (54.2%) normal esophageal tissues. The GSK3β high-expression of Kazaks and Hans in tumors was 8.9% (13/115) and 6.2% (7/150) respectively. The statistic intimated that the expression status of GSK3β was closely associated with the tumor location (P = 0.02), but not associated with the ethnic, tumor size, degree of differentiation, AJCC stage, lymph node status (Table 2).
The β-catenin expression exhibited four staining patterns (Figure 1D-I). The β-catenin expression is localized to the membrane in normal epithelium, but aberrant expression was shown in 144 (54.3%) carcinoma tissues. The altered β-catenin expression rates in Kazak patients and Han patients were 54.8% (63/115) and 60.0% (90/150).
When the GSK3β expressions were down-regulated, the rate of the altered β-catenin expression in Kazak patients and Han patients were 55.6% and 47.1%. Obviously, the expression of β-catenin implicated the nation difference (P = 0.019). Among the GSK3β low-expressions, in Kazak ESCC patients, altered β-catenin expression was closely correlated with the AJCC stage (P = 0.05), but not with tumor size, tumor location, degree of differentiation, lymph node status. At the same time, the data showed the altered β-catenin expression in all GSK3β-negative samples was significantly correlated with the tumor location, tumor size, degree of differentiation, AJCC stage, lymph node status (Table 3). However, the aberrant expression of β-catenin in Han GSK3β-negative ESCC was not associated with ethnic group, tumor size, tumor location, degree of differentiation, AJCC stage and lymph node status (Table 3).
Table 3.
Relationship between the β-catenin in GSK3β-negative and clinicopathological characteristics
| Kazakh | P value | Han | P value | Total | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| β-catenin | β-catenin | β-catenin | |||||||
|
|
|
|
|||||||
| preserved | aberrant | preserved | aberrant | preserved | aberrant | ||||
| Tumor location | |||||||||
| Upper | 2 | 2 | 1 | 0 | 3 | 2 | |||
| Middle | 13 | 14 | 0.964 | 29 | 28 | 0.621 | 42 | 42 | 0.000 |
| Lower | 36 | 48 | 48 | 44 | 84 | 92 | |||
| Tumor size | |||||||||
| ≤ 3 cm | 15 | 13 | 0.686 | 28 | 22 | 0.614 | 43 | 35 | 0.002 |
| > 3 cm | 36 | 51 | 50 | 50 | 86 | 101 | |||
| Degree of differentiation | |||||||||
| Well | 13 | 13 | 13 | 0 | 26 | 13 | |||
| Moderately | 24 | 37 | 0.699 | 48 | 53 | 0.230 | 72 | 90 | 0.000 |
| Poorly | 14 | 14 | 18 | 18 | 32 | 32 | |||
| AJCC stage | |||||||||
| T0 | 0 | 4 | 2 | 8 | 2 | 12 | |||
| T1 | 22 | 31 | 0.05 | 40 | 30 | 0.152 | 62 | 61 | 0.000 |
| T2 | 16 | 5 | 0 | 8 | 16 | 13 | |||
| T3 | 13 | 24 | 40 | 22 | 53 | 46 | |||
| Lymph node metastasis | |||||||||
| No | 39 | 33 | 0.181 | 70 | 53 | 109 | 86 | ||
| Yes | 13 | 30 | 9 | 18 | 0.387 | 22 | 48 | 0.000 | |
Significant prognostic value of GSK3β and β-catenin expression patterns for ESCC
Survival dates were analyzed for 100 follow-up patients during follow-up periods of 1-72 months (median, 15 months). The ESCC-5-year survival rate was 3.7%. Overall Survival curves based on GSK3β and β-catenin expression were constructed using the Kaplan-Meier method. The β-catenin-altered-patients had significantly poorer survival in comparison to the β-catenin-preserved-patients (Figure 2A), but was not found statistically significant (P > 0.05). In addition, when the GSK3β was low-expression, the prognosis of the β-catenin-altered-patients was no obvious difference with the β-catenin-preserved-patients (Figure 2B).
