Abstract
Objective: Esophageal squamous cell carcinoma (ESCC) is one of the leading causes of cancer deaths worldwide. CD9 has been reported to play a critical role in cell motility, growth and metastasis of multiple cancers. The present study investigated the clinicopathological features of CD9, and its biological characteristics in ESCC. Methods: Fifteen normal esophageal tissue specimens, fifty-three ESCC adjacent tissues and one hundred and four ESCC tissues were included in this study. Using immunohistochemistry (IHC), the expression levels of CD9 were evaluated among different samples. And its clinicopathological parameters and its prognostic factors were analyzed. Western blotting was used to measure CD9 expression and colony formation was performed to determine the effect of CD9 on cell growth in ESCC TE-1 cells. Results: Compared with normal esophageal tissues and tumor adjacent tissues, CD9 expression level is significantly higher in ESCC tissues. CD9 expression correlated with tumor stage (P = 0.022) and lymph node metastasis (P = 0.019) in ESCC patients. Furthermore, the small interfering RNA-mediated silencing of CD9 expression in TE-1 cells resulted in increased proliferation as evidenced by increased colony number and colony size. Conclusion: CD9 expression is upregulated in ESCC tissues and its expression is correlated with tumor stage and lymph node metastasis in ESCC patients. CD9 suppresses the proliferation of TE-1 cells. CD9 may present a potential in tumor progression in ESCC.
Keywords: Esophageal squamous cell carcinoma (ESCC), CD9, proliferation, metastasis
Introduction
The tetraspanins represent a large superfamily of four membrane-spanning proteins that are expressed in the plasma membrane of all mammalian cells [1,2]. Tetraspanins can interact with a diverse array of key proteins, including immune receptors, integrins, and signaling molecules [1,2]. CD9, a member of the tetraspanin superfamily, is the third most abundant protein on the platelet surface and required for the release of microparticles from coated-platelets [3,4]. The tetraspanin CD9 has been shown to be participated in diverse functions, such as cell motility, growth and immune response [5]. In recent years, CD9 is found to be involved in tumor progression [6]. Accumulating evidence supports a role for CD9 in metastatic cancers both in vitro and in vivo [7-9]. For example, CD9 expression upregulates pro-MMP-9 expression and release and promotes cellular invasion in a human fibrosarcoma cell line HT1080 [10]. However, controversial evidence was also reported showing the metastasis-inhibitory effect of CD9. Chen et al found that down-regulation of CD9 expression was more frequently observed in metastatic and invasive tumors [11]. CD9 overexpression of small-cell lung cancer (SCLC) cells reduces the metastatic spread of cancer cells via the inhibition of cell proliferation and motility [5,12]. CD9 expression is inversely correlated with tumor cell motility, proliferation and survival of patients in several solid tumors, including oral, pancreatic and gastrointestinal tumors [13-17]. Ectopic expression of CD9 can inhibit the proliferation and tumorigenicity in colon carcinoma cells [13]. Hashida et al found that CD9-positive patients had a higher survival rate than that of CD9-negative patients in 146 colon cancer patients. They also showed that nodal status and pathological status were inversely correlated with CD9 expression [18]. The above evidence suggests that the effect of CD9 in cancer may differ and depend on cancer types.
Esophageal cancer is the eighth most common cancer by incidence and ranks the sixth most common cause of cancer-related death worldwide [19,20]. This cancer has distinct geographic feature around the world and is highly prevalent in China. The 1- and 5-year survival rates are 50% and 15%, respectively and 95% of esophageal cancer patients are esophageal squamous cell carcinoma (ESCC) in China [20,21]. The absence of early symptoms, the metastasis of lymphadenopathy, hepatomegaly and a pleural effusion in the late stage make it difficult for the surveillance and treatment of this disease [21]. Metastasis plays a pivotal role in tumor progression, and is the major cause of ESCC-related death. It is warranted to find new biomarkers for evaluating the invasiveness, metastasis potential and prognosis of this tumor.
However, the specific function of CD9 in ESCC has not been reported. Uchida et al reported a relationship between CD9 expression and lymph node metastasis in ESCC [22]. To explore the clinicopathological significance and possible role of CD9 in ESCC development, we analyzed its expression in normal esophageal tissues and in ESCC tissues. Furthermore, we performed clone formation assays to investigate the role of CD9 in ESCC progression.
Materials and methods
Tissue samples
The ESCC samples were collected from 104 patients who had not received chemotherapy or radiotherapy prior to surgery. For these patients, if preoperative ultrasonography or computed tomography (CT) does not show any enlarged cervical lymph nodes (minor axis < 0.5 cm), patients were underwent a subtotal esophagectomy with two-field lymphadenectomy through a right thoracotomy, followed by a laparotomy. If enlarged cervical lymph nodes were shown by preoperative ultrasonography or CT, patients were to undergo radical oesophagectomy with cervico-thoraco-abdominal three-field lymphadenectomy through a right thoracotomy, followed by a laparotomy and a cervical incision. The resection extent includes nodes along the cervical part of the esophagus and deep cervix. Fifteen normal esophageal tissue samples were obtained from surgical resections of trauma patients. These tissues were obtained postoperatively in 2010 from the Gastrointestinal Center, Jiangyin People’s Hospital, Medical School of University of Southeast of China (Jiangyin, China) as reported previously [23]. All patients provided signed, informed consent for their tissue samples to be used for scientific research. The ethical approval for the study was obtained from the Jiangyin People’s Hospital, Medical School of University of Southeast of China. All diagnoses were based on pathological and/or cytological evidence. The histological features of the specimens were evaluated by a senior pathologist according to the classification criteria from the World Health Organization [24]. Tissue samples were obtained prior to chemotherapy and radiation therapy and were immediately fixed in 10% neutral buffered formalin prior to immunohistochemistry analysis.
Immunohistochemistry staining (IHC)
The tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin. Three-micrometer thick paraffin sections were deparaffinized and heat-treated with citrate buffer (pH 6.0) for 7 min as an epitope retrieval protocol. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 15 min at room temperature and tissue non-specific-binding sites were blocked with skim milk powder at 4% applied for 30 min. Sections were then incubated with the CD9 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h (dilution 1:60) and mixed with skim milk powder at 2% again to reduce the non-specific staining. Biotinylated secondary antibody was then added for 30 min. IHC staining was visualized with substrate solution containing diaminobenzidine (DAB) and hydrogen peroxide. The counter-staining was performed with haematoxylin. All steps were performed at room temperature. Negative controls consisted of tissue sections undergoing similar staining procedures in the absence of the primary antibody.
The criteria for scoring the stained sections were as follows: negative (0), < 10% of the whole tissue section stained positive; weakly positive (+ 1), 10-25% of the whole tissue section stained positive; moderately positive (+ 2), 25-75% of the whole tissue section stained positive; and strongly positive (+ 3), 75% of the tissue section stained positive.
Cell culture and transfection
The human esophageal cancer cell line, TE-1, was maintained in DMEM supplemented with 10% FBS and antibiotics (Gibco, Grand Island, NY). Cells were grown in a 37°C incubator with 5% CO2. The cells were grown in a 6-well culture plate to 70-80% confluence and then transfected with either siRNA-NC or siRNA-CD9 (Santa Cruz Biotechnology, Santa Cruz, CA) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. After 48 hours, the cells were collected for Western blotting.
Western blotting
After treatment, cells were harvested and lysed in RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) containing protease inhibitor cocktail (Sigma, St. Louis, MO) for 30 min at 4°C. Forty micrograms of protein from each lysate were fractionated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). After blocking with 5% nonfat milk in PBS-Tween-20 for 1 h at room temperature, the membranes were blotted with CD9 primary antibody (Sangon Biotech, Shanghai, China). α-Tubulin (Beyotime, Nantong, China) was used as a loading control. After washing four times with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary antibody (Santa Cruz Technology, Santa Cruz, CA) for 2 h. The proteins were visualized using the enhanced chemiluminescence (ECL, Beyotime, Nantong, China).