Figure 2.
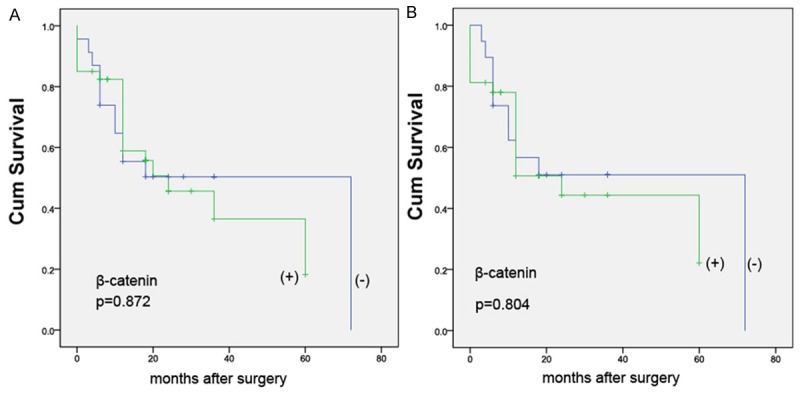
The relationship between the expression of β-catenin and survival curve. A. The aberrant β-catenin expression was not correlated with poor prognosis (P = 0.872); B. In the GSK3β-negative ESCCs, the prognosis of the β-catenin-altered-patients was no obvious difference with the β-catenin-preserved-patients (P = 0.804).
Discussion
Most patients with ESCC are diagnosed at an advanced stage [22]. Despite recent improvements in its treatment, the clinical outcome of ESCC patients remains unsatisfactory [23]. It is urgently needed to investigate the underlying molecular markers to improve the outcome of patients with ESCC. In recent years, numerous studies have demonstrated that Wnt canonical signaling pathway played an important role in carcinogenesis and progression in ESCC [23-25]. In the current study, we found that Wnt2 and β-catenin was up-regulated while GSK3β was down-regulated in ESCC tissue. Our result was concordant with the findings by Fu et al, He et al, and Wang et al [9,24,26].
Wnt2, a ligand protein, has been reported as an oncogene with the potential to activate the Wnt canonical signaling pathway [9]. The Wnt pathway is a central mechanism in cancer biology [27]. High-expression of Wnt2 has been observed in colorectal cancer, gastric cancer and esophageal adenocarcinoma [28-30], while there are a few studies concerning Wnt2 expression in ESCC. In our study, Wnt2 was not expressed in normal esophageal epithelial tissues. However, 56.3% (147/265) ESCCs were Wnt2 positive. It suggested that Wnt2 regulated ESCC progression by activating the Wnt canonical signaling pathway. Consistent with the idea that higher expression of Wnt2 in moderate- and high-grade malignant in comparison to low-grade malignant [31], we found that the positive rate of Wnt2 increased with the malignant degree.
GSK3β is an essential component of the Wnt signaling pathway and plays a critical role in sequestrating β-catenin, GSK3β has joined APC, Axin as a part of β-catenin destruction complex [32,33]. The main function of the destruction complex is to promote phosphorylacstion of β-catenin. The positive rate of GSK3β in ESCC tissues was 7.2%, but the normal esophageal tissue was 54.2%. It suggested that GSK3β poorly expressed in ESCC, which reconfirmed the findings by He et al [26]. Here, we novel found that the expression status of GSK3β was closely associated with the tumor location. Further studies on the relationship between the expression of GSK3β and tumor location would be very rewarding. GSK-3 is an essential component of the Wnt signaling pathway and plays important roles in regulating cell proliferation, differentiation, and apoptosis [34]. However, the GSK3β expression showed no significant association with tumor size, degree of differentiation, AJCC stage, lymph node status in ESCC.