Colony formation
Cells transfected with the indicated siRNAs were plated at low density (1,000 cells per 6 cm plate). After transfection, cells were incubated for 10 days and fixed and stained with crystal violet. Foci and colonies containing more than 50 cells were counted using a microscope.
Statistical analysis
The differences in the demographic characteristics between the tumour patients and non-cancer subjects were evaluated using the X2 test. The differences between two groups were assessed using the Mann-Whitney U-test and the data involving more than two groups were analyzed using the Kruskal-Wallis test. The quantification of colony size and number was analyzed using ImageJ image analysis software (NIH, Bethesda, MD). The statistical analysis was performed using the SPSS software (Release 19.0, SPSS Inc.). The data were considered significant if P < 0.05.
Results
Expression of CD9 is upregulated in esophageal cancer tissues
To investigate the expression of CD9 in human ESCC tissue, we performed IHC analysis on tissue section of normal esophageal tissue specimens, ESCC and tumor adjacent tissues. The demographics features of patients for the immunohistochemistry staining are summarized in Table 1. 15 patients (9 males, 6 females) with stage I ESCC were included. Their age ranges from 55~69 years old, and the mean age is 62.8 years old. Stage II included 52 ESCC patients (37 males and 15 females), and their age ranges from 33~78 years old, with a mean age of 60.5 years old. Stage III had a total of 33 ESCC patients (23 males, 10 females) with age ranging from 32~75 years old, mean age of 59.2 years old. Stage IV ESCC patients (4 males, while no female) were collected, with age ranging from 52~67 years old and a mean age of 61.8 years old. Among the 104 ESCC tissues, 53 of which had corresponding adjacent tumor tissues. Besides, 15 paraffin-embedded normal esophageal tissue samples were also collected. Using immunohistochemistry, the expression of CD9 was detected in these tissue samples. An overall stronger staining for CD9 was frequently observed in the ESCC tissues, whereas very weak staining of CD9 was observed in the normal samples and tumor-adjacent tissues (Figure 1A). The expression of CD9 was observed in the plasma membrane of ESCC cancer cells (Figure 1A). The samples were further scored by a senior pathologist (Representative image of each score is shown in Figure 1B), as described in the Materials and Methods section. As shown in Figure 1B, CD9 expression in normal esophageal tissues was similar to that in adjacent tumor tissues; however, there were significant differences in the expression of CD9 among tumor tissues and between tumor tissues and adjacent tumor tissues (P < 0.01). These results indicated an increase in the expression of CD9 in esophageal carcinoma, suggesting its involvement in ESCC.
Table 1.
Patient demographics for the IHC analysis
| Normal | Grade I | Grade II | Grade III | Grade IV | All Tumors | |
|---|---|---|---|---|---|---|
| Number | 15 | 15 | 52 | 33 | 4 | 104 |
| Mean age (year) | 58.7 | 62.8 | 60.5 | 59.2 | 61.8 | 60.3 |
| Age range (year) | 32-72 | 55-69 | 33-78 | 32-75 | 52-67 | 32-78 |
| Sex | ||||||
| Male | 10 | 9 | 37 | 23 | 4 | 73 |
| Female | 5 | 6 | 15 | 10 | 0 | 31 |
Figure 1.
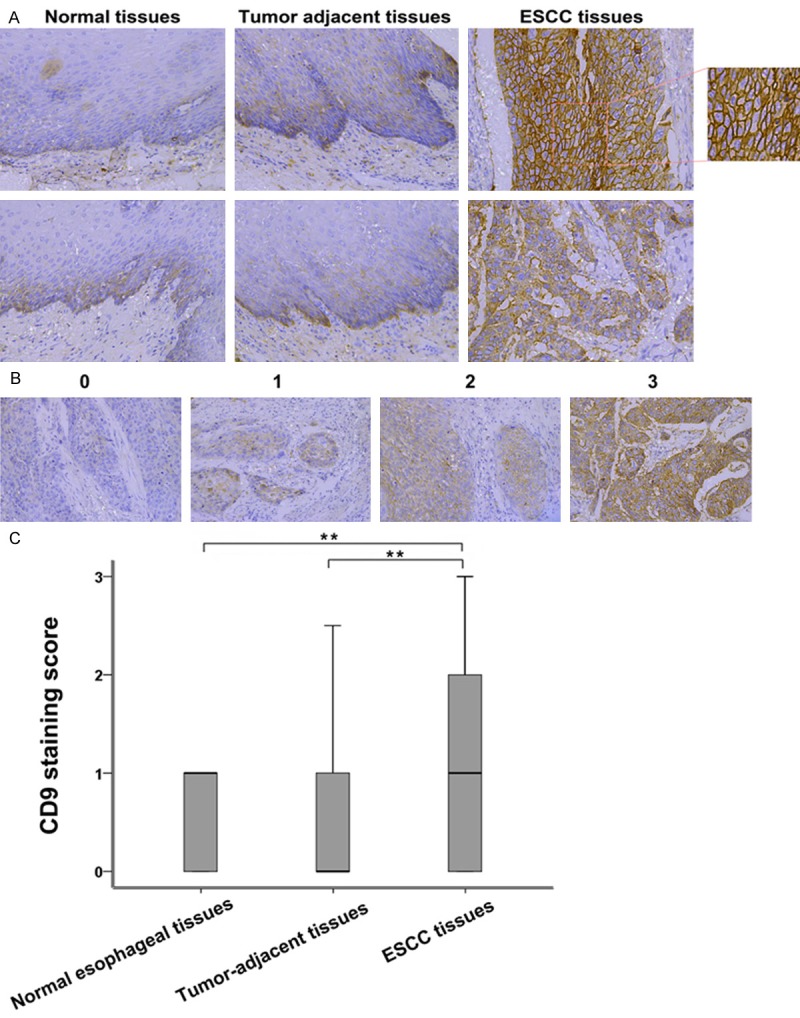
Overexpression of CD9 in ESCC tissues. A. Representatives IHC analysis of CD9 expression in normal esophageal tissue specimens, ESCC adjacent tissues and ESCC tissues. B. Representative images of IHC staining illustrating the scoring of CD9 in ESCC tissues (magnification, × 200). C. Box plot representing the range of CD9 immunohistochemical (IHC) staining score in normal esophageal tissues (n = 15), ESCC tissues (n = 104) and tumor adjacent tissues (n = 52), **(P < 0.01).
Correlation of CD9 expression with clinicopathological factors of ESCC
The relationship between clinicopathological and CD9 IHC staining score in ESCC is shown in Table 2. The results showed a non-significant correlation between CD9 expression and age, gender, histological grade and lymphatic invasion of ESCC patients. CD9 expression correlated with ESCC stage (P = 0.022) and lymph node metastasis (P = 0.019). The expression of CD9 protein level was higher in stage III-IV and metastasis ESCC tissues than in stage I-II tumors (1.210 ± 0.199 Vs. 1.084 ± 0.132, P < 0.05). The metastatic ESCC tissues showed significant higher expression than from non-metastatic tissues (1.583 ± 0.194 vs. 0.992 ± 0.131, P < 0.01). No other clinicopathological parameter is showed significant correlation with CD9 expression. These results suggest CD9 as a characteristic of this malignancy.
Table 2.