β-catenin is not only an important structural component of both normal epithelium and malignant cells, but also a pivotal component of the canonical Wnt signaling pathway [35]. In normal esophageal, membrane β-catenin blinding to membrane E-cadherin forms a complex that promotes cell adhesion. The β-catenin expression in the cytoplasm and/or nuclear could be defined as the aberrant expression. The aberrant β-catenin accumulates the nucleus to interact with TCF/LEF transcription factors which are involved in oncogenic transformation [26,36]. Then, it was reported that β-catenin was significantly correlated with invasion depth and lymph node status [37]. This study also found that in GSK3β-negative ESCCs the aberrant expression of β-catenin significantly correlated with the clinicopathological features. The results demonstrated that the aberrant expression of β-catenin can be regarded as an indication for activated, oncogenic, Wnt signaling and β-catenin/TCF transcription. As Ninomiya et al. reported, GSK3β mediated phosphorylation function as a switch in regulating the stabilization of β-catenin [38]. However, the results never referred to the correlation between GSK3β and β-catenin. To further verify the relationship between GSK3β and β-catenin, further study should be reformed by other methods. There was a subgroup of patients who had alteration of β-catenin but negative GSK3β .One reason might be that β-catenin activated through other pathways besides Wnt2. Moreover, One critical findings in this study that the aberrant expression of β-catenin in GSK3β-negative ESCCs was significantly higher in Kazak ESCC patients. It was thought that there was compared difference of β-catenin expression in Kazak and Han. So the further research with a large sample size would be worth doing.
In this study, we did not investigate that patients survival was correlated with the expressions of GSK3β, β-catenin. The limitation of our study is high loss to follow-up, which may influence the results. The communication difficulties with most of Kazakhs, who usually spoke ethnic languages and lived in remote country from the First Affiliated Hospital, Xinjiang Medical University, leaded to lose contact or received some error message. Moreover, the wrong or missing phone numbers also caused some ESCCs could not be contacted. For these reasons, only 100 (37.7%) patients of ESCC were followed up. Therefore, the relationship between the prognoses with the Wnt canonical signaling pathway may be investigated by further study with a large sample size.
In summary, we found the positive rate of Wnt2 significantly increased with the malignant degree of Kazak ESCC patients. Our present study also demonstrated that in GSK3β-negative ESCCs, β-catenin was significantly correlated with the tumor location, tumor size, degree of differentiation, AJCC stage, lymph node metastasis. This report provided a novel indication that the expression of β-catenin implicated the ethnic difference. These finding suggests that the aberrant β-catenin expression could be an adverse underlying factor in carcinogenesis and progression of ESCC. Although Wnt2, GSK3β, β-catenin may not be an independent prognostic factor, it should be further verified by prospective analysis and more comprehensive follow-up.
Acknowledgements
This study was supported by the grant from “The National Natural Science Foundation of China (81260308) and Postgraduate Research Projects in Xinjiang Uygur Autonomous Region (XJGRI2014085)”.
Disclosure of conflict of interest
None.