Relationship between clinicopathological and CD9 expression in esophageal squamous cell carcinoma (ESCC) (n = 104)
| Clinicopathological parameters | CD9 expression | P-Value | ||
|---|---|---|---|---|
|
| ||||
| Case | Mean | ± SEM | ||
| Age (years) | 0.467 | |||
| < 64 | 61 | 1.303 | 0.149 | |
| ≥ 64 | 43 | 1.127 | 0.178 | |
| Gender | 0.592 | |||
| Male | 73 | 1.281 | 0.141 | |
| Female | 31 | 1.113 | 0.189 | |
| Histological grade | 0.139 | |||
| Well | 25 | 1.200 | 0.206 | |
| Moderately | 48 | 1.031 | 0.168 | |
| Poorly | 31 | 1.565 | 0.218 | |
| Stage | 0.022 | |||
| 1 + 2 | 67 | 1.084 | 0.132 | |
| 3 + 4 | 37 | 1.210 | 0.199 | |
| pN | 0.019 | |||
| (+) | 42 | 1.583 | 0.194 | |
| (-) | 62 | 0.992 | 0.131 | |
| Lymphatic invasion | 0.079 | |||
| (+) | 49 | 1.459 | 0.176 | |
| (-) | 55 | 1.027 | 0.144 | |
pN, lymph node metastasis
CD9 suppresses the proliferation in TE-1 cells
To explore the role of CD9 in esophageal cancer cell growth, cells were first transfected with a siRNA targeting CD9 (siRNA-CD9) or a control siRNA (siRNA-NC) and the effective downregulation of CD9 was confirmed by Western blot. Our data revealed that CD9 protein expression was much lower in siRNA-CD9 group than that in siRNA-NC-transfected group (Figure 2A). And the colony formation assay showed that the number of TE-1 cell colonies formed in siRNA-CD9 group was significantly increased compared to the siRNA-NC-transfected group (P < 0.05, Figure 2B and 2C). The CD9 silencing cells also showed increased (Figure 2D). These results suggest that CD9 inhibits the proliferation of esophageal tumor cells.
Figure 2.
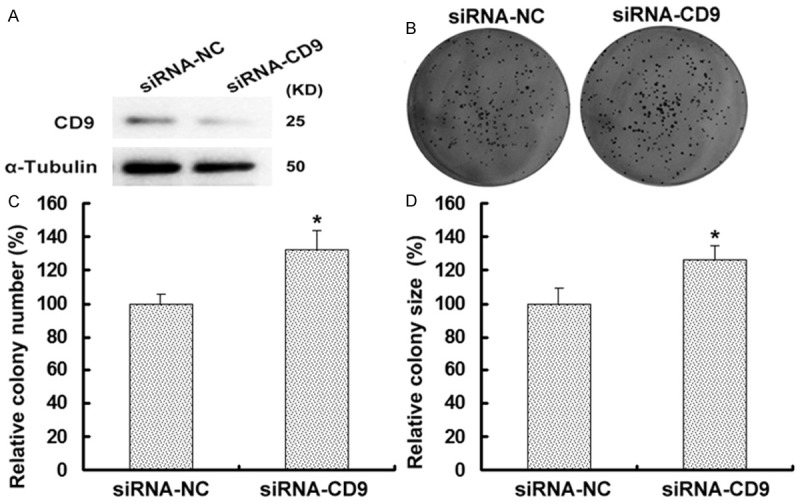
CD9 inhibits the growth of TE-1 cells. A. Western blot analysis of CD9 was performed on TE-1 cells with transfection with siRNA-NC or siRNA-CD9. CD9 protein level was higher in siRNA-NC group than that in siRNA-CD9 group. α-Tubulin was used as an internal control to ensure equal loading. B. TE-1 cells were analysis for their ability to form colonies via colony formation assay. Cells were transfected with siRNA-NC or siRNA-CD9 and incubated for 10 days. The figure showed one representative experiment repeated for three times with the similar results. C. The relative number of colonies formed after 10 days’ culture in siRNA-CD9 group was significantly more than that in siRNA-NC group (P < 0.05). D. Downregulation of CD9 increased the size of TE-1 cell size compared with the controls (P < 0.05).
Discussion
CD9 encodes a member of the transmembrane 4 superfamily, which is located at chromosome 12 (12 p13) in humans [25]. It contains 8 exons and 7 introns, spanning approximately 24- to 27-kDa, and plays major roles in cell signaling, proliferation, adhesion, motility and metastasis [4,6,26]. CD9, also called as motility related protein-1 (MRP-1), was firstly recognized by a monoclonal antibody M31-15 [27]. The expression and function of CD9 is widely studied in colon, lung, ovarian, breast and renal cell carcinomas [5,13,28-31]. CD9 expression is inversely correlated with metastasis status and patient survival rate in renal cancer [5]. Overexpression of human CD9/MRP-1 suppressed cell motility and metastasis into human lung adenocarcinoma cells and human myeloma cells [16]. Ectopic expression of CD9 caused the inhibition of cell proliferation and induced apoptosis in small cell lung cancer cells [26]. In this study, we employed the transfected TE-1 cell lines with different expression level of CD9 to illustrate its influence on tumor cell growth. We found that the small interfering RNA-mediated silencing of CD9 expression promotes cell proliferation of ESCC cells, which is consistent with other studies demonstrating a potent inhibitory of CD9 protein in other tumor cell growth [12,13,26].
Our study also describes the clinicopathological significance of CD9 in ESCC. Using IHC for human ESCC tissues, we found that weak expression of CD9 in normal tissues and adjacent tumor tissues, whereas increased expression in ESCC tissues. We assume that CD9 may be involved in esophageal oncogenesis and tumor development. ESCC is one of the most aggressive cancers and no effective measure has been developed to completely cure this malignancy so far. And the metastasis of lymphadenopathy is major pathway for ESCC cells to spread. Results revealed that CD9 level is correlated with tumor stage and lymph node metastasis. CD9 expression in patients with TNM stage III-IV and metastatic ESCC was significantly higher than that in patients with TNM stage I-II, non-metastatic ESCC. These results suggest that CD9 may be a predicator of malignant degree and may act as a predictive and prognostic factor. Furthermore, our results are consistent with the known function of CD9 as a tumor suppressor in other tumors. Uchida et al demonstrated that reduced expression of CD9 correlated to lymph node metastasis in ESCC, and they also found an inverse correlation between CD9 expression and lymphatic invasion [22]. Adenoviral infection of CD9/MRP-1 in orthotopic lung cancer could dramatically inhibit the lymph nodes metastasis to the mediastinum [32]. In human fibrosarcoma cell line HT1080, a reduction of CD9 resulted in the promotion of tumor metastasis potential, and CD9 could inhibit cell transformation via the downregulation of Wnt1, and it may suppress tumor metastasis via the downregulation of Wnt5a [33]. They also found that CD9 could downregulate VEGF-A expression [33]. Therefore, CD9 may be a potential target for the diagnosis and therapy of ESCC as reported in other cancer types [24]. Further studies are needed to demonstrate the molecule mechanism by which CD9 modulates the malignant behavior of ESCC.
In summary, our study provide evidence that CD9 expression is upregulated in ESCC tissues And CD9 level is correlated with tumor stage lymph node metastasis and lymphatic invasion, which may present a potential target for ESCC treatment.
Acknowledgements
This work is supported by the the National Natural Science Foundation of China (81372411), Jiangsu Provincial Special Program of Clinical Medical Science (BL2014040) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Disclosure of conflict of interest
None.
References
- 1.van Spriel AB. Tetraspanins in the humoral immune response. Biochem Soc Trans. 2011;39:512–527. doi: 10.1042/BST0390512. [DOI] [PubMed] [Google Scholar]
- 2.Lazo PA. Functional implications of tetraspanin proteins in cancer biology. Cancer Sci. 2007;98:1666–77. doi: 10.1111/j.1349-7006.2007.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herr MJ, Longhurst CM, Baker B, Homayouni R, Speich HE, Kotha J, Jennings LK. Tetraspanin CD9 modulates human lymphoma cellular proliferation via histone deacetylase activity. Biochem Biophys Res Commun. 2014;447:616–620. doi: 10.1016/j.bbrc.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 4.Dale GL, Remenyi G, Friese P. Tetraspanin CD9 is required for microparticle release from coated-platelets. Platelets. 2009;20:361–366. doi: 10.1080/09537100903096692. [DOI] [PubMed] [Google Scholar]
- 5.Kwon HJ, Min SY, Park MJ, Lee C, Park JH, Chae JY, Moon KC. Expression of CD9 and CD82 in clear cell renal cell carcinoma and its clinical significance. Pathol Res Pract. 2014;210:285–290. doi: 10.1016/j.prp.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Wang HX, Li Q, Sharma C, Knoblich K, Hemler ME. Tetraspanin protein contributions to cancer. Biochem Soc Trans. 2011;39:547–552. doi: 10.1042/BST0390547. [DOI] [PubMed] [Google Scholar]