References
- 1.Vallbohmer D, Brabender J, Metzger R, Holscher AH. Genetics in the pathogenesis of esophageal cancer: possible predictive and prognostic factors. J Gastrointest Surg. 2010;14(Suppl 1):S75–80. doi: 10.1007/s11605-009-1021-5. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Xi M, Li QQ, He LR, Liu SL, Zhao L, Shen JX, Liu MZ. The modified glasgow prognostic score is an independent prognostic factor in patients with inoperable thoracic esophageal squamous cell carcinoma undergoing chemoradiotherapy. J Cancer. 2014;5:689–695. doi: 10.7150/jca.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang AH, Liu Y, Wang B, He YX, Fang YX, Yan YP. Epidemiological studies of esophageal cancer in the era of genome-wide association studies. World J Gastrointest Pathophysiol. 2014;5:335–343. doi: 10.4291/wjgp.v5.i3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui XB, Chen YZ, Pang XL, Liu W, Hu JM, Li SG, Yang L, Zhang WJ, Liu CX, Cao YW, Jiang JF, Gu WY, Pang J, Yang L, Yuan XL, Yu SY, Li F. Multiple polymorphisms within the PLCE1 are associated with esophageal cancer via promoting the gene expression in a Chinese Kazakh population. Gene. 2013;530:315–322. doi: 10.1016/j.gene.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 5.Chai J, Modak C, Ouyang Y, Wu SY, Jamal MM. CCN1 induces β-Catenin translocation in esophageal squamous cell carcinoma through integrinα11. ISRN Gastroenterol. 2012;2012:207235. doi: 10.5402/2012/207235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol. 2004;48:477–487. doi: 10.1387/ijdb.041815jb. [DOI] [PubMed] [Google Scholar]
- 7.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23:111–6. doi: 10.1080/08977190500125746. [DOI] [PubMed] [Google Scholar]
- 9.Fu L, Zhang C, Zhang LY, Dong SS, Lu LH, Chen J, Dai Y, Li Y, Kong KL, Kwong DL, Guan XY. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/β-catenin signalling pathway. Gut. 2011;60:1635–1643. doi: 10.1136/gut.2011.241638. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Fu L, Fu J, Hu L, Yang H, Rong TH, Li Y, Liu H, Fu SB, Zeng YX, Guan XY. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res. 2009;15:4017–27. doi: 10.1158/1078-0432.CCR-08-2824. [DOI] [PubMed] [Google Scholar]
- 11.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 12.Gkretsi V, Zhang Y, Tu Y, Chen K, Stolz DB, Yang Y, Watkins SC, Wu C. Physical and functional association of migfilin with cell-cell adhesions. J Cell Sci. 2005;118:697–710. doi: 10.1242/jcs.01638. [DOI] [PubMed] [Google Scholar]
- 13.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 14.Kudo Y, Kitajima S, Ogawa I, Hiraoka M, Sargolzaei S, Keikhaee MR, Sato S, Miyauchi M, Takata T. Invasion and metastasis of oral cancer cells require methylation of E-cadherin and/or degradation of membranous beta-catenin. Clin Cancer Res. 2004;10:5455–5463. doi: 10.1158/1078-0432.CCR-04-0372. [DOI] [PubMed] [Google Scholar]
- 15.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 16.Krieghoff E, Behrens J, Mayr B. Nucleo-cytoplasmic distributionof β-catenin is regulated by retention. J Cell Sci. 2006;119:1453–63. doi: 10.1242/jcs.02864. [DOI] [PubMed] [Google Scholar]
- 17.Ishiguro H, Furukawa Y, Daigo Y, Miyoshi Y, Nagasawa Y, Nishiwaki T, Kawasoe T, Fujita M, Satoh S, Miwa N, Fujii Y, Nakamura Y. Isolation and characterization of human NBL4, a gene involved in the betacatenin/tcf signaling pathway. Jpn J Cancer Res. 2000;91:597–603. doi: 10.1111/j.1349-7006.2000.tb00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang X, Zheng D, Hu P, Zeng Z, Li M, Tucker L, Monahan R, Resnick MB, Liu M, Ramratnam B. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the β-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Res. 2014;42:2988–98. doi: 10.1093/nar/gkt1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu PK, Wu YC, Chou TY, Huang CS, Hsu WH. Comparison of the 6th and 7th editions of the American Joint Committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg. 2010;89:1024–1031. doi: 10.1016/j.athoracsur.