- 7.Herr MJ, Kotha J, Hagedorn N, Smith B, Jennings LK. Tetraspanin CD9 promotes the invasive phenotype of human fibrosarcoma cells via upregulation of matrix metalloproteinase-9. PLoS One. 2013;8:e67766. doi: 10.1371/journal.pone.0067766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kischel P, Bellahcene A, Deux B, Lamour V, Dobson R, DE Pauw E, Clezardin P, Castronovo V. Overexpression of CD9 in Human Breast Cancer Cells Promotes the Development of Bone Metastases. Anticancer Res. 2012;32:5211–5220. [PubMed] [Google Scholar]
- 9.Iwasaki T, Takeda Y, Maruyama K, Yokosaki Y, Tsujino K, Tetsumoto S, Kuhara H, Nakanishi K, Otani Y, Jin Y. Deletion of tetraspanin CD9 diminishes lymphangiogenesis in vivo and in vitro. J Biol Chem. 2013;288:2118–2131. doi: 10.1074/jbc.M112.424291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herr MJ, Mabry SE, Jameson JF, Jennings LK. Pro-MMP-9 upregulation in HT1080 cells expressing CD9 is regulated by epidermal growth factor receptor. Biochem Biophys Res Commun. 2013;442:99–104. doi: 10.1016/j.bbrc.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Gu S, Trojanowicz B, Liu N, Zhu G, Dralle H, Hoang-Vu C. Down-regulation of TM4SF is associated with the metastatic potential of gastric carcinoma TM4SF members in gastric carcinoma. World J Surg Oncol. 2011;9:43. doi: 10.1186/1477-7819-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng R, Yano S, Zhang H, Nakataki E, Tachibana I, Kawase I, Hayashi S, Sone S. CD9 overexpression suppressed the liver metastasis and malignant ascites via inhibition of proliferation and motility of small-cell lung cancer cells in NK cell-depleted SCID mice. Oncol Res. 2005;15:365–372. doi: 10.3727/096504005776449699. [DOI] [PubMed] [Google Scholar]
- 13.Ovalle S, Gutierrez-Lopez MD, Olmo N, Turnay J, Lizarbe MA, Majano P, Molina-Jimenez F, Lopez-Cabrera M, Yanez-Mo M, Sanchez-Madrid F, Cabanas C. The tetraspanin CD9 inhibits the proliferation and tumorigenicity of human colon carcinoma cells. Int J Cancer. 2007;121:2140–2152. doi: 10.1002/ijc.22902. [DOI] [PubMed] [Google Scholar]
- 14.Mori M, Mimori K, Shiraishi T, Haraguchi M, Ueo H, Barnard GF, Akiyoshi T. Motility related protein 1 (MRP1/CD9) expression in colon cancer. Clin Cancer Res. 1998;4:1507–1510. [PubMed] [Google Scholar]
- 15.Higashiyama M, Taki T, Ieki Y, Adachi M, Huang CL, Koh T, Kodama K, Doi O, Miyake M. Reduced motility related protein-1 (MRP-1/CD9) gene expression as a factor of poor prognosis in non-small cell lung cancer. Cancer Res. 1995;55:6040–6044. [PubMed] [Google Scholar]
- 16.Ikeyama S, Koyama M, Yamaoko M, Sasada R, Miyake M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J Exp Med. 1993;177:1231–1237. doi: 10.1084/jem.177.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sho M, Adachi M, Taki T, Hashida H, Konishi T, Huang Cl, Ikeda N, Nakajima Y, Kanehiro H, Hisanaga M. Transmembrane 4 superfamily as a prognostic factor in pancreatic cancer. Int J Cancer. 1998;79:509–516. doi: 10.1002/(sici)1097-0215(19981023)79:5<509::aid-ijc11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Hashida H, Takabayashi A, Tokuhara T, Hattori N, Taki T, Hasegawa H, Satoh S, Kobayashi N, Yamaoka Y, Miyake M. Clinical significance of transmembrane 4 superfamily in colon cancer. Br J Cancer. 2003;89:158–167. doi: 10.1038/sj.bjc.6601015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 20.Tang WR, Chen ZJ, Lin K, Su M, Au WW. Development of esophageal cancer in Chaoshan region, China: association with environmental, genetic and cultural factors. Int J Hyg Environ Health. 2015;218:12–18. doi: 10.1016/j.ijheh.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 21.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 22.Uchida S, Shimada Y, Watanabe G, Li ZG, Hong T, Miyake M, Imamura M. Motility-related protein (MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 1999;79:1168–1173. doi: 10.1038/sj.bjc.6690186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Zhou X, Ge X, Liu P, Cao J, Lu X, Ling Y, Zhang S. Upregulation of Ying Yang 1 (YY1) suppresses esophageal squamous cell carcinoma development through heme oxygenase-1. Cancer Sci. 2013;104:1544–1551. doi: 10.1111/cas.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobin LH, Wittekind C. TNM Classification of Malignant Tumor. 6th edition. New Jersey: John Wiley and Sons; 2002. [Google Scholar]
- 25.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 26.Saito Y, Tachibana I, Takeda Y, Yamane H, He P, Suzuki M, Minami S, Kijima T, Yoshida M, Kumagai T. Absence of CD9 enhances adhesion-dependent morphologic differentiation, survival, and matrix metalloproteinase-2 production in small cell lung cancer cells. Cancer Res. 2006;66:9557–9565. doi: 10.1158/0008-5472.CAN-06-1131. [DOI] [PubMed] [Google Scholar]
- 27.Miyake M, Koyama M, Seno M, Ikeyama S. Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J Exp Med. 1991;174:1347–1354. doi: 10.1084/jem.174.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang JR, Jo K, Lee Y, Sung BJ, Park YW, Lee JH. Upregulation of CD9 in ovarian cancer is related to the induction of TNF-α gene expression and constitutive NF-κB activation. Carcinogenesis. 2012;33:77–83. doi: 10.1093/carcin/bgr257. [DOI] [PubMed] [Google Scholar]
- 29.Kohmo S, Kijima T, Otani Y, Mori M, Minami T, Takahashi R, Nagatomo I, Takeda Y, Kida H, Goya S. Cell surface tetraspanin CD9 mediates chemoresistance in small cell lung cancer. Cancer Res. 2010;70:8025–8035. doi: 10.1158/0008-5472.CAN-10-0996. [DOI] [PubMed] [Google Scholar]
- 30.Si Z, Hersey P. Expression of the neuroglandular antigen and analogues in melanoma. CD9 expression appears inversely related to metastatic potential of melanoma. Int J Cancer. 1993;54:37–43. doi: 10.1002/ijc.2910540107. [DOI] [PubMed] [Google Scholar]
- 31.Miyake M, Nakano K, Itoi SI, Koh T, Taki T. Motility-related protein-1 (MRP-1/CD9) reduction as a factor of poor prognosis in breast cancer. Cancer Res. 1996;56:1244–1249. [PubMed] [Google Scholar]
- 32.Takeda T, Hattori N, Tokuhara T, Nishimura Y, Yokoyama M, Miyake M. Adenoviral transduction of MRP-1/CD9 and KAI1/CD82 inhibits lymph node metastasis in orthotopic lung cancer model. Cancer Res. 2007;67:1744–1749. doi: 10.1158/0008-5472.CAN-06-3090. [DOI] [PubMed] [Google Scholar]
- 33.Huang Cl, Liu D, Masuya D, Kameyama K, Nakashima T, Yokomise H, Ueno M, Miyake M. MRP-1/CD9 gene transduction downregulates Wnt signal pathways. Oncogene. 2004;23:7475–7483. doi: 10.1038/sj.onc.1208063. [DOI] [PubMed] [Google Scholar]
- 34.Zhang BH, Liu W, Li L, Lu JG, Sun YN, Jin DJ, Xu XY. KAI1/CD82 and MRP1/CD9 Serve as Markers of Infiltration, Metastasis, and Prognosis in Laryngeal Squamous Cell Carcinomas. Asian Pac J Cancer Prev. 2013;14:3521–3526. doi: 10.7314/apjcp.2013.14.6.3521. [DOI] [PubMed] [Google Scholar]