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Lv J, Cao XF, Ji L, Zhu B, Wang DD, Tao L, Li SQ. Association of β-catenin, Wnt1, Smad4, Hoxa9, and Bmi-1 with the prognosis of esophageal squamous cell carcinoma. Med Oncol. 2012;29:151–160. doi: 10.1007/s12032-010-9816-5. [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, Chang X, LeBron C, Nagpal JK, Lee J, Huang Y, Yamashita K, Trink B, Ratovitski EA, Sidransky D. Neurofilament heavy polypeptide regulates the akt-β-catenin pathway in human esophageal squamous cell carcinoma. PLoS One. 2010;5:e9003. doi: 10.1371/journal.pone.0009003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao XW, Zhu ST, He YL, Li P, Wang YJ, Zhang ST. Epigeneticin activation of secreted frizzled-related protein2 in esophageal squamous cell carcinoma. World J Gastroenterol. 2012;18:532–540. doi: 10.3748/wjg.v18.i6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Xue L, Wang P. Prognostic value of β-catenin, c-myc, and cyclin D1 expressions in patients with esophageal squamous cell carcinoma. Med Oncol. 2011;28:163–169. doi: 10.1007/s12032-010-9436-0. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Lu Q, Han Y, Li Z, Zhang Z, Li X. ABCG2/V-ATPase was associated with the drug resistance and tumor metastasis of esophageal squamous cancer cells. Diagn Pathol. 2012;7:1746–1596. doi: 10.1186/1746-1596-7-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He H, Ding F, Li Y, Luo A, Chen H, Wu C, Liu Z. Migfilin regulates esophageal cancer cell motility through promoting GSK-3b-mediated degradation of β-catenin. Mol Cancer Res. 2012;7:180. doi: 10.1158/1541-7786.MCR-11-0419. [DOI] [PubMed] [Google Scholar]
- 27.Anastas JN, Moon RT. WNT signaling pathways as therapeutic target in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 28.Park JK, Song JH, He TC, Nam SW, Lee JY, Park WS. Overexpression of Wnt-2 in colorectal cancers. Neoplasma. 2009;56:119–23. doi: 10.4149/neo_2009_02_119. [DOI] [PubMed] [Google Scholar]
- 29.Cheng XX, Wang ZC, Chen XY, Sun Y, Kong QY, Liu J, Gao X, Guan HW, Li H. Frequent loss of membranous E-cadherin in gastric cancers: a cross-talk with Wnt in determining the fate of beta-catenin. Clin Exp Metastasis. 2005;22:85–93. doi: 10.1007/s10585-005-4578-8. [DOI] [PubMed] [Google Scholar]
- 30.Clement G, Braunschweig R, Pasquier N, Bosman FT, Benhattar J. Alterations of the Wnt signaling pathway during the neoplastic progression of Barrett’s esophagus. Oncogene. 2006;25:3084–92. doi: 10.1038/sj.onc.1209338. [DOI] [PubMed] [Google Scholar]
- 31.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Sun PL, Li JZ, Jheon S, Lee CT, Chung JH. Aberrant Wnt1/β-catenin expression is an independent poor prognostic marker of non-small cell lung cancer after surgery. J Thorac Oncol. 2011;6:716–724. doi: 10.1097/JTO.0b013e31820c5189. [DOI] [PubMed] [Google Scholar]
- 35.Abrahamsson AE, Geron I, Gotlib J, Dao KH, Barroga CF, Newton IG, Giles FJ, Durocher J, Creusot RS, Karimi M, Jones C, Zehnder JL, Keating A, Negrin RS, Weissman IL, Jamieson CH. Glycogen synthase kinase 3bmissplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci U S A. 2009;106:3925–3929. doi: 10.1073/pnas.0900189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nusse R. Wnt Signaling. Cold Spring Harb Perspect Biol. 2012;4:a011163. doi: 10.1101/cshperspect.a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu P, Li A, Wang Y, Hsieh CC, Huang MH, Hsu WH, Hsu HS. Reduced membranous β-catenin protein expression is associated with metastasis and poor prog-nosis in squamous cell carcinoma of the esophagus. J Thorac Cardiovasc Surg. 2008;135:1029–35. doi: 10.1016/j.jtcvs.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Ninomiya I, Endo Y, Fushida S, Sasagawa T, Miyashita T, Fujimura T, Nishimura G, Tani T, Hashimoto T, Yagi M, Shimizu K, Ohta T, Yonemura Y, Inoue M, Sasaki T, Miwa K. Alteration of beta-catenin expression in esophageal squamous-cell carcinoma. Int J Cancer. 2000;85:757–61. doi: 10.1002/(sici)1097-0215(20000315)85:6<757::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]